DOI: 10.11817/j.issn.1672-7207.2016.07.003
大洋多金属结核高压低质量分数碱浸过程中SiO2溶出行为
冯雅丽1,刘鹏伟1,李浩然2,郭成林1,吴梦妮1,李洪珊1,袁飞1
(1. 北京科技大学 土木与环境工程学院,北京,100083;
2. 中国科学院过程工程研究所,生化国家重点实验室,北京,100080)
摘要:为提高综合利用率,对大洋多金属结核碱浸进行预处理研究,考察大洋多金属结核高压低质量分数碱浸过程中NaOH初始质量分数、反应温度、液固比、浸出时间等因素对SiO2溶出影响,并对高压碱浸脱硅动力学进行研究。研究结果表明:在浸出温度为180 ℃、质量分数为10%NaOH溶液、液固比为3.0:1.0条件下反应70 min,可制得118.51 g/L的Na2SiO3溶液,其余Na2SiO3以方沸石(Na (Si2Al)O6·H2O)形式析出,SiO2高压浸出反应的表观活化能为50.08 kJ/mol,遵循化学反应控制的收缩核模型。通过高压低质量分数碱浸可选择性浸出其中的SiO2,并生成有良好吸附性能的方沸石,而不破坏有用金属元素晶格结构,所得浸渣锰质量分数为30.72%以上。
关键词:大洋多金属结核;SiO2;低质量分数碱浸;高压浸出
中图分类号:TF111.3 文献标志码:A 文章编号:1672-7207(2016)07-2196-09
Dissolving behavior of silica of ocean polymetallic nodules in low mass fraction of alkali leaching under high pressure
FENG Yali1, LIU Pengwei1, LI Haoran2, GUO Chenglin1, WU Mengni1, LI Hongshan1, YUAN Fei1
(1. School of Civil and Environmental Engineering, University of Science and Technology Beijing,
Beijing 100083, China;
2. State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Science, Beijing 100080, China)
Abstract: In order to improve the comprehensive utilization, an alkali leaching pretreatment of ocean polymetallic nodules was studied. The effects of initial mass fraction of NaOH, leaching temperature, mass ratio of liquid-to-solid, leaching time, and the pressure leaching kinetic on the dissolution of silica were investigated during the low mass fraction of alkali leaching process of ocean polymetallic nodules with low mass fraction of NaOH solution under high pressure. And the high pressure alkali leaching kinetics of desilication was also investigated. The results show that the mass concentration of Na2SiO3 in the leachate reaches 118.51 g/L with the leaching temperature of 180 oC, the mass fraction NaOH of 10%, the liquid-to-solid mass ratio of 3.0:1.0 and the leaching time of 70 min, and the rest of Na2SiO3 changes to analcime Na(Si2Al)O6·H2O. The apparent activation energy of SiO2 in the