DEHPA在Petrofin中萃取分离La(III)和Ni(Ⅱ)的动力学与机理
来源期刊:中国有色金属学报(英文版)2019年第7期
论文作者:Swagatika SATPATHY Sujata Mishra
文章页码:1538 - 1548
关键词:萃取;分离;动力学;机理;La(III);Ni(II)
Key words:extraction; separation; kinetics; mechanism; La(III); Ni(II)
摘 要:采用界面积恒定的连续搅拌萃取池,研究在乳酸存在条件下,以用Petrofin稀释的二-2-乙基己基磷酸(DEHPA)作萃取剂从硝酸盐介质中萃取分离La(III)与Ni(Ⅱ)的动力学。考察搅拌速度、界面积、pH值、乳酸浓度、萃取剂浓度、金属离子浓度和温度对萃取速率的影响。结果表明,该萃取体系是受扩散控制的,界面反应为速率控制步骤。两种金属离子的萃取速率均与pH值无关。La(III)和Ni(II)的萃取速率与乳酸浓度和金属离子(La(III)或Ni(II))浓度呈线性关系。La(III)的萃取速率与DEHPA浓度呈线性关系,而Ni(Ⅱ) 的萃取速率则与DEHPA浓度的1.5次方呈线性关系。在低界面积和低搅拌速度的条件下,从硝酸盐溶液中分离La(III)和Ni(Ⅱ)是可行的。
Abstract: The kinetics of extractive separation of La(III) and Ni(II) from nitrate medium in the presence of lactic acid (HLac) using di-2-ethylhexyl phosphoric acid (DEHPA) diluted in petrofin was investigated using a cell with constant interfacial area and continuous stirring. The effects of stirring speed, interfacial area, pH, HLac concentration, extractant concentration, concentrations of metal ions and temperature on the extraction rate were examined. Results suggested that the extraction regime is diffusion-controlled. The reaction which occurred at the interface was found to be the rate-determining step. The extraction rates of both metal ions are found to be independent of pH. The extraction rates of La(III) and Ni(II) are first-order dependent with respect to lactic acid and metal ions (La(III) and Ni(II)) concentrations. The extraction rate of La(III) is first-order dependent on DEHPA concentration and for Ni(II), it varies to the power of 1.5. The separation of La(III) and Ni(II) from nitrate solution is possible at low interfacial area and low stirring speed.
Trans. Nonferrous Met. Soc. China 29(2019) 1538-1548
Swagatika SATPATHY, Sujata MISHRA
Department of Chemistry, Institute of Technical Education and Research, Siksha ‘O’ Anusandhan Deemed to be University, Khandagiri Square, Bhubaneswar-751030, Odisha, India
Received 14 July 2018; accepted 21 May 2019
Abstract: The kinetics of extractive separation of La(III) and Ni(II) from nitrate medium in the presence of lactic acid (HLac) using di-2-ethylhexyl phosphoric acid (DEHPA) diluted in petrofin was investigated using a cell with constant interfacial area and continuous stirring. The effects of stirring speed, interfacial area, pH, HLac concentration, extractant concentration, concentrations of metal ions and temperature on the extraction rate were examined. Results suggested that the extraction regime is diffusion-controlled. The reaction which occurred at the interface was found to be the rate-determining step. The extraction rates of both metal ions are found to be independent of pH. The extraction rates of La(III) and Ni(II) are first-order dependent with respect to lactic acid and metal ions (La(III) and Ni(II)) concentrations. The extraction rate of La(III) is first-order dependent on DEHPA concentration and for Ni(II), it varies to the power of 1.5. The separation of La(III) and Ni(II) from nitrate solution is possible at low interfacial area and low stirring speed.
Key words: extraction; separation; kinetics; mechanism; La(III); Ni(II)
1 Introduction
New processes are needed to be explored for the recovery of rare earths and nickel from the electrodes of used nickel metal hydride (NiMH) batteries [1,2]. The quantitative separation of metal ions is difficult in the equilibrium state and can be made easier on the basis of difference in their extraction kinetics [3-5]. The study of equilibrium provides information regarding reactions occurring in the aqueous phase, organic phase or at the interface, but it does not furnish idea about formation of intermediates which can be obtained from the kinetics studies [6]. The knowledge about rate-controlling step and overall mechanism help in achieving quantitative separation [7]. The rate studies of solvent extraction systems are vital to obtain clear information regarding the nature of these processes. The idea about limitations and advantages of the extraction rate could be known from the knowledge of overall rate of mass transfer and of the extraction mechanism in an extraction system. It plays an important role in evaluation of system’s industrial application and optimization of the operating conditions [8]. Researchers have carried out solvent extraction of rare earths using various extractants [9-11]. The kinetics of solvent extraction of rare earths has been studied by many researchers [12-15]. The effect of HLac and citric acid on the extraction kinetics of Nd(III) using DEHPA has been reported [16]. The extraction kinetics of Nd3+ using DIDPA (diisodecylphosphoric acid) has been studied at 303 K [17]. The extraction equilibrium constants for diluents n-heptane, toluene and benzene were determined and the process was found to be exothermic based on the study of thermodynamics. The extraction rate of Sm(III) from aqueous nitrate solution with DEHPA and bis (2, 4, 4-trimethylpentyl) dithiophosphinic acid (Cyanex 301) in kerosene was studied by single drop-column method. The extraction rate shows direct dependence on the concentrations of Sm(III), DEHPA and Cyanex 301 and inversely varies with aqueous phase acid concentration. The extraction rate using Cyanex 301 was lower as compared to that with DEHPA [18]. Studies on the extraction rate of La(III) from nitrate-acetato medium by bis-(2, 4, 4- trimethylpentyl) phosphinic acid (Cyanex 272) using single drop-column have also been reported [19]. Mass transfer kinetics of La(III) extraction in chloride medium by bifunctional ionic liquid [A336] [CA-12] (tricaprylmethylammonium sec-octylphenoxy acetic acid) using a constant interfacial cell with the laminar flow was investigated and the results indicated that the extraction was a mixed-controlled process influenced by interfacial reaction [20]. Researchers have studied the forward and backward extraction kinetics of Nd(III) using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC 88A) in xylene and found that the extraction depends on different parameters such as pH of the aqueous solution, metal ion concentration, extractant concentration, stirring speed and other parameters[21]. High value of activation energy supported the fact that the extraction regime was chemical reaction controlled which took place at the interface. Extraction kinetics of La(III) from chloride medium with two complexing agents HLac and citric acid in the aqueous phase by DEHPA has been carried out using constant interfacial area cell with laminar flow. The extraction rate has been found to follow pseudo-first-order kinetics. Factors affecting extraction rates such as stirring speed, temperature and specific interfacial area have been investigated [22].
