文章编号:1004-0609(2009)12-2243-08
高铁三水铝石型铝土矿烧结过程中氧化铝反应热力学
朱忠平,姜 涛,李光辉,黄柱成
(中南大学 资源加工与生物工程学院,长沙 410083)
摘 要:研究了高铁三水铝石型铝土矿烧结过程中Al2O3与CaCO3、CaO、SiO2及FeO反应的热力学规律。结果表明:在1 473~1 673 K温度下,Al2O3比Fe2O3更易与CaCO3反应;Al2O3与铁酸钙(2CaO?Fe2O3和CaO?Fe2O3)反应不能生成3CaO?Al2O3,当烧结温度大于1 000 K时,可以与2CaO?Fe2O3反应生成12CaO?7Al2O3;SiO2比Al2O3更易与CaO结合,Al2O3与SiO2直接反应生成硅酸铝的可能性较小;当烧结温度为1 473~1 673 K时,除CaO?2Al2O3和CaO?Al2O3不能向3CaO?SiO2转变外,其余铝酸钙均可在SiO2的作用下向硅酸钙转变;2CaO?Al2O3·SiO2是CaO、Al2O3和SiO2三者直接反应的产物,不能由硅酸钙和铝酸钙相互反应生成;CaO、Fe2O3、Al2O3和SiO2四元矿物存在时,烧结过程优先生成2CaO·Al2O3·SiO2和4CaO·Al2O3·Fe2O3,这与烧结实验结果相符。
关键词:三水铝石; 铝土矿;铁矿石;烧结;氧化铝
中图法分类号:TF521; TF821 文献标识:A
Thermodynamics of reaction of alumina during sintering process of high-iron gibbsite-type bauxite
ZHU Zhong-ping, JIANG Tao, LI Guang-hui, HUANG Zhu-cheng
(School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The thermodynamics of the reactions of alumina(Al2O3) with CaCO3, CaO, SiO2 and FeO in the sintering process of high-iron gibbsite-type bauxite was investigated. The results show that alumina reacts with calcium carbonate much easier than ferri oxide(Fe2O3) at the industrial sintering temperature of 1 473-1 673 K. Alumina can not react with calcium ferrites(2CaO?Fe2O3 and CaO?Fe2O3) to form 3CaO?Al2O3, but when temperature is over 1 000 K alumina reacts with 2CaO?Fe2O3 to form 12CaO?7Al2O3. SiO2 reacts with CaO much easier than Al2O3. The possibility of alumina silicate from direct reaction of Al2O3 with SiO2 is little. Except for CaO?2Al2O3 and CaO?Al2O3, the other calcium aluminates can transform into calcium silicate by reacting with SiO2. Gehlenite(2CaO?Al2O3?SiO2) can not be formed from the reaction of calcium silicate(CaO?SiO2) with calcium aluminate(CaO?Al2O3), but it can be directly formed from the reaction of CaO, Al2O3 and SiO2. When CaO, Fe2O3, Al2O3 and SiO2 coexist in the sintering process, they are more likely to form ternary compound 2CaO·Al2O3·SiO2 and 4CaO?Al2O3?Fe2O3, which is consistent with the sintering results in laboratory.
Key words: gibbsite; bauxite; iron ore; sintering; alumina
随着我国经济的快速发展,对支持国民经济可持续发展的第一、二大金属铁、铝的消耗也越来越大,铁矿和铝土矿的资源供应量严重短缺[1-3]。20世纪80年代,我国在广西等地发现大量高铁三水铝石型铝土矿(以下简称为高铁铝土矿),资源储量超过1.6亿t。该矿中铝、铁含量较低(总铁含量24%~37%,Al2O3含量20%~37%)、铝硅比较低(A/S=2.6~5.4),而且由于铁矿物与铝矿物嵌布粒度细,相互胶结,矿物的单体解离性能极差,难以选别,若以单一铁矿或铝土矿开发,均未达到一般冶炼品位要求[1]。但由于该矿含有价金属品种多(主要含铁、铝、钒、镓、锰和钛等),国内外科研工作者一直没有间断对此矿的研究。