high pressure leaching reaction is 50.08 kJ/mol, which follows the shrinking core model with surface chemical reaction control. Through the low mass fraction of alkali leaching with high pressure leaching, SiO2 can be selectively leached by the leaching process with low mass fraction of NaOH solution under high pressure, while analcime with high adsorption property can be produced. At the same time, the lattice structures of useful metal elements will not be damaged, and the mass fraction of Mn of residue reaches above 30.72%.
Key words: ocean polymetallic nodules; SiO2; low mass fraction of alkali leaching; pressure leaching
大洋多金属结核作为国际海底区域极具开发前景的固体结核矿物,是低质量分数、多金属共生的复杂微晶态氧化物[1],在大洋多金属结核早期矿物学研究中,研究者多用陆地上锰矿物或者人工合成矿物来命名结核中各种锰矿物,如结核中1 nm锰矿相和0.7 nm锰矿相常以陆地上钡镁锰矿(Todorokite)和钠水锰矿(Birnesite)命名,但随研究的不断深入,许多研究者发现海洋锰矿与陆上锰矿的空间结构有很大差异,目前国内外关于多金属结核的文献报道普遍认为多金属结核中锰及铁以水羟锰矿(δ-MnO2)和针铁矿(FeOOH)形式存在[2-5]。另外,其所含Cu,Ni和Co等80余种金属元素以物理、化学吸附或晶格占据等方式嵌布于锰或铁的氧化物内,具有结晶程度差、嵌布粒度细、孔隙发达、含水量高等特点,李国胜[6]对东太平洋CC区多金属结核矿物学特征研究表明氧化锰与SiO2,Al2O3,K2O和Na2O等常量元素氧化物之间内在关系较为密切,亲和力强,并在适宜条件下极易相互结合,难以使用常规物理选矿方法进行富集分离。目前对大洋多金属结核主要集中在冶炼和非传统冶炼领域,冶炼方面主要集中在提取Mn,Cu,Co和Ni及稀有金属等,WANG等[7]采用碱浸三相氧化法将大洋多金属结核中氧化锰转化为锰酸钾,可使其他有用金属元素从氧化锰晶格中解离,回收利用有用金属,与此同时,孙传尧等[8-10]研究发现在制备锰酸钾过程中,原料中SiO2对锰转化率有显著影响,原料中SiO2的存在不但会增加碱耗还影响结晶过程和产品质量;非传统冶金领域主要根据大洋金属锰结核较大的比表面积和多孔结构而用作化工工业催化剂及吸附处理工业废水Cu,Mn和Ni等重金属离子,同时因其优良电化学性能也可用作电源材料。碱浸脱硅作为一种绿色冶金工艺,对矿物中二氧化硅具有较好的浸出效果。赵昌明等[11]采用常压高质量分数氢氧化钠浸出红土镍矿中的二氧化硅,其脱除率达80%以上。XIAO等[12]研究了采用亚熔盐脱除含钒钢渣中的二氧化硅,可使二氧化硅的脱除率达95.72%。杨波等[13]采用高质量分数氢氧化钠对铝土矿进行了预脱硅,使铝硅比由7.6提高到12.0以上,满足了拜耳法生产对铝土矿的要求。常压高质量分数碱浸可有效脱除矿物中二氧化硅[14-16],但高质量分数碱浸存在腐蚀性强、碱液回收困难等缺点,基于此,本文作者针对大洋多金属结核中矿物学特点,初步研究大洋多金属结核中二氧化硅在高压低质量分数碱液中的溶出行为,并建立浸出动力学模型。
1 实验
1.1 实验材料
本实验所用的大洋多金属结核由“大洋一号”在东太平洋CC区采得,其主要化学组成和XRD分析结果分别如表1和图1所示。
表1 大洋多金属结核的主要化学组成(质量分数)
Table 1 Main chemical composition of ocean polymetallic nodule %

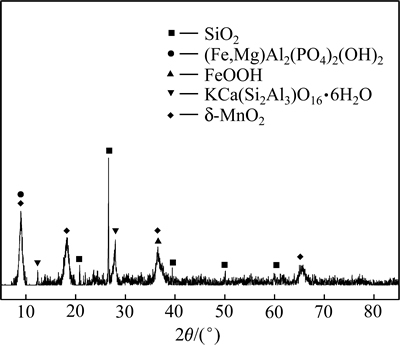
图1 大洋多金属结核的XRD谱
Fig. 1 XRD pattern of polymetallic nodule