Kinetic studies are of great importance in the design, operation, control and optimization of reactors in chemical industry. Chemical kinetics of extraction process throws light on extraction and mechanism rates involved there. The difference in extraction kinetics of two metals was used for the improvement of separation of metals [23-25].
The rate of a chemical reaction in an extraction process depends primarily on stirring speed, interfacial area, chemical compositions of aqueous and organic phases. It is quite challenging to analyze systems consisting of a number of complex chemical species. In our recent report, we have investigated the separation possibility of La(III) and Ni(II) using HLac in the aqueous phase by solvent extraction under various experimental conditions [26]. The modification of aqueous phase with addition of a water-soluble complexing agent like HLac leads to the enhanced separation of La(III) and Ni(II). Therefore, kinetics and mechanisms involved in the solvent extraction separation of La(III) and Ni(II) have been investigated using HLac in the aqueous medium. So far, few attempts have been made to study the kinetics of separation of lanthanides and transition metals. Petrofin (n-tridecane) is a less-volatile, cheap, non-polar, non-toxic diluent having flash point (94 °C) higher than that of the conventionally used diluent kerosene (37-65 °C). Therefore, there is minimum risk of volume loss due to evaporation by using petrofin as diluent. The present research described the kinetics of extractive separation of La(III) and Ni(II) in the presence of HLac using DEHPA and petrofin in the organic phase with the help of a cell having constant interfacial area and continuous stirring. The influence of stirring speed, pH, temperature, concentrations of metal ions, HLac and DEHPA on the extraction rate was analyzed in detail.
2 Experimental
2.1 Chemicals
The extractant DEHPA having purity >98%, procured from Merck was diluted in desired concentration of petrofin obtained from Sri Ashok Petro Products, Fine Par Oil. La(NO3)3·6H2O (99%), Ni(NO3)3·6H2O (99%), HLac (92%) and the spectrophotometric reagent Arsenazo III were supplied by Merck. All other reagents used in this experiment were of analytical reagent grade.
2.2 Instrument and analysis procedures
The metal salts were weighed accurately with the help of a citizen digital balance (CY 320C) with a precision of ±0.001 g. The pH measurement was done with the aid of a pH meter (Systronics 335). The complete phase disengagement of the aqueous and organic mixtures after extraction was achieved using a REMI R-4C centrifuge. The concentrations of La(III) in the aqueous phase before and after extraction were estimated at 655 nm with a double-beam (ELICO SL-244, India) UV-Vis spectrophotometer using Arsenazo III as the chromophoric reagent while the estimation of Ni(II) concentration was carried out with an atomic absorption spectrophotometer (ELICO SL-176, India).
2.3 Kinetics study
For kinetics studies the concentrations of La(III) and Ni(II) in the aqueous phase were taken as 1.616 g/L Ni(II) (0.0275 mol/L) and 1.389 g/L La(III) (0.01 mol/L, respectively), which were the same as the amounts present in leached solutions of spent nickel metal hydride batteries [26]. Double-distilled water was used for dilution wherever desired. The complexing agent used in the present study was HLac. Dilute solutions of HNO3 and NaOH were used each time for adjusting the pH of the aqueous phase. The variations of parameters where the percentage of extraction is almost 50% can help to investigate the details of effect of these variables on extraction [6]. Keeping this in view all the levels of variations are chosen. The kinetics of extractive separation of La(III) and Ni(II) was determined with the help of a constant interface stirred cell made up of glass with interfacial area (Q) of 10.17 cm2. However, to know the influence of interfacial area on extraction rate, the cells with different interfacial areas were used. Equal volumes of aqueous and organic phases were taken in the cell and the stirring speed was fixed at 500 r/min throughout the course of experiments to keep the hydrodynamic conditions constant. The stirring speed was varied only in the case of experiments designed to study its effect on the extraction rate.
The contents of the cell were stirred for different time intervals ranging from 30 to 300 s. After extraction, the aqueous-organic mixture was left undisturbed for some time and was centrifuged thereafter for complete separation of the two phases. The loaded organic solvent was separated from the raffinate. The La(III) and Ni(II) concentrations in the aqueous phase before and after extraction at different time intervals were determined. The experiments were performed in triplicate and the reproducibility of the results obtained was obtained by taking the average values of the measurements. The experimental conditions for kinetics study of La(III) and Ni(II) have been presented in Table 1 and the schematic representation of a cell having constant interfacial area and continuous stirring is shown in Fig. 1.
