文章编号:1004-0609(2012)05-1482-06
CaWO4-NaCl-CaCl2体系熔盐电解制备钨粉的表征与电化学分析
王 旭,廖春发,杨文强,谢泉文
(江西理工大学 材料与化学工程学院,赣州 341000)
摘 要:通过熔盐电解法从CaWO4-NaCl-CaCl2体系制备出金属钨粉,采用X射线衍射仪(XRD)、电子扫描显微镜(SEM)和能谱分析仪(EDS)对钨粉的形貌和相组成进行表征。在试验数据的基础上计算电流效率,通过分析电解过程中形成的中间产物,讨论电解过程中的电极反应,采用循环伏安法和方波伏安法分析体系的基本热力学性质。结果表明:在750 ℃、槽电压2.5 V下经5 h以上电解可制备出纯度达到99%、平均粒度低于2 μm的金属钨粉,实验中的电流效率可达到80%;电解过程的电极反应不可逆,浓差极化是体系电解速率的主要阻力。
关键词:钨酸钙;熔盐电解;钨粉;电极过程;浓差极化
中图分类号:TF111.52 文献标志码:A
Characterization and electrochemical analysis of tungsten powder prepared by molten salt electrolysis in CaWO4-CaCl2-NaCl system
WANG Xu, LIAO Chun-fa, YANG Wen-qiang, XIE Quan-wen
(College of Materials and Chemical Engineering, Jiangxi University of Technology, Ganzhou 341000, China)
Abstract: Tungsten powders were prepared by molten salt electrolysis in CaWO4-CaCl2-NaCl system and its morphologies and phases composition were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and electron spectroscopy (EDS), respectively. The electrolytic efficiency was calculated based on experimental data. The electrode reactions during electrolysis were discussed by analyzing intermediate products formed in the electrolysis process. The thermodynamic properties of electrolysis process were studied by cyclic voltammetry and square wave voltammetry. The results show that tungsten powders can be prepared by electrochemical electrolysis under 750 ℃ with a cell voltage of 2.5 V for 5 h, which have a purity of 99% and particle sizes less than 2 μm, the current efficiency reaches 80%. The electrode reactions in the stable electrolysis region is irreversible and the concentration polarization process of cathode is an important factor of restricting electrolysis rate
Key words: calcium tungstate; molten salt electrolysis; tungsten powder; electrode process; concentration polarization