由图1可知:大洋多金属结核锰、铁主要以水羟锰矿(δ-MnO2)和针铁矿(FeOOH)形式存在,脉石矿物主要是石英,另有部分钙十字沸石及云母、黏土等铝质脉石矿物。
1.2 实验装置
对大洋多金属结核进行高压碱浸预脱硅的实验装置有0.5 L特制釜体、炉体分离式高压釜;桨叶式搅拌器及可控硅温度控制箱,主要的分析检测装置是UV-1750分光光度计分析液相中Si质量浓度及X线衍射分析仪(Rigaku, 日本理学)分析浸渣物相。
1.3 实验方法
将风干大洋多金属结核经锤式破碎机破碎至小于6 mm后,再经棒磨机磨细至小于0.074 mm,放入烘箱烘干,取40 g物料加入高压釜,并往高压釜中加入分析纯NaOH,用去离子水稀释至设定质量分数。

图2 高压碱浸反应装置图
Fig. 2 Reaction device of alkali leaching with high pressure
高压釜炉体加热到指定温度后,将高压釜釜体放入炉体中开始计时,反应指定时间后从取料口取料,并通冷凝水冷却至室温,过滤,用硅钼蓝分光光度法测定滤液中的Si的质量浓度[17-18]。
2 结果与讨论
2.1 NaOH初始质量分数的影响
在反应温度为180 ℃、液固比为3.0:1.0的条件下,NaOH初始质量分数对大洋多金属结核中SiO2浸出及锰质量分数的影响,如图3所示。
由图3(a)可知:在反应前70 min,浸出液中Na2SiO3质量浓度随NaOH初始质量分数增加而升高,这是由于NaOH初始质量分数升高使体系中OH-活度增大,利于SiO2溶出。由图3(b)可以看出:经过碱浸锰质量分数提高,NaOH初始质量分数为10%时浸渣中锰质量分数达到30.72%,继续增加NaOH质量分数,锰质量分数增幅减缓。
同时在SiO2浸出反应过程中有一个最高点,其后有下降趋势,这是因为大洋多金属结核中有部分铝质脉石,体系中同时存在如下反应:
nSiO2+2NaOH→Na2O·nSiO2+H2O (1)
Al2O3+2NaOH→2NaAlO2+H2O (2)
yNa2O·nSiO2+xNaAlO2+(m+y)H2O→Nax[(SinyAlx)O(2ny+2x)]·mH2O+2yNaOH (3)
生成的水合铝硅酸钠存在不稳定形态的溶解过程和稳定形态的析出过程[19-20],即常见的“脱硅副反应”,浸液中有足够质量分数的Al2O3就会与其中的SiO2反应生成水合铝硅酸钠结晶沉淀物,浸出过程中SiO2的溶解反应与Na(Si2Al)O6·H2O的沉淀反应同时进行,二者之间的竞争作用直接影响浸液中Na2SiO3质量浓度变化。
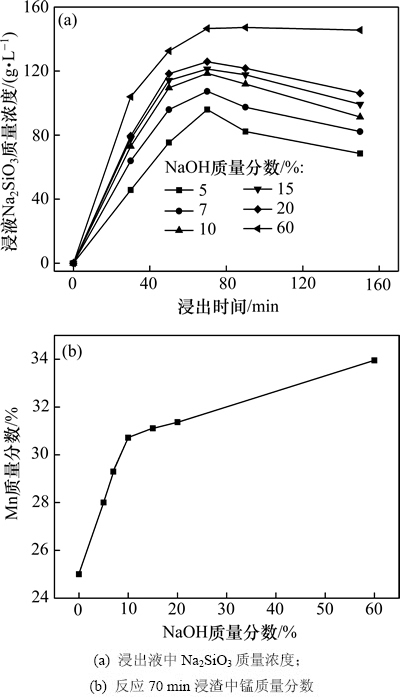
图3 NaOH初始质量分数对浸出液中Na2SiO3质量浓度及反应70 min浸渣中锰质量分数的影响
Fig. 3 Effects of NaOH mass fraction on mass concentration of Na2SiO3 in leachate and mass fraction of Mn in leaching residue after 70 min
图4所示为原矿及NaOH初始质量分数为7%,10%及60%浸出70 min后浸渣XRD谱。与原矿XRD谱相比,碱浸处理后出现了反应式(3)生成的Na(Si2Al)O6·H2O的衍射峰,且随碱质量分数升高衍射峰减弱至消失;比较NaOH初始质量分数为7%和10%浸渣XRD谱,前者仍有较弱的SiO2衍射峰,后者未出现。
由图4可看出:当NaOH初始质量分数为7%时,浸渣中仍有部分SiO2,当NaOH质量分数大于10%时SiO2完全脱除,表明经高压碱浸后,原矿中SiO2溶入浸液,并有部分以Na(Si2Al)O6·H2O析出。罗琳等[21]的研究表明在Na2O质量分数为20%以下的碱溶液体系中,只要混杂少量的Al2O3,便使Na2SiO3平衡质量浓度急剧降低。结合图3(a)可知:反应(3)在低质量分数碱浸过程中影响明显,在高质量分数碱浸出过程中影响较弱,低质量分数NaOH浸出过程中,溶液中OH-活度低,反应(3)易正向进行,NaOH质量分数升高,OH-活度增大,使浸液中Na2SiO3平衡质量浓度增大,使反应(3)不易正向进行。但高质量分数NaOH浸出存在苛性高、黏度大、普通过滤介质过滤难、浸出选择性差等缺点,综合考虑锰质量分数和SiO2脱除效果及NaOH利用率的影响,在NaOH初始质量分数为10%时,反应曲线较稳定,且浸液中Na2SiO3质量浓度较高。