3 Results and discussion
In order to demonstrate the kinetics of solvent extraction separation of La(III) and Ni(II) in the presence of HLac using DEHPA in petrofin, a series of experiments were performed by varying each of the operating parameters. The concentrations of La(III) and Ni(II) in the organic phase after extraction were estimated at 30, 90, 150, 210, 270 and 300 s using material balance. The extraction rates (r) were calculated from the slopes of the plots of concentration versus time and expressed as Eqs. (1) and (2):
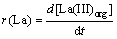 (1)
(1)
 (2)
(2)
3.1 Identification of extraction regime
In the kinetics study, the recognition of extraction regime opens the way to analyze the process of mass transfer in the extraction. In order to establish the extraction regime which rules the kinetics of extraction, adequate information concerning solubility of extractant, and composition of aqueous and organic phases are required. At given chemical composition of the extraction system and constant hydrodynamic conditions, different experimental variables are evaluated to decide the extraction regime. The lower solubility of organic extractant in the aqueous phase confines the location of rate-determining step of the reaction to interfacial zone and diffusion aqueous film [27]. When the kinetics is in diffusion regime, it is well explained that mass transfer is accompanied with instantaneous chemical reaction. If slow chemical reaction occurs at the interface the resistance to mass transfer process increases [28].
3.1.1 Effect of stirring speed
The study of effect of stirring speed on the extraction rate helps to find the rate-determining step of chemical reaction involved in the extraction. The extraction regime in the kinetics study is usually identified from the dependency of extraction rate on the stirring speed at a fixed interfacial area [29]. Usually, the extraction process taking place with diffusion contribution is identified through an enhancement in the extraction rate, with the increase in the stirring speed of the aqueous and organic phases [30]. The influence of stirring speed on the extraction rate has been investigated keeping all other parameters unchanged. The plots of La(III) and Ni(II) concentrations in the organic phase versus time at different stirring speeds are shown in Figs. 2 and 3, respectively. Linear dependence of extraction rates on the stirring speed has been observed for both La(III) and Ni(II) shown in Fig. 4. The values of r(La) and r(Ni) have been calculated from the slopes of figures for different stirring speeds given in Figs. 2 and 3. The increase in extraction rate of La(III) with increase in stirring speed from 200 to 500 r/min substantiates the fact that the process is diffusion- controlled rather than chemically-controlled [22]. The plateau region observed in Fig. 4 reveals that the extraction is diffusion-controlled and chemical reactions occurring here are very fast. Furthermore, if a slow interfacial or bulk chemical reaction occurs at low stirring speed, the thickness of the interfacial region becomes large so that the process of diffusion becomes the slowest and rate-controlled one [18]. The same has been observed in the case of Ni(II). At low stirring speed, there seems to be a large difference in the extraction rates of La(III) and Ni(II) which gives a better possibility option for separation.
Table 1 Operating conditions used in rate studies of extractive separation of La(III) and Ni(II)

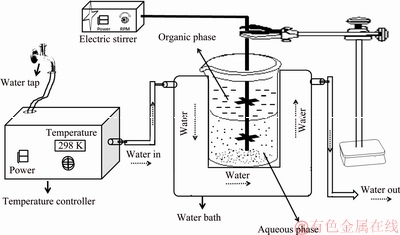
Fig. 1 Schematic representation of continuously stirred cell with constant interfacial area

Fig. 2 Plot of [La(III)]org versus time at various stirring speeds
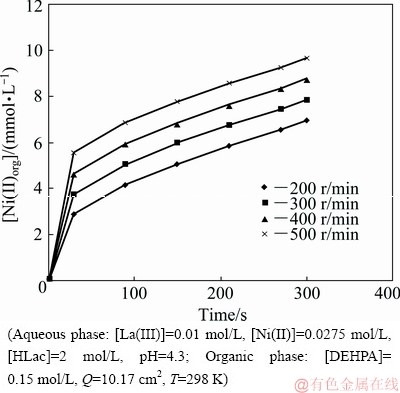
Fig. 3 Plot of [Ni(II)]org versus time at various stirring speeds
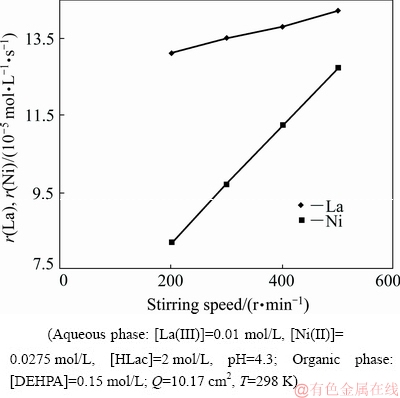
Fig. 4 Dependence of extraction rates of La(III) and Ni(II) on stirring speed
3.1.2 Effect of interfacial area
The solvent extraction kinetics which has industrial relevance is best explained by the interfacial chemistry. Phase transfer becomes enhanced by maximizing interfacial area [30]. The extractant generally prefers the interfacial region rather than the bulk as free energy of the solution is minimum in this zone [8]. The change in the interfacial area is used to distinguish chemical reactions occurring in the bulk phase and at the interface. If it takes place at the interface, extraction rate would certainly increase with the increase in interfacial area. Experiments were performed by increasing the interfacial area from about 10 to 33 cm2. Figures 5 and 6 represent plots of organic phase concentrations of both metal ions against time at various interfacial areas. Based on the experiments conducted, a linear relationship was obtained between the interfacial area and the extraction rates of La(III) and Ni(II), as shown in Fig. 7, which is the characteristic of an interfacial reaction for La(III) and Ni(II) extraction with DEHPA. So, it can be deduced that the rate-determining step reaction occurs in the interfacial region [13,19]. From the experimental results it seems that the separation of La(III) and Ni(II) is possible based on the large difference in their extraction rates with low interfacial area.