钨具有熔点高、硬度高、耐酸腐蚀性好等优异的物理化学性能,在国民经济各部门及国防工业中的使用范围非常广泛。目前,处理钨精矿到制取金属钨粉要经过钨精矿的分解制取钨酸钠、APT工艺制成仲钨酸铵结晶、煅烧成三氧化钨。而后,采用氢还原三氧化钨的方法制取钨粉。可见,获得钨粉的全部生产工艺流程长而复杂。尽管我国的钨矿资源居世界首位,但是,开发短流程,提高资源利用率,降低生产成本已成为钨冶金技术发展的趋势[1-2]。作为制备高纯物质的手段,国内外许多研究[3-9]认为采用熔盐电解法在制备难熔金属方面有很好的应用前景。许多学者对熔盐电解制备金属钨进行了探索,据文献[10]介绍,20世纪60~70年代有采用磷酸钠-硼酸钠-氧化钨、氟化物-氯化物-氧化钨体系制取钨粉的研究。到20世纪90年代,冯乃祥等[11-12]采用KCl-NaCl-Na2WO4-WO3和NaCl-Na2WO4-WO3体系电解制备出金属钨粉。这些研究结果表明,选择适当的电解体系,以钨的氧化物作为活性物质,可以直接制备出一定粒度和纯度的金属钨粉,能够简化传统工艺由氢气还原WO3的过程。本文作者提出采用熔盐电解法以白钨精矿的主要成分钨酸钙为活性物,以对碳质电极的腐蚀性小的CaCl2-NaCl熔盐体系为电解质[13],直接电解制取金属钨粉,可进一步缩短由钨酸盐制取钨粉的传统工艺,对降低生产成本,提高钨的回收率具有重要意义。
1 实验
1.1 试验方法
化学纯CaCl2、NaCl、CaWO4经300 ℃烘干24 h备用。取CaCl2-NaCl混合盐(摩尔比1:1,510 g)和40 g CaWO4充分混合,在温度750 ℃、槽电压2.5 V、以直径10 mm的石墨棒为电极条件下分别电解2.5、5 h,先用热蒸馏水清洗阴极周围富集物并保留清洗液,固体不溶物用5%氢氧化钠清洗后得到电解产物。分别采用日本理学Miniflex型X射线衍射分析仪、荷兰飞利浦XL30W/TMP型扫描电子显微镜、美国伊达克斯公司EDAX型能谱仪对产物进行XRD和SEM及EDS分析。
采用纯钨丝(长直径2 mm)作为工作电极,高纯石墨棒(直径4 mm)作为辅助电极,纯铂丝(直径1 mm)为参比电极,对750 ℃的NaCl-CaCl2(摩尔比1:1,150 g)混合熔盐进行循环伏安分析,随后加入15 g CaWO4后进行循环伏安分析和方波伏安分析,设备采用荷兰AUTOLAB公司(PGSTAT301型)电化学工作站。
1.2 试验装置
试验装置如图1所示,电解过程中的电压和电流数据可以通过稳压电源的RS485接口导入计算机。
2 结果与讨论
2.1 电解产物的表征
图2所示为不同电解时间电解阴极产物的XRD谱。由图2可知,谱线(a)表明经2.5 h的电解后,钨已在阴极沉积,同时夹杂有CaWO4及钨的+4价氧化物WO2;谱线(b)中钨的特征峰形尖锐、突出,未见其它物质明显的特征峰,表明经过5 h的电解后,产物的主要物相为钨。
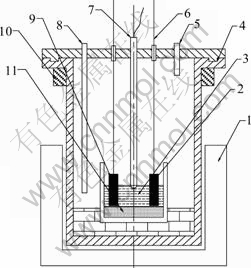
图1 试验装置示意图
Fig. 1 Schematic diagram of experimental apparatus: 1—Electric furnace; 2—Graphite cathode; 3—Molten salt; 4—Reactor; 5—Ar gas outlet; 6—Electrode extension; 7—Thermocouple; 8—Ar gas inlet; 9—Graphite anode; 10—Cooling water; 11—Corundum crucible
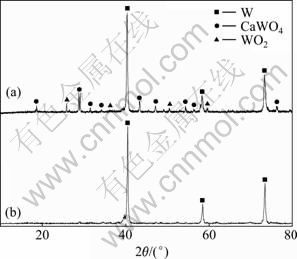
图2 不同电解时间电解阴极产物的XRD谱
Fig. 2 XRD patterns of cathodic products at 2.5 V and 750 ℃ for different electrolysis times: (a) 2.5 h; (b) 5 h
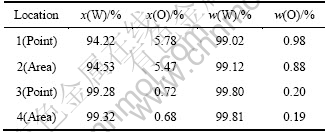