图4 原矿及不同NaOH初始质量分数浸出70 min后浸渣XRD谱
Fig. 4 XRD patterns of ore and residue after 70 min leaching with different NaOH mass fractions
2.2 反应温度的影响
为考察高压釜内不同温度下蒸汽压变化规律,在NaOH初始质量分数为10%、液固比为3.0:1.0的条件下研究反应温度对大洋多金属结核(40 g)中SiO2浸出及锰质量分数的影响,结果如图5所示。
从图5可以看出:高压釜中压强随反应温度的升高而升高,从120 ℃加热到200 ℃,高压釜内压力从0.1 MPa提高到1.3 MPa。
在大量预实验的基础上,当反应温度>120 ℃时,大洋多金属结核中的SiO2在高压釜中水汽所产生的压力下能较好地浸出。不同温度条件下浸出液中Na2SiO3质量浓度随时间的变化趋势及反应70 min浸渣中锰质量分数的变化如图6所示。
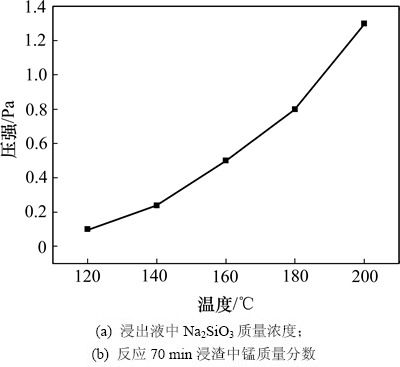
图5 温度对高压釜中压强的影响
Fig. 5 Effect of temperature on pressure in autoclave

图6 反应温度对浸出液中Na2SiO3质量浓度及反应70 min浸渣中锰质量分数的影响
Fig. 6 Effects of temperature on mass concentration of Na2SiO3 in leachate and mass fraction of Mn in leaching residue after 70 min
由图6可以看出:升高温度使浸液Na2SiO3质量浓度达到最大值所需时间缩短,对SiO2浸出有明显的促进作用,但反应约70 min后温度越高,浸液Na2SiO3质量浓度降低越快。因随温度升高,体系中更多的分子越过势垒,成为活化分子,而且加剧了分子的热运动,增大了扩散速率,促进SiO2浸出;但提高温度同样会提高反应(3)的反应速率,特别是脱硅过程后期时,SiO2浸出速率降低,而Na(Si2Al)O6·H2O生成速率则因溶液中Na2SiO3质量浓度升高而加快,升高温度对反应(3)的促进作用比反应(1)的作用大,加快了已溶SiO2析出,导致70 min后温度升高有加剧浸液Na2SiO3质量浓度降低的趋势,在180 ℃时曲线相对较平稳且能获得118.51 g/L的Na2SiO3浸液。低于180 ℃时,随着温度升高,温度对浸渣中锰质量分数的影响越来越明显,而190 ℃相比180 ℃时锰质量分数提高不足1%。
图7所示为原矿及在170,180和190 ℃反应70 min后浸渣XRD谱。与原矿相比,后三者均出现Na(Si2Al)O6·H2O衍射峰,且随温度升高峰强度有升高趋势;170 ℃时浸渣中仍有部分SiO2,180 ℃和190 ℃时SiO2衍射峰消失。
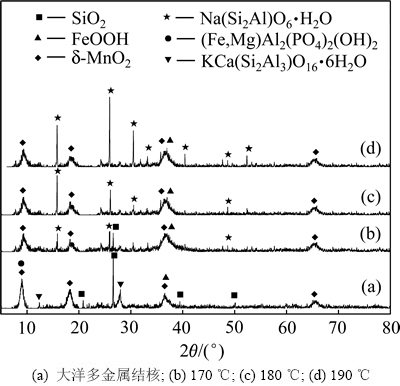
图7 原矿及不同反应温度浸出70 min后浸渣XRD谱
Fig. 7 XRD patterns of ore and residue after 70 min leaching at different temperatures
由图7得出:在170 ℃时未能将大洋多金属结核中SiO2完全溶出,大于180 ℃时SiO2可完全溶出,一部分进入溶液,一部分以Na(Si2Al)O6·H2O形式存在渣中,且升高温度加剧方沸石(Na(Si2Al)O6·H2O)沉淀反应。
2.3 液固比的影响
在反应温度为180 ℃、NaOH初始质量分数为10%条件下研究液固比对大洋多金属结核中SiO2浸出及锰质量分数的影响,如图8所示。

图8 不同液固比对浸出液中Na2SiO3质量及反应70 min浸渣中锰质量分数的影响
Fig. 8 Effect of liquid-to-solid ratio on mass of Na2SiO3 in leachate and mass fraction of Mn in leaching residue after 70 min
由图8可看出:随液固比提高,浸液中Na2SiO3质量增加,这是由于增大液固比可以提高矿浆的流动性,使矿浆中游离OH-增加,一定程度上增加了与SiO2接触的概率,从而加快传质速率,利于SiO2溶出。同时在一定范围内提高液固比,可降低溶出体系中Na2SiO3及Al2O3质量浓度,使其质量浓度不易达到饱和值,抑制Na(Si2Al)O6·H2O沉淀反应,使浸液中Na2SiO3的绝对量增多。当溶液中OH-达到浸出反应饱和值后继续增加液固比对SiO2浸出作用影响变小,液固比为3.0:1.0,3.5:1.0和4.0:1.0时浸液中Na2SiO3变化曲线几乎相同。由图8(b)可以看出:浸渣锰质量分数随液固比增加而提高,在液固比3.0:1.0时浸渣锰质量分数大于30%,大于3.0:1.0时锰质量分数增速减缓。