Fig. 5 Plot of [La(III)]org versus time at various interfacial area

Fig. 6 Plot of [Ni(II)]org versus time at various interfacial area
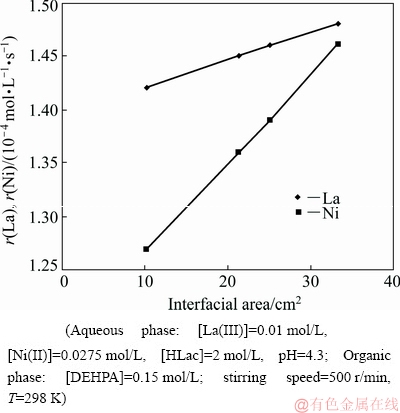
Fig. 7 Dependence of extraction rates of La(III) and Ni(II) on interfacial area
3.2 Effect of temperature
For the extraction which falls in diffusion regime, temperature has negligible effect on the extraction rate. The variation of temperature in the range of 298-328 K was studied in order to observe its influence on the extraction rates of La(III) and Ni(II) under the experimental conditions given in Table 1. Temperature has negative influence on the extraction rates (Figs. 8 and 9). The activation energies have been calculated using Arrhenius equation from the slopes of the plots of lg r against 1000/T (Fig. 10). The data have been given in Table 2. The activation energies were found to be -1.34 and -3.44 kJ/mol for La(III) and Ni(II), respectively. The magnitude of activation energy, Ea, is less than 20 kJ/mol, indicating that extraction rate is diffusion-controlled [16]. Negative value of activation energy supports the assertion that it is a barrier-less and spontaneous process [31]. The reaction process depends on the capture of the molecules in the potential well. The increase in temperature reduces the probability of complex forming reaction which leads to less extraction. As seen from Fig. 10, the separation of La(III) and Ni(II) is feasible at high temperatures due to large difference in the extraction rates.

Fig. 8 Plot of [La(III)]org versus time at various temperatures

Fig. 9 Plot of [Ni(II)]org versus time at various temperatures
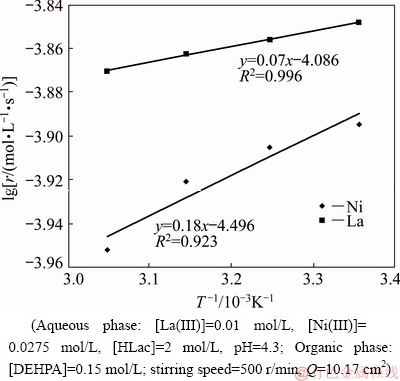
Fig. 10 Effect of temperature on extraction rates of La(III) and Ni(II)
3.3 Effect of aqueous pH
The effect of aqueous pH on the extraction rate has been investigated by varying it in the range from 2.5 to 4.3. Plot of [La(III)]org versus time at various aqueous pH is shown in Fig. 11. The extraction rate constants obtained for La(III) and Ni(II) have been found to be nearly constant with variation of pH. The values of extraction rate obtained for La(III) at pH 2.5, 3, 3.5 and 4.3 have been calculated to be 6×10-6, 5×10-6, 4×10-6 and 3×10-6 mol/(L·s), respectively. For Ni(II), no change in the extraction rate was obtained with change in pH. The extraction percentage of Ni(II) varied from 0.25 to 3.7 at extraction time from 30 to 300 s at pH 2.5, 3.0, 3.5 and 4.3. This may be due to the fact that HLac added to the aqueous phase acts as a buffer which resists the change in pH. This indicates that under the present experimental conditions extraction rate is independent of the aqueous phase pH (Fig. 12) (slope is 0.08 in the case of La(III)). In the case of Ni(II) there was no change in the extraction rate with pH.
Table 2 Extraction rate and activation energy data for La(III) and Ni(II)

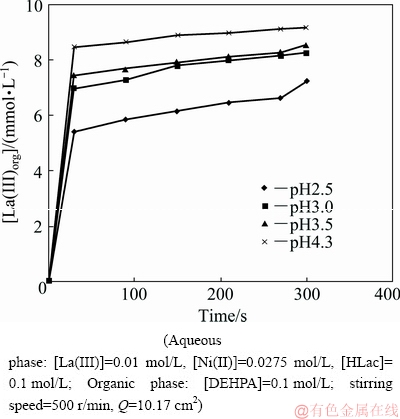
Fig. 11 Plot of [La(III)]org versus time at various pH

Fig. 12 Effect of pH on extraction rate of La(III)
3.4 Effect of HLac concentration
The concentration of HLac was varied in the range of 0.005-0.1 mol/L and 0.1-2 mol/L for studying extraction rate dependence of La(III) and Ni(II), respectively while all other parameters remained constant as presented in Table 1. To study the effect of HLac concentration on the extraction rate of La(III), lower concentrations of HLac were used in the aqueous phase since with higher concentrations, the extraction rate of La(III) was very high. In the case of Ni(II), with low concentration of HLac in the aqueous feed, very low extraction rate was found. Therefore, higher concentrations of HLac were considered for studying its effect on the extraction rate of Ni(II). Figures 13 and 14 represent the plots of [La(III)]org and [Ni(II)]org versus time at various HLac concentrations, respectively. The plots of lg r against lg [HLac] shown in Figs. 15 and 16 give straight lines with slopes of 0.91 and 0.87 for La(III) and Ni(II), respectively. As the extraction rate is directly proportional to the concentration of HLac, the reaction order with respect to [HLac] is said to be 1 for both La(III) and Ni(II).