图3所示为不同电解时间电解阴极产物的SEM像。为进一步了解产物的特征,分别对图3(a)中点1和区域2及图3(b)中点3和区域4进行了EDS分析,结果见表1。由图3(a)可见,产物中结晶颗粒粒径基本小于2 μm,但并不均匀,晶粒并非成串连续析出,结晶颗粒较为分散,对应图2中谱线(a)可推断,此状态是由于产物中存在单质钨和CaWO4及钨的氧化物等多相混合物。由图3(b)可见,经过5 h的连续电解后。形成的结晶颗粒粒径均小于2 μm,而且是成串均匀地连续析出,形状规则,说明阴极产物钨在电解过程中优先在已形成的钨颗粒上继续形核而形成新颗粒的生长点,而不是继续长大,这将有利于形成细晶粒的钨粉。由表1的数据可估测钨粉的纯度。假设图3(a)中的点1和区域2中的氧元素以WO3形式存在,按表1中的摩尔分数估算,则点1和区域2中的单质钨含量分别为97.43%和97.20%;按质量分数计算,则点1和区域2中的钨含量分别为95.27%和95.75%。这说明经2.5 h的电解,钨粉含量仅能达到95%,有较高的含氧化合物杂质,电解脱氧率较低。同理分析图3(b) 中的点3和区域4,可知经5 h的电解后,阴极产物中钨粉含量可达到99%以上,且氧化合物杂质较少,电解脱氧率较高。
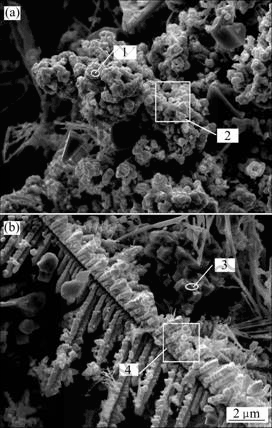
图3 不同电解时间电解阴极产物的SEM像
Fig. 3 SEM images of cathodic products at 2.5 V and 750 ℃ for different electrolysis times: (a) 2.5 h; (b) 5 h
表1 阴极产物部分区域EDS结果
Table 1 EDS results of cathodic products in Fig.3
2.2 电化学分析
2.2.1 电极过程
如图2谱线(a)所示,钨酸钙作为体系的活性物,在电解过程中元素W6+并非由+6价直接变为零价,WO2的存在说明有W6+→W4+的价态变化过程。由于没有检测到WO 的存在,不能证明存在W4+→W2+的变化。为考察阳极产物的成分,实验过程中,尾气经便携式气体检测管可检测到CO2与CO气体,且CO2与CO的体积比约为10:1。阴极周围产物经蒸馏水后的上清液经pH计检测为强碱性,通入CO2后混浊,可以推断CaWO4电解后钙元素以CaO的形式沉积在阴极周围。
由于CaWO4密度为5.98 g/cm3,而CaCl2-NaCl混合熔盐在750 ℃的密度不高于2 g/cm3(CaCl2和NaCl固态分别为2.15和2.165 g/cm3,熔化体积膨胀率分别为0.9%和25%),所以在电解开始之前,CaWO4会在熔体中沉积。通电后,在电流作用下搅拌熔盐,一定量溶解在熔体中的CaWO4扩散至阴极和阳极附近进而吸附于电极表面。CaWO4分子中的W6+经式(1)、(2)的电极过程使钨在阴极沉积,而CaWO4分子中一部分氧离子在熔体中扩散至阳极表面发生电极反应(3)而形成氧原子;随后形成的氧原子与碳质的阳极发生式(4)、(5)的化学反应而在阳极表面析出CO及CO2气体。一部分氧离子与钙离子在阴极附近形成CaO,根据文献[14]数据可推算CaO的理论分解电压在750 ℃下为2.7 V,未参与电极反应而残留于阴极周围及熔体中。
W6++2e→W4+ (1)
W4++4e→W (2)
O2--2e→O (3)
C+O→CO (4)
C+2O→CO2 (5)
2.2.2 电流效率
图4所示为电解过程的时间—电流曲线。图4表明,在温度750 ℃、2.5 V电压下,电流经250 min以后逐渐趋于稳定。随着电解的进行,电流整体呈下降趋势,其中的波动可以认为是吸附在阳极表面的氧原子与石墨阳极作用产生的气体 (CO, CO2)后脱附和扩散而引起的反电动势的结果。另外,钨在阴极表面形成的过程中会改变阴极表面的结构,也会引起电流的波动。
在试验过程中共收集到了阴极产物为20.91g,若忽略收集过程中的损失,纯度按95%计,可估算最终获得的产物中钨约为19.86 g。电解过程中,通过的极间电量、生成19.86 g的钨所需电量和电流效率[15]可由以下公式计算:
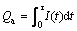 (6)
(6)
 (7)
(7)
 (8)
(8)
式中:Qa为电解过程中通过的极间电量,积分上限τ为电解时间,取250 min;I(t)为电解过程中电流随时间变化的函数。Qc为生成19.86 g的钨所需电量;m为生成钨的质量,取19.86 g;M为钨的摩尔质量,取183.85 g/mol;n为生成一个钨原子的转移电子数(电解前钨的化合价为+6价,故n=6);F为Faraday常数;η为电流效率。
根据式(6)的原理,由Origin软件对图4的时间与电流数据进行统计,可以得到Qa=75 303.6 C。同时,通过式(7)可计算出Qc = 62 445.5 C,按式(8)可初步估算电流效率约为82.9%。取电解后电解槽底部白色不溶物,经烘干取样,经XRD分析为CaWO4,说明CaWO4在经过5 h后电解仍不完全。
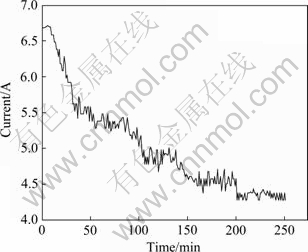
图4 电解过程中时间—电流曲线
Fig. 4 Time—current curve during electrolylic process (Electrolytic cell voltage is 2.5 V, temperature is 750 ℃, current is recorded by DP-4 ammeter)
2.2.3 电化学机理
图5所示为实验获得的循环伏安曲线。图5中的曲线1为CaCl2-NaCl(摩尔比1:1)150 g在750 ℃熔化后扫描的结果,曲线2为随后加入20 g CaWO4的扫描结果,直径分别为2 mm和4 mm的钨棒和石墨棒分别为工作电极和辅助电极,纯铂丝(直径1 mm)为参比电极,为防止阳极气体CO2和CO在熔体中扩散而影响测量结果,阳极外罩用底部带有直径1 mm气孔,内径为6 mm的氮化硼管,扫描的速率v=20 mV/s,步长为10 mV。曲线1表明CaCl2-NaCl混合熔盐在扫描范围为-3~1.5 V范围内电流未出现明显波动,工作电流基本对称,表明熔盐的阻抗稳定,电极附近未出现电极反应,熔盐的成分也稳定,没有杂质的影响。
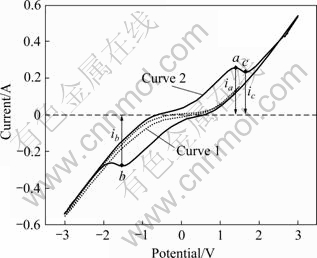
图5 循环伏安曲线
Fig. 5 Cyclic voltammetry (CaCl2-NaCl molten; 750 ℃; Scan rate 20 mV/s)
加入CaWO4后,体系扫描曲线2出现了明显的还原峰b和氧化峰a,由热力学数据可估算WO3和WO2在750 ℃下的理论分解电压为-0.98 V和-1.03 V,图中氧化峰a的峰电压Ea=1.384 V,对应的电流ia= 0.258 4 A;还原峰b的峰电压Eb=-1.531 V,对应电流大小ib= 0.279 A;循环伏安曲线1和2都是在多次扫描后取其中的稳态曲线,从波形和波峰的位置上看是单电子波的特征,并不能直接显示WO3或WO2得电子转移过程。结合实验,可以判断整个电解过程是一个多元体系多步骤的电荷转移过程,此时的还原峰和氧化峰应该包含多个峰的合并,电流峰则对应多个电子传递过程。虽然不能定量分析是活性物质扩散迁移还是电子电极转移控制反应速率,但可以初步计算电极过程的基本热力学性质。假设体系的氧化峰和还原峰电流分别为ibp和iap,则峰电流比ibp/iap可以判断体系的部分特征,由于实际的循环伏安曲线2中,法拉第响应叠加在近似为常数的充电电流上,必须对ib和ia做相应校正,根据文献[16]提供的方法,可以用基于零电流基线校正阳极峰电流ia和换向点c处的换向电流icp来按式(9)计算:
ibp/iap=icp/ia+0.485icp/ia+0.086 (9)
式中:icp=0.227 A,因此可得ibp/iap=1.391,对于稳定的可逆波峰电流比ibp/iap=1,与扫描速度、扩散系数无关,体系的ibp/iap比值偏离1,说明阴极电极反应非可逆。