图9所示为原矿及在液固比为2.5:1.0和3.0:1.0条件下反应70 min后浸渣XRD谱。
与原矿相比,经高压碱浸后均有Na(Si2Al)O6·H2O衍射峰出现,液固比为2.5:1.0条件下浸渣中仍有较弱的SiO2衍射峰,而液固比为3.0:1.0时SiO2衍射峰消失,说明液固比为3.0:1.0时原矿中SiO2才能完全溶出并转化。

图9 原矿及不同液固比浸出70 min后浸渣XRD谱
Fig. 9 XRD patterns of ore and residue after 70 min leaching with different liquid-to-solid ratios
2.4 反应时间的影响
在反应温度为180 ℃、NaOH初始质量分数为10%、液固比为3.0:1.0条件下研究反应时间对大洋多金属结核中SiO2浸出及锰质量分数的影响,如图10所示。
由图10可以看出:70 min之前浸出液中Na2SiO3质量浓度随反应进行而提高,70 min时浸液中Na2SiO3质量浓度为118.51 g/L,计算可得大洋多金属结核中的SiO2有73.1%溶出到浸液中,在70 min后Na2SiO3质量浓度有下降趋势,150 min后趋于平稳。由图10(b)可知:浸渣中锰质量分数变化与SiO2的浸出趋势相同,在70 min时锰质量分数达到30.72%,其后由于已溶SiO2在反应(3)的作用下析出,锰质量分数有下降趋势。
图11所示为原矿及浸出30,70和150 min后浸渣的XRD谱。由图11可以得出:与原矿的XRD相比,碱浸处理30 min后仍有较弱SiO2衍射峰,碱浸70 min和150 min后衍射峰消失,均出现Na(Si2Al)O6·H2O衍射峰,且反应150 min时明显增强。
碱浸70 min可将SiO2完全脱除,在大洋多金属结核进行碱浸脱硅的同时,溶出的SiO2及Al2O3会继续反应,在碱浸反应的初期,SiO2溶出速率快,Al2O3相对较难解离溶出[22],随着反应的进行,溶液中Na2SiO3和Al2O3的质量浓度增高,达到反应(3)平衡质量浓度后,溶液中Na2SiO3和Al2O3开始形成非晶态铝硅酸钠盐,非晶态相初期呈薄膜状覆盖于颗粒表面,之后形成球状非晶质,在此基础上形成晶核,并逐渐生长为方沸石(Na(Si2Al)O6·H2O)晶体[23],当反应(3)反应速率大于反应(1)的速率时,浸液中Na2SiO3质量浓度降低,因此超过平衡质量浓度后反应时间的延长不利于SiO2的浸出,控制反应时间可有效地降低反应(3)的影响。在180 ℃时,70 min之前可以获得SiO2的高浸出率和Al2O3的低浸出率,实验结果表明70 min左右时反应(3)的作用最小,SiO2的浸出效果最好。
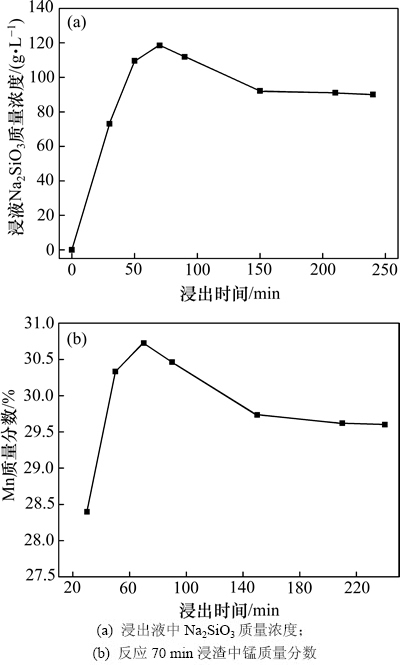
图10 反应时间对浸出液中Na2SiO3质量浓度及反应70 min浸渣中锰质量分数的影响
Fig. 10 Effects of leaching time on mass concentration of Na2SiO3 in leachate and mass fraction of Mn in leaching residue after 70 min

图11 原矿及不同反应时间浸渣XRD谱
Fig. 11 XRD patterns of ore and residue leaching with different time
分析图11中原矿、反应70 min和150 min浸渣的XRD谱可以看出:后两者中SiO2,KCa(Si2Al3)O16·6H2O和(Fe,Mg)Al2(PO4)2(OH)2的衍射峰消失,有用金属的衍射峰没有变化,说明经过碱浸反应只将原矿中的脉石脱出,并未损失有用矿物。
2.5 SiO2及主要金属元素的浸出
在180 ℃、NaOH初始质量分数为10%、液固比为3.0:1.0的条件下,研究大洋多金属结核中SiO2及主要金属元素的浸出,实验结果如图12所示。
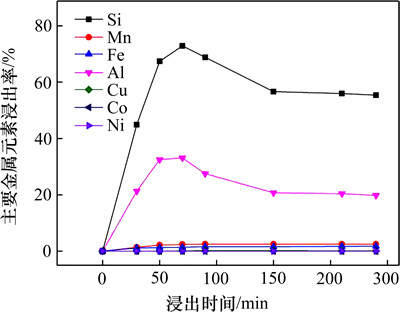
图12 高压碱浸过程中大洋多金属结核中各元素浸出率