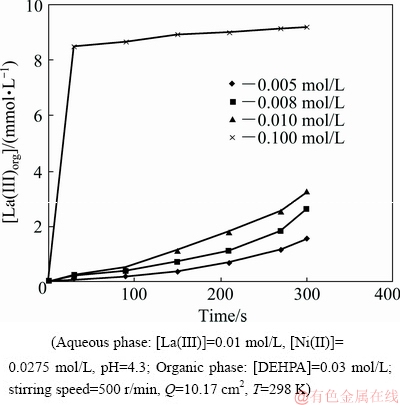
Fig. 13 Plot of [La(III)]org versus time at various HLac concentrations
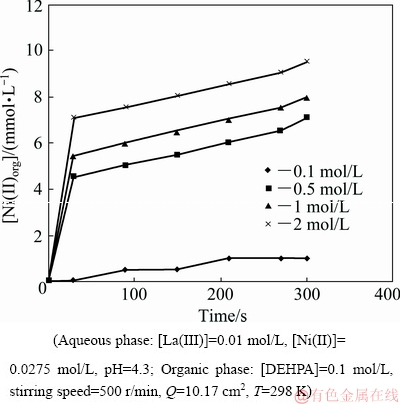
Fig. 14 Plot of [Ni(II)]org versus time at various HLac concentrations

Fig. 15 Effects of [M] (M=HLac, DEHPA and La(III)) concentrations on extraction rate of La(III)

Fig. 16 Effects of [M] (M=HLac, DEHPA and Ni(II)) concentration on extraction rate of Ni(II)
3.5 Effect of DEHPA concentration
The extraction rates of La(III) and Ni(II) were measured at various DEHPA concentrations in the range from 0.02 to 0.2 mol/L for La(III) and from 0.1 to 0.4 mol/L for Ni(II). Lower concentrations of DEHPA were considered for studying its effect on the extraction rate of La(III) as in the presence of higher DEHPA concentration the extraction rate becomes very high. On the contrary, Ni(II) could not be extracted using low concentration of DEHPA and thus higher concentrations were chosen for examining the influence on extraction rate. Figures 17 and 18 represent the plots of [La(III)]org and [Ni(II)]org versus time at various DEHPA concentrations, respectively. The dependence of extraction rate on DEHPA concentration has been shown in Fig. 15 for La(III) and in Fig. 16 for Ni(II). The curves of lg r versus lg [DEHPA] with slopes of 1.12 and 1.52 indicate that the extraction rates are directly proportional to the first power of DEHPA concentration for La(III), and for Ni(II) the order is 1.5. It is concluded that theorder of extraction reaction with respect to DEHPA concentration is 1 for La(III) and 1.5 for Ni(II).
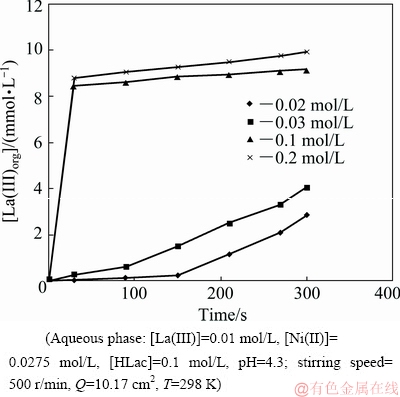
Fig. 17 Plot of [La(III)]org versus time at various DEHPA concentrations

Fig. 18 Plot of [Ni(II)]org versus time at various DEHPA concentrations
3.6 Effect of lanthanum concentration
Lanthanum concentration varied in the aqueous phase in the range of 0.008-0.011 mol/L in order to have a clear insight into the dependence of extraction rates of La(III) and Ni(II) on La(III) concentration. It was seen that extraction rate of La(III) increased with increasing La(III) concentration. There was no extraction of Ni(II) under the chosen experimental conditions. Figure 19 shows plot of La(III)org versus time at various lanthanum concentrations. The plot of lg r(La(III)) versus lg [La(III)] shown in Fig. 15 yielding a slope of 1.39 reveals the extraction rate of La(III) to be first-order dependent on La(III) concentration.
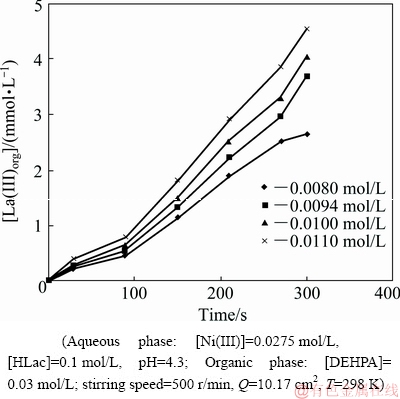
Fig. 19 Plot of [La(III)]org versus time at various La(III) concentrations
3.7 Effect of nickel concentration
The impact of nickel concentration in the aqueous feed on the extraction rates of La(III) and Ni(II) was investigated by varying it in the range of 0.0275-0.0325 mol/L. Figures 20 and 21 represent plots of La(III)org and [Ni(II)]org versus time at various nickel ion concentrations, respectively. A linear dependence was observed as can be seen from the slope value of 1.40 obtained between lg r(Ni(II)) versus lg [Ni(II)] (Fig. 16). So, the extraction rate is first-order with respect to Ni(II) concentration.