由于方波伏安法具有更高的灵敏度和有效的抑制背景电流的影响,对体系进行了方波伏安扫描,曲线如图6所示。脉冲高度为4 mV,脉冲宽度为25 ms,阶梯步进为0.5 mV/s,扫描方向由-0.5~-2.0 V。图6所示方波伏安曲线是在反复循环多次得到的稳态曲线,可分解为在台阶为0.5 mV/s的阶梯波叠加对称的脉冲高度为4 mV,脉冲宽度为25 ms的双脉冲构成。对称的双脉冲在电极上进行的一系列循环使系统在步进波的循环过程中工作电极周围没有扩散层的恢复和更新,明显减少了扩散电流和充电电流的影响。图5也表明点a和b的电流平均值为0.029 5 A,循环伏安曲线在-1.157 V电流大小为0.235 A,可见方波伏安曲线的电流基数远小于循环伏安法,可以认为电解以极限扩散速度发生。但由于体系涉及慢速异相电子传递、复杂的传质形式,只能通过观察波形的整体定性研究体系的性质,图6表明,体系的电流的变化从点a起始经b达到峰值c后回落,表明电极的电子传递过程集中在a(-1.157 V, 0.028 A)—b(-1.291 V, 0.028 A)—c(-1.504 V, 0.031 A),从a点到b点的过渡时间为34.7 s,较长的过渡时间表明体系的活性物质扩散速度缓慢,阴极的浓差极化是体系电解速率的主要阻力。为进一步确定主因,对CaWO4在熔体实验条件下的溶解度进行了粗略的估测,将CaCl2-NaCl混合电解质 100 g放入刚玉坩埚中加热到750 ℃完全熔化后,加入过量的块状CaWO4 (把粉末状CaWO4在10 MPa下压成块状),缓慢搅拌混合后恒温2 h,取上清熔盐约30 g,冷却后溶于蒸馏水中,取其中的CaWO4沉淀物称量。结果表明:CaWO4溶解度约为0.2%~0.4%。可见,CaWO4在CaCl2-NaCl混合熔盐中的溶解度较低,但实验条件下CaWO4在熔体中的扩散系数尚需进一步实验确定。
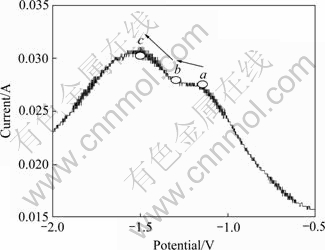
图6 方波伏安曲线
Fig. 6 Square wave voltammetry (CaCl2-NaCl-CaWO4 solutions; 750 ℃; Pulse height Eh=4 mV; Pulse width is 25 ms, frequency 50 Hz, scan increment rate 0.5 mV/s)
3 结论
1) 在CaCl2-NaCl混合盐(摩尔比1:1),CaWO4质量占5%~10%的体系,以石墨棒为电极材料;基本外部条件为温度750 ℃,槽电压2.5 V下经5 h以上电解可在阴极附近析出金属钨粉,纯度可达到99%,平均粒度小于2 μm。
2) 分析电解过程的中间产物表明在电解过程中的元素W并非由+6价直接变为零价,存在W6+→W4+的价态变化过程;通过时间-电流数据估算电流效率可达到82.9%。
3) 电化学分析分析表明电解过程不可逆,整个电解过程是一个多元体系多步骤的电荷转移过程,CaWO4在阴极还原为金属钨的实际电位在-1.5 V左右,而CaWO4在阴极表面区的富集和扩散(浓差极化)过程是体系电解速率的主要阻力。
REFERENCES
[1] 李洪桂. 稀有金属冶金学[M]. 北京: 冶金工业出版社, 1990: 64-66.
LI Hong-gui. Metallurgical of rare metals[M]. Beijing: Metallurgical Industry Press, 1990: 64-66.
[2] 罗章青, 廖小英, 王文华, 陈树茂. 从APT结晶尾气中回收氨的工业实践[J]. 稀有金属, 2007, 31(6): 90-92.
LUO Zhang-qing, LIAO Xiao-ying, WANG Wen-hua, CHEN Shu-mao. Industrial practice of recycling ammonia from APT tail gas crystal[J]. Chinese Journal of Rare Metals, 2007, 31(6): 90-92.
[3] OMEL’CHUK A A. Electrorefining of heavy nonferrous metals in molten electrolytes[J]. Russian Journal of Electrochemistry, 2010, 46: 680-690.