Fig. 12 Dissolving rate of elements in ocean polymetallic nodules during alkali leaching under high pressure
由图12可以看出:高压碱浸过程中Si和Al浸出率最大值分别可达73.1%和32.5%,因两者在溶液中反应生成方沸石(Na(Si2Al)O6·H2O),导致在70 min后浸出率有下降趋势,同时高压低浓度碱浸过程中Mn,Fe,Cu,Co和Ni几乎未浸出,可以看出高压低质量分数碱浸可选择性浸出多金属结核中的二氧化硅而几乎未损失有用矿物。
2.6 浸出动力学研究
NaOH浸出SiO2的过程主要进行以下反应:
nSiO2(s)+2NaOH(aq)→Na2O·nSiO2(aq)+H2O(aq) (4)
因反应中存在“脱硅副反应”的影响,因此,只取反应前70 min数据进行分析研究。
假设NaOH质量分数及矿物颗粒粒径保持恒定,反应(4)浸出过程动力学模型可以用收缩核模型来描述。无固体产生的收缩核模型按不同控制步骤可分为化学反应控制、液体边界层扩散控制[12, 24-25],当反应为化学反应控制时,动力学方程为
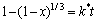 (5)
(5)
当反应为液体边界层扩散控制时,动力学方程为
 (6)
(6)
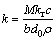 (7)
(7)
式中:x为反应t时刻时SiO2的脱出率,%;b为NaOH的化学计量系数;d0为球形颗粒的初始直径,cm;ρ为SiO2的密度,g/cm3;M为SiO2的相对分子质量;k 为反应速率常数,s-1; 与
与 分别为化学反应控制与液体边界层扩散控制的反应速率常数,s-1;kr为本征反应速率常数,s-1;c为溶液中NaOH的浓度,mol/L。
分别为化学反应控制与液体边界层扩散控制的反应速率常数,s-1;kr为本征反应速率常数,s-1;c为溶液中NaOH的浓度,mol/L。
为验证并明确SiO2的浸出过程的动力学控制因素,将实验所得数据分别代入化学反应控制、液体边界层扩散控制的动力学方程式中,结果如图13和表2所示。

图13 不同温度下浸出动力学与时间的线性关系
Fig. 13 Relationship between leaching kinetics and time at different temperatures
表2 不同温度下浸出动力学参数
Table 2 Parameter of leaching kinetics under different temperatures

由图13及表2可以看出:只有化学反应控制的动力学曲线1-(1-x)1/3=kt才具有良好的线性回归关系,因此,在此反应过程中,SiO2的浸出过程受化学反应控制。
在化学反应中,温度对反应速率常数的影响可用Arrhenius方程表示:
 (8)
(8)
式中:k为反应速率常数;A为频率因子;R为摩尔气体常数;Ea为反应的表观活化能,kJ/mol;T为反应温度,K。
以ln k对1/T作图,结果如图14所示。
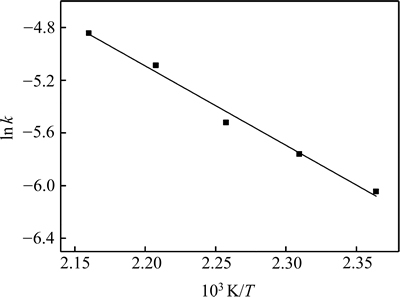
图14 浸出SiO2的ln k-T-1关系曲线
Fig. 14 Plot of ln k-T-1 for leaching of SiO2
由图14可知:ln k与1/T呈线性关系,其直线斜率即为-Ea/R,其中R取8.314,线性方程式为
 (9)
(9)
可求得表观活化能为50.08 kJ/mol。这一表观活化能表明此反应属于化学反应控制。
3 结论
1) 在NaOH质量分数为10%、液固比为3.0:1.0、180 ℃条件下反应70 min,SiO2脱出率达73.1%,同时可得质量浓度为118.51 g/L的Na2SiO3溶液和锰质量分数为30.72%的浸渣。
2) 在碱浸过程中铝质脉石的溶解会与已溶SiO2反应生成吸附材料Na(Si2Al)O6·H2O(方沸石),结果表明延长反应时间、降低NaOH质量分数、适当减小液固比可促进Na(Si2Al)O6·H2O析出。
3) 大洋多金属结核高温高压低质量分数NaOH浸出SiO2反应的表观活化能为50.08 kJ/mol,遵循化学反应控制的收缩核模型。
参考文献:
[1] 王纪学, 王鹏, 李浩然. 流态化还原大洋多金属结核[J]. 中南大学学报(自然科学版), 2011, 42(S2): 361-364.