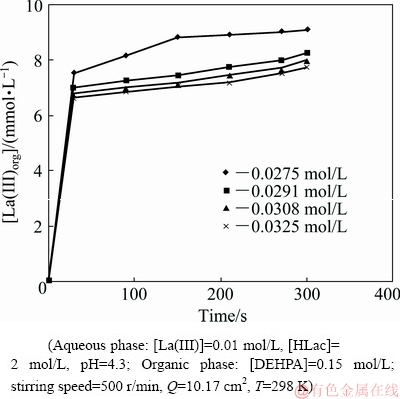
Fig. 20 Plot of [La(III)]org versus time at various Ni(II) concentrations
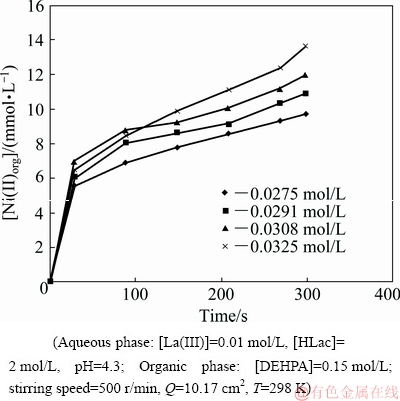
Fig. 21 Plot of [Ni(II)]org versus time at various Ni(II) concentrations
3.8 Extraction rate equation and reaction order
The plots of lg r against lg [HLac], lg [La(III)], lg [Ni(II)] and lg [DEHPA] shown in Figs. 15 and 16 give straight lines with slopes of 0.91 and 0.87,1.12 and 1.52, 1.39 and 1.40 for La(III) and Ni(II), respectively. The extraction follows the first-order with respect to HLac, metal ion and DEHPA concentrations for La(III) while order is 1.5 with respect to DEHPA concentration in the case of Ni(II) extraction. From the results obtained in the above investigation the extraction rates of La(III) and Ni(II) can be represented by the following expressions (Eq. (3) and Eq. (4)):
r(La)=10-2.516[La(III)]1.39[H+]0.08[HLac]0.91[DEHPA]1.12 (3)
r(Ni)=10-3.051[Ni(II)]1.40[H+]0[HLac]0.87[DEHPA]1.52 (4)
The values of rate constants obtained from the intercepts of dependency graphs (Figs. 15 and 16) are listed in Table 3 for La(III) and in Table 4 for Ni(II).
Table 3 Rate constants of extraction of La(III)
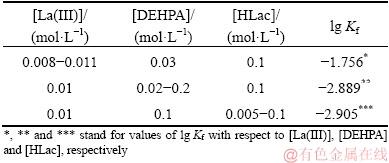
Table 4 Rate constants of extraction of Ni(II)

3.9 Extraction mechanism from rate studies
The dependence of extraction rate on various parameters was studied in order to determine the reaction mechanism (Eqs. (5) and (6)). The above discussion indicates that the diffusion regime controls the extraction kinetics. The reaction between metal ion and the extractant which takes place at the interface is the rate-determining one.
For La3+,
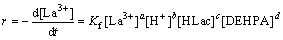 (5)
(5)
 lg r=lg Kf+alg[La3+]+blg[H+]+clg[HLac]+dlg[DEHPA] (6)
lg r=lg Kf+alg[La3+]+blg[H+]+clg[HLac]+dlg[DEHPA] (6)
where a=1.39, b=0.08, c=0.91 and d=1.12.
Extraction mechanism for La(III) can be expressed as fallows (Eqs. (7)-(16)):
H2A2(org)  2HA(i) (7)
2HA(i) (7)
 +HLac(aq)
+HLac(aq) 
 +
+ (fast) (8)
(fast) (8)
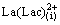 +
+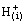
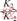
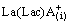 +
+ (slow) (9)
(slow) (9)
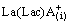 +HA(i)
+HA(i)  La(Lac)A2(i)+
La(Lac)A2(i)+ (fast) (10)
(fast) (10)
La(Lac)A2(i)+2H2A2(org)  La(Lac)A2·4HA(org) (fast) (11)
La(Lac)A2·4HA(org) (fast) (11)
Overall rate can be written as
rf=Kf[La(Lac)2+](i)[HA](i) (12)
Rate formation of La(Lac)A+(i) will be

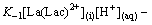
 (13)
(13)
 (14)
(14)
Thus,
 (15)
(15)
K2[HA(i)]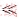 K-1[H+(aq)]
K-1[H+(aq)]
Therefore,
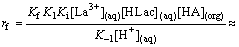
 (16)
(16)
(As rate is independent of [H+] due to buffer action of HLac)
For Ni2+, rate can be written using Eqs. (17) and (18) as follows:
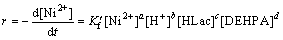 (17)
(17)
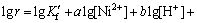
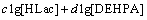 (18)
(18)
where a=1.40, b=0, c=0.87 and d=1.52.