[4] MOHANDAS K S, FRAY D J. FFC Cambridge process and removal of oxygen from metal-oxygen systems by molten salt electrolysis[J]. Trans Indian Inst Met, 2005, 57: 579-592.
[5] TRIPATHY P K, GAUTHIER M, FRAY D J. Electrochemical deoxidation of titanium foam in molten calciumchloride[J]. Metallurgical and Material Transition B, 2007, 38: 893-900.
[6] 张庆军, 屈梅玲, 王 岭, 戴 磊, 田 颖, 崔春翔. 熔盐电脱氧法制备CoSn合金[J]. 中国有色金属学报, 2010, 20(8): 1578-1582.
ZHANG Qing-jun, QU Mei-ling, WANG Ling, DAI Lei, TIAN Ying, CUI Chun-xiang. Preparation of CoSn alloy by electro-deoxidization in molten salt[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(8): 1578-1582.
[7] MOHANDAS K S, FRAY D J. Electrochemical deoxidation of solid zirconium dioxide in molten calciumchloride[J]. Metallurgical and Material Transition B, 2009, 40: 685-699.
[8] QIU G H, WANG D H, JIN X B, CHEN G Z. A direct electrochemical route from oxide precursors to the terbium-nickel intermetallic compound TbNi5[J]. Electrochimica Acta, 2006, 51: 5785-5793.
[9] 邓丽琴, 许 茜, 李 兵, 翟玉春, 黄振奇. 电脱氧法由Nb2O5直接制备金属铌[J]. 中国有色金属学报, 2005, 15(4): 541-545.
DENG Li-qin, XU Qian, LI Bing, ZHAI Yu-chun, HUANG Zhen-qi. Preparation of niobium by direct electrochemical reduction of solid Nb2O5[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(4): 541-545.
[10] 彭少方. 钨冶金学[M]. 北京: 冶金工业出版社, 1981: 25-30.
PENG Shao-fang. Tungsten metallurgy[M]. Beijing: Metallurgical Industry Press, 1981: 25-30.
[11] 冯乃祥, 刘希诚, 孙 阳. 用熔盐电解法制备超细钨粉[J]. 材料研究学报, 2001, 15: 459-462.
FENG Nai-xiang, LIU Xi-chen, SUN Yang. Preparation of ultrafine tungsten power by electrolyzing in molten salts[J]. Chinese Journal of Material Research, 2001, 15: 459-462.
[12] 冯乃祥, 孙 阳, 葛贵军. NaCl-Na2WO4-WO3系熔盐电解法制备超细钨粉的研究[J]. 稀有金属, 2001, 25: 374-377.
FENG Nai-xiang, SUN Yang, GE Gui-jun. Study on producing ultrafine tungsten powder by electrolysis in molten salts of NaCl-Na2WO4-WO3 system[J]. Chinese Journal of Rare Metals, 2001, 25: 347-377.
[13] FREIDINA E B, FRAY D J. Study of the ternary system CaCl2-NaCl-CaO by DSC[J]. Thermochim Acta, 2000, 356: 97-99.
[14] 梁英教, 车荫昌. 无机物热力学数据手册[M]. 沈阳: 东北大学出版社: 1994: 45-50.
LIANG Ying-jiao, CHE Yin-chang. Manual of inorganic thermodynamic data[M]. Shenyang: Northeastern University Press, 1994: 45-50.
[15] WANG X, ZHAI Y C. An electrochemical method for the preparation of CaB6 crystal powder[J]. Journal of Applied Electrochemist, 2009, 39: 1797-1802.
[16] 阿伦·J·巴德, 拉里·R·福克纳. 电化学原理: 方法与应用[M]. 北京: 化学工业出版社, 2005: 121-138.
BARD A J, FAULKNER L R. Electrochemical methods: Fundamentals and applications[M]. Beijing: Chemical Industry Press, 2005: 121-138.
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51074081)
收稿日期:2011-06-27;修订日期:2012-02-22
通信作者:廖春发,教授,博士;电话:0797-8312243;E-mail: liaochfa@163.com