WANG Jixue, WANG Peng, LI Haoran. Fluidizing reduction roasting of ocean polymetallic nodule[J]. Journal of Central South University (Science and Technology), 2011, 42(S2): 361-364.
[2] 冯旭文, 钱江易, 张培志. 大洋多金属结核中1 nm锰矿相的相变及其主要控制因素研究[J]. 矿物学报, 2003, 23(2): 109-114.
FENG Xuwen, QIAN Jiangyi, ZHANG Peizhi. The study of phase change of 1 nm manganate in polymetallic nodules and its main controlling factors[J]. Acta Mineralogica Sinica, 2003, 23(2): 109-114.
[3] 刘淑琴, 潘家华. 对大洋多金属结核10 A锰矿物相的研究[J]. 地球学报, 1998(3): 65-74.
LIU Shuqin, PAN Jiahua. The research on 10 A manganese mineral phase in the oceanic polymetallic nodules[J]. Acta Geoscientia Sinica, 1998(3): 65-74.
[4] USUI A, MELLIN T. Structural stability of marine 10 A manganite from the ogasawara arc. Implication for low- temperature hydrothermal activity[J]. Marine Geology, 1989, 86(1): 41-54.
[5] 胡大千, 初凤友, 姚杰. 中太平洋富钴锰结壳水羟锰矿研究[J]. 吉林大学学报(地球科学版), 2009, 39(4): 706-710, 748.
HU Daqian, CHU Fengyou, YAO Jie. Study on vernadite in Co-rich crust from the Central Pacific Ocean[J]. Journal of Jilin University (Earth Science Edition), 2009, 39(4): 706-710, 748.
[6] 李国胜. 东太平洋CC区多金属结核矿物学特征及物源[J]. 物探与化探, 2009, 33(6): 613-619.
LI Guosheng. Mineralogical characteristics and material source of polymetallic nodules in CC area of east Pacific[J]. Geophysical and Geochemical Exploration, 2009, 33(6): 613-619.
[7] WANG Y, LI Z, LI H. A new process for leaching metal values from ocean polymetallic nodules[J]. Minerals Engineering, 2005, 18(11): 1093-1098.
[8] 孙传尧, 谭欣, 周秀英, 等. 大洋多金属结核及富钴结壳矿物材料的研究述评(Ⅰ)[J]. 国外金属矿选矿, 2003, 40(9): 4-11.
SUN Chuanyao, TAN Xin, ZHOU Xiuying, et al. Polymetallic nodules and rich cobalt crust mineral materials research review(Ⅰ) [J]. Metallic Ore Dressing Abroad, 2003, 40(9): 4-11.
[9] 冯林永. 大洋多金属结核合成锂离子筛与吸附基础研究[D]. 昆明: 昆明理工大学冶金与能源工程学院, 2009: 13-14.
FENG Linyong. Polymetallic nodules synthesis of lithium ion sieve adsorption of basic research[D]. Kunming: Kunming University of Science and Technology. College of Metallurgy and Energy Engineering, 2009: 13-14.
[10] 王云山, 李佐虎, 李浩然. 大洋多金属结核的非冶金加工应用[J]. 化工新型材料, 2005, 33(5): 62-64.
WANG Yunshan, LI Zuohu, LI Haoran. Non-metallurgical processing applications of ocean polymetallic nodules[J]. New Chemical Materials, 2005, 33(5): 62-64.
[11] 赵昌明, 翟玉春, 刘岩, 等. 红土镍矿在NaOH 亚熔盐体系中的预脱硅[J]. 中国有色金属学报, 2009, 19(5): 948-954.
ZHAO Changming, ZHAI Yuchun, LIU Yan, et al. Pre-desilication of laterite in NaOH sub-molten salt system[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(5): 948-954.
[12] XIAO Qinggui, CHEN Yin, GAO Yiying, et al. Leaching of silica from vanadium-bearing steel slag in sodium hydroxide solution[J]. Hydrometallurgy, 2010, 104(2): 216-221.
[13] 杨波, 王京刚, 张亦飞, 等. 常压下高浓度NaOH浸取铝土矿预脱硅[J]. 过程工程学报, 2007, 7(5): 922-927.
YANG Bo, WANG Jinggang, ZHANG Yifei, et al. Pre-desiliconization of a bauxite ore through leaching by high concentration NaOH solution under atmospheric pressure[J]. The Chinese Journal of Process Engineering, 2007, 7(5): 922-927.
[14] 陈超, 韦萍, 粟海锋, 等. 常压碱溶法提取软锰矿酸浸渣中的硅[J]. 无机盐工业, 2012, 44(9): 36-38.