Extraction mechanism for Ni(II) can be expressed as follows (Eqs. (19)-(26)):
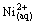 +HLac(aq)
+HLac(aq) 
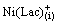 +
+ (fast) (19)
(fast) (19)
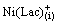 +2HA(i)
+2HA(i) Ni(Lac)A·HA(i)+H+(aq) (slow) (20)
Ni(Lac)A·HA(i)+H+(aq) (slow) (20)
Ni(Lac)A·HA(i)+H2A2(org) Ni(Lac)A·3HA(org) (fast) (21)
Ni(Lac)A·3HA(org) (fast) (21)
 (22)
(22)
The formation rate of Ni(Lac)+(i) is given as


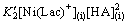 (23)
(23)
 (24)
(24)
Thus,
 (25)
(25)
As  <<
<< and due to buffer action of HLac, the rate is independent of [H+]. Therefore, we obtain
and due to buffer action of HLac, the rate is independent of [H+]. Therefore, we obtain
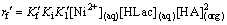 (26)
(26)
where subscript “i” in Eqs. (7)-(26) is used to denote the reactions occurring at the interface.
The above interpretation about mechanism agrees well with the experimental results.
4 Conclusions
The extraction regime which controls the rate is diffusion-controlled as evident from the kinetics data analysis. The extraction rate of both the metal ions increases with the enhancement in stirring speed as well as interfacial area. Based on the dependency of extraction rate on La(III), Ni(II), HLac and DEHPA concentrations, the rate equations have been deduced. The mechanism of the extraction reaction has also been presented. The extraction rate equations are expressed as r=10-2.516[H+]0.08[La(III)]1.39[HLac]0.91[DEHPA]1.12 and r=10-3.051[Ni(II)]1.40[HLac]0.87[DEHPA]1.52 for La(III) and Ni(II), respectively. The rates are independent of pH since the HLac in the aqueous phase acts as buffer. From the kinetics studies, it is concluded that the separation of La(III) and Ni(II) is possible based on the large difference in their extraction rates with low interfacial area and low stirring speed.
Acknowledgements
The authors express their sincere gratitude to authorities of Siksha ‘O’ Anusandhan Deemed to be University for their kind support to complete the experimental work. One of the authors (Swagatika SATPATHY) is thankful to DST, Govt. of India for the award of INSPIRE fellowship. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
[1] BISAKA K, THOBADI I C, PAWLIK C. Extraction of rare-earth elements from iron-rich rare-earth deposits [J]. Journal of the South African Institute of Mining and Metallurgy, 2017, 117: 731-739.
[2] BINNEMANS K, JONES P T, BLANPAIN B, van GERVEN T, YANG Y, WALTON A, BUCHERT M. Recycling of rare earths: A critical review [J]. Journal of Cleaner Production, 2013, 51: 1-22.
[3] LOU Zhen-ning, XIONG Ying, SONG Jun-jun, SHAN Wei-jun, HAN Guang-xi, XING Zhi-qiang, KONG Yu-xia. Kinetics and mechanism of Re(III) extraction and separation from Mo(II) with trialkyl amine [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s10-s14.
[4] ITABASHI H, TAKAZAWA Y, NIIBE N, KAWAMOTO H. Kinetic separation of cadmium(II) from zinc(II) with dithizone by back-extraction [J]. Analytical Sciences, 1997, 13: 921-924.
[5] KOKUSEN H, SUZAKI K, OHASHI K, YAMAMOTO K. Kinetic separation of nickel(II) and cobalt(II) with 5-octyloxymethyl-8- quinolinol by differential extraction [J]. Analytical Sciences, 1988, 4: 617-622.
[6] RYDBERG J. Solvent extraction principles and practice: revised and expanded [M]. Boca Raton: CRC Press, 2004:112.
[7] KAWAMOTO H, AKAIW H. Separation of cobalt (II) from nickel (II) by using dithizone extraction and rate-controlling effect of 1, 10-phenanthroline [J]. Analytical Sciences, 1987, 3: 573-574.
[8] DANESI P R, CHIARIZIA R. The kinetics of metal solvent extraction [J]. CRC Critical Reviews in Analytical Chemistry, 1980, 10: 1-126.
[9] FENG Xing-liang, LONG Zhi-qi, CUI Da-li, WANG Liang-shi, HUANG Xiao-wei, ZHANG Guo-cheng. Kinetics of rare earth leaching from roasted ore of bastnaesite with sulfuric acid [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 849-854.
[10] NAYL A A, ALY H F. Solvent extraction of V(V) and Cr(III) from acidic leach liquors of ilmenite using Aliquat 336 [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 4183-4191.
[11] YANG Hua-ling, CHEN Ji, ZHANG Dong-li, WANG Wei, CUI Hong-min, LIU Yu. Kinetics of cerium(IV) and fluoride extraction from sulfuric solutions using bifunctional ionic liquid extractant (Bif-ILE) [A336][P204] [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 1937-1945.
[12] GAO J, PENG B, FAN H, KANG J. Solvent extraction kinetics of rare earth elements [J]. Talanta, 1996, 43: 1721-1725.
[13] CHEN Z, WANG Y. Solvent extraction kinetics of Sm(III), Eu(III) and Gd(III) with 2-ethylhexyl phosphoric acid-2-ethylhexyl ester [J]. Chinese Journal of Chemical Engineering, 2018, 26: 317-321.
[14] MIYAKE Y, BABA Y. Rate processes in solvent extraction of metal ion [J]. Mineral Processing and Extractive Metallurgy Review, 2000, 21: 351-380.
[15] STEVENS G W, PERERA J M. Kinetics of solvent extraction processes [J]. Mineral Processing and Extractive Metallurgy Review, 1997, 17: 205-226.