CHEN Chao, WEI Ping, SU Haifeng, et al. Leaching silicon from acid leaching residue of pyrolusite by alkaline leaching method at atmospheric pressure[J]. Inorganic Chemicals Industry, 2012, 44(9): 36-38.
[15] 王佳东, 申晓毅, 翟玉春. 碱溶粉煤灰提硅工艺条件的优化[J]. 矿产综合利用, 2010(4): 42-44.
WANG Jiadong, SHEN Xiaoyi, ZHAI Yuchun. Optimization of the technological conditions for extracting silica from fly ash[J]. Multipurpose Utilization of Mineral Resources, 2010(4): 42-44.
[16] 王学诗. 脱硅技术的创新与脱硅概念的拓展[J]. 轻金属, 2001(2): 27-29.
WANG Xueshi. Desilication technology innovation and development of the concept of desilication[J]. Light Metals, 2001(2): 27-29.
[17] 李海燕, 孙宝莲, 禄妮, 等. 加压氧化钼精矿浸出液中硅的测定[J]. 稀有金属材料与工程, 2011, 40(S2): 124-126.
LI Haiyan, SUN Baolian, LU Ni, et al. Determination of silicon in leaching solution of molybdenum oxide under pressure[J]. Rare Metal Materials and Engineering, 2011, 40(S2): 124-126.
[18] 沙德仁, 颜科, 吴旦. 光化学还原—硅钼蓝分光光度法测定硅的研究[J]. 光谱实验室, 2010, 27(6): 2336-2345.
SHA Deren, YAN Ke, WU Dan. Determination of silicon by photochemical reduction-silicon-molybdenum blue spectro- photometry[J]. Chinese Journal of Spectroscopy Laboratory, 2010, 27(6): 2336-2345.
[19] MATIJEVIC E, MANGRAVITE F, CASSELL A. Stability of colloidal silica (Ⅳ): the silica-alumina system[J]. Journal of Colloid & Interface Science, 1971, 35(4): 560-568.
[20] JAMIALAHMADI M, MULLER H. Determining silica solubility in bayer process liquor[J]. Journal of the Minerals Metals & Materials Society, 1998, 50(11): 44-49.
[21] 罗琳, 何伯泉, 刘永康. 国内外高硅铝土矿焙烧预脱硅工艺的评述[J]. 国外金属矿选矿, 1999(1): 38-41, 32.
LUO Lin, HE Boquan, LIU Yongkang. The review of high silica bauxite roasting desilication process in China and abroad[J]. Metallic Ore Dressing Abroad, 1991(1): 38-41, 32.
[22] 李光辉. 铝硅矿物的热行为及铝土矿石的热化学活化脱硅[D]. 长沙: 中南大学资源加工与生物工程学院, 2002: 101-104.
LI Guanghui. Thermal behaviors of silicon aluminum minerals and desilication from bauxite ores by thermochemical activation (TCA) process[D]. Changsha: Central South University. School of Minerals Processing and Bioengineering, 2002: 101-104.
[23] 王华, 张强, 宋存义. 莫来石在粉煤灰碱性溶液中的反应行为[J]. 粉煤灰综合利用, 2001(5): 24-27.
WANG Hua, ZHANG Qiang, SONG Cunyi. The reaction behavior of mullite in alkaline solution of fly ash[J]. Fly Ash Comprehensive Utilization, 2001(5): 24-27.
[24] SANTOS F M F, PINA P S, PORCARO R. The kinetics of zinc silicate leaching in sodium hydroxide[J]. Hydrometallurgy, 2010, 102(1/2/3/4): 43-49.
[25] 徐志峰, 邱定蕃, 王海北. 铁闪锌矿加压浸出动力学[J]. 过程工程学报, 2008, 8(1): 28-34.
XU Zhifeng, QIU Dingfan, WANG Haibei. Pressure leaching kinetics of marmatite[J]. The Chinese Journal of Process Engineering, 2008, 8(1): 28-34.
(编辑 刘锦伟)
收稿日期:2015-07-18;修回日期:2015-09-20
基金项目(Foundation item):水体污染与治理科技重大专项(2015ZX07205-003);中国大洋矿产资源研究计划项目(DY125-15-T-08);国家自然科学基金资助项目(21176026,21176242) (Project(2015ZX07205-003) supported by the Major Science and Technology Program for Water Pollution Control and Treatment; Project(DY125-15-T-08) supported by the China Ocean Mineral Resources Research & Development Program; Projects(21176026, 21176242) supported by the National Natural Science Foundation of China)
通信作者:李浩然,博士,从事非传统资源利用研究;E-mail: hrli@home.ipe.ac.cn