[16] YIN S, LI S, ZHANG B, PENG J, ZHANG L. Extraction kinetics of neodymium(III) from chloride medium in the presence of two complexing agents by D2EHPA using a constant interfacial area cell with laminar flow [J]. Hydrometallurgy, 2016, 161: 160-165.
[17] KONDO K, TAO L X, MATSUMOTO M. Extraction equilibrium and kinetics of neodymium with diisodecylphosphoric acid [J]. Hydrometallurgy, 1997, 44: 321-330.
[18] TORKAMAN R, SAFDARI J, TORAB-MOSTAEDI M, MOOSAVIAN M A. A kinetic study on solvent extraction of samarium from nitrate solution with D2EHPA and Cyanex 301 by the single drop technique [J]. Hydrometallurgy, 2014, 150: 123-129.
[19] SALEH M I, BARI M F, JAB M S, SAAD B. Kinetics of lanthanum (III) extraction from nitrate-acetato medium by Cyanex 272 in toluene using the single drop technique [J]. Hydrometallurgy, 2002, 67: 45-52.
[20] YANG H, CHEN J, WANG W, CUI H, ZHANG D, LIU Y. Extraction kinetics of lanthanum in chloride medium by bifunctional ionic liquid [A336][CA-12] using a constant interfacial cell with laminar flow [J]. Chinese Journal of Chemical Engineering, 2014, 22: 1174-1177.
[21] CHITRA K R, GAIKWAD A G, SURENDER G D, DAMODARAN A D. Studies on kinetics of forward and backward extraction of neodymium by using phosphonic acid monoester as an acidic extractant [J]. The Chemical Engineering Journal and the Biochemical Engineering Journal, 1995, 60: 63-73.
[22] YIN S, LI S, ZHANG B, PENG J, ZHANG L. Mass transfer kinetics of lanthanum(III) extraction in the presence of two complexing agents by D2EHPA using a constant interfacial area cell with laminar flow [J]. Chemical Engineering Research and Design, 2015, 104: 92-97.
[23] NASH K L. A review of the basic chemistry and recent developments in trivalent f-elements separations [J]. Solvent Extraction and Ion Exchange, 1993, 11: 729-768.
[24] DAOUD J A, ALY H F. Kinetic approach for Eu(III) and Am(III) separation using selective thenoyltrifluoroacetone-triphenylarsine oxide systems [C]//Proc International Solvent Extraction Conference 1996. Melbourne, Australia, PA: ISEC, 1996: 475-480.
[25] DAOUD J A, ABDEL-RAHMAN N, ALY H F. Kinetic studies on the extraction of U(IV) by TBP in kerosene from nitrate medium [J]. Journal of Radio Analytical and Nuclear Chemistry, 1997, 221: 41-44.
[26] SATPATHY S, MISHRA S. Extractive separation studies of La(III) and Ni(II) in the presence of HLac using DEHPA in petrofin [J]. Separation and Purification Technology, 2017, 179: 513-522.
[27] IRABIEN A, ORTIZ I, PEREZ ORTIZ E S. Kinetics of metal extraction: Model discrimination and parameter estimation [J]. Chemical Engineering Processing, 1990, 27: 13-18.
[28] XING P, WANG C Y, XU S M, JU Z J. Kinetics of cobalt (II) extraction from sulfate aqueous solution by sodium salt of di-decylphosphinic acid (DDPA) [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 517-523.
[29] EL-HEFNY N E. Chemical kinetics and reaction mechanisms in solvent extraction: New Trends and Applications [J]. Journal of Physical Sciences, 2017, 28: 129-156.
[30] LEWIS J B. The mechanism of mass transfer of solutes across liquid-liquid interfaces. Part I: The determination of individual transfer coefficients for binary systems [J]. Chemical Engineering Sciences, 1954, 3: 248-259.
[31] MOZURKEWICH M, BENSON S W. Negative activation energies and curved Arrhenius plots. 1: Theory of reactions over potential wells [J]. The Journal of Physical Chemistry, 1984, 88: 6429-6435.
Swagatika SATPATHY, Sujata MISHRA
Department of Chemistry, Institute of Technical Education and Research, Siksha ‘O’ Anusandhan Deemed to be University, Khandagiri Square, Bhubaneswar-751030, Odisha, India
摘 要:采用界面积恒定的连续搅拌萃取池,研究在乳酸存在条件下,以用Petrofin稀释的二-2-乙基己基磷酸(DEHPA)作萃取剂从硝酸盐介质中萃取分离La(III)与Ni(Ⅱ)的动力学。考察搅拌速度、界面积、pH值、乳酸浓度、萃取剂浓度、金属离子浓度和温度对萃取速率的影响。结果表明,该萃取体系是受扩散控制的,界面反应为速率控制步骤。两种金属离子的萃取速率均与pH值无关。La(III)和Ni(II)的萃取速率与乳酸浓度和金属离子(La(III)或Ni(II))浓度呈线性关系。La(III)的萃取速率与DEHPA浓度呈线性关系,而Ni(Ⅱ) 的萃取速率则与DEHPA浓度的1.5次方呈线性关系。在低界面积和低搅拌速度的条件下,从硝酸盐溶液中分离La(III)和Ni(Ⅱ)是可行的。
关键词:萃取;分离;动力学;机理;La(III);Ni(II)
(Edited by Wei-ping CHEN)
Corresponding author: Sujata MISHRA; E-mail: drsujatamishra97@gmail.com
DOI: 10.1016/S1003-6326(19)65061-2