东北大学对广西高铁铝土矿“高炉冶炼─炉渣浸出提铝”工艺的研究表明,通过严格控制造渣条件,可在炼铁高炉中实现铁铝的有效分离[4]。本课题组[5]针对该矿的烧结特性进行了系统的研究,结果表明:高铁铝土矿超高碱度烧结矿,机械强度高,还原性能良好,基本能满足高炉冶炼要求。在烧结过程中,一般通过添加大量的石灰石熔剂,以使烧结混合料中的氧化铁转化为铁酸钙,氧化铝转化铝酸钙。氧化铁在烧结过程中的行为已有研究[6],本文作者基于已有热力学数据[7-13],对氧化铝在烧结过程中的反应行为进行分析研究。
1 热力学分析
1.1 Al2O3与含钙化合物的反应
烧结过程中涉及的含钙化合物主要有碳酸钙、氧化钙、铝酸钙、铁酸钙和硅酸钙等。在高铁铝土矿与石灰石的烧结过程中,高铁铝土矿中的Al2O3、Fe2O3均与碳酸钙发生化学反应生成相应的铝酸盐和铁酸盐。在Al2O3-CaO-Fe2O3系中,烧结过程同时存在Al2O3、Fe2O3与CaCO3的反应,其反应方程式如下:
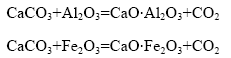
经热力学计算,其吉布斯自由能(?G)与温度(T)的关系如图1所示。由图1可知:Al2O3、Fe2O3与CaCO3反应的吉布斯自由能都随温度的升高而降低,在正常烧结过程中(由于1 200 K以上CaCO3发生分解,因而1 200 K以上没有作曲线),反应(1)和(2)均能自动向右进行,生成相应的铝酸钙和铁酸钙;在同一温度下,Al2O3与CaCO3反应的吉布斯自由能比Fe2O3与CaCO3反应的更负。在1 473~1 673 K烧结温度下,CaCO3实际已发生分解,因此,在以下的分析中只按CaO 考虑。
由于烧结的最终目的是要满足高炉需要的烧结矿,使铁铝在高炉冶炼过程中分离后,再由炉渣浸出提取氧化铝。如果不考虑炉渣中MgO和TiO2等少量组分,则炉渣可简化为CaO-Al2O3-SiO2三元系。在该三元系中的含铝矿物中,只有12CaO?7Al2O3(C12A7)
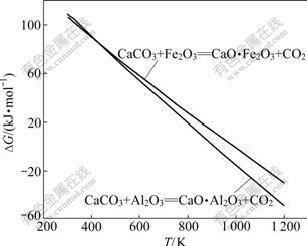
图1 反应式(1)和(2)的反应吉布斯自由能与温度的关系
Fig.1 Relationship between Gibbs free energy changes of reactions (1) and (2) and temperature
能完全溶于碳酸钠溶液中,3CaO?Al2O3(C3A)和CaO?Al2O3(CA)溶解速度较慢,而2CaO?Al2O3?SiO2 (C2AS)等矿物则完全不溶。假定高炉不再添加熔剂,则烧结配料时应按照高炉冶炼和炉渣浸出的要求,考虑尽量避免这些三元化合物的生成,并应使Al2O3尽可能地形成C12A7,且避开C2AS的初晶区。Al2O3与CaO反应生成C3A、C12A7、CA、CaO?2Al2O3(CA2)的方程式如下:

反应式(3)~(6)的反应吉布斯自由能与温度的关系如图2所示。由图2可知,Al2O3与CaO反应的吉布斯自由能随着温度的升高而降低,在正常烧结温度区间(1 473~1 673 K,下同)下,反应(3)~(6)均能自动向右进行,生成相应的铝酸钙;在热力学上,在同一烧结温度下,1 mol Al2O3与CaO生成铝酸钙由易至难的顺序为:C12A7、C3A、CA和CA2。
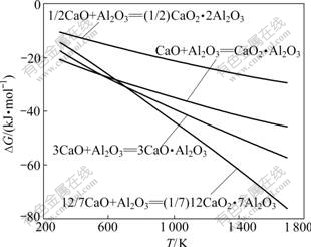
图2 反应式(3)~(6)的反应吉布斯自由能与温度的关系
Fig.2 Relationship between Gibbs free energy changes of reactions (3)-(6) and temperature
当原料中CaO用量不足时,多余的Al2O3可能会与新生成的高钙铝比(CaO与Al2O3摩尔比)的铝酸钙向低钙铝比的铝酸钙转变,其反应方程式如下:

反应式(7)~(12)的反应吉布斯自由能与温度的关系如图3所示。由图3可知,在正常烧结温度下,反应式(7)~(12)的反应吉布斯自由能均为负,反应均能向右进行,生成相应的低钙铝比的铝酸钙;除反应式(9)和(10)外,其余反应式的吉布斯自由能随着温度的升高而更负;在热力学上,在正常烧结温度下,各反应式由易到难进行的顺序依次为(7)、(8)、(9)、(10)、(11)和(12)。对比图(2)和(3),在热力学上,Al2O3与CaO反应更容易生成C12A7。
在高铁铝土矿烧结过程中,SiO2在烧结过程中可能与CaO形成CaO·SiO2(CS)、3CaO·2SiO2(C3S2)、2CaO·SiO2(C2S)、和3CaO·SiO2(C3S),其相应的反应吉布斯自由能随温度的变化曲线见图4。
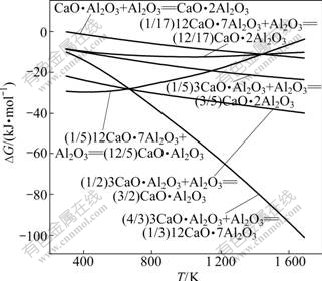
图3 反应式(7)~(12)的反应吉布斯自由能与温度的关系
Fig.3 Relationship between Gibbs free energy changes of reactions (7)-(12) and temperature
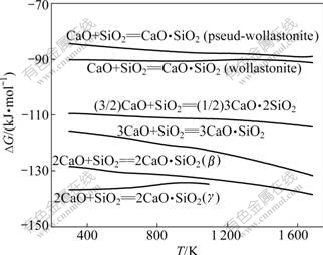
图4 硅酸钙的吉布斯自由能与温度的关系
Fig.4 Relationship between Gibbs free energy changes of calcium silicate and temperature
由图4可知:在热力学上,当温度低于1 100 K时,SiO2与CaO先形成γ-C2S,当温度高于1 100 K时,SiO2与CaO先形成β-C2S;在正常烧结温度下,在热力学上,硅酸钙形成的先后顺序为C2S, C3S, C3S2, CS。
由于SiO2的酸性比Al2O3的强,因此,新生成的铝酸钙有可能与SiO2反应生成硅酸钙和铝氧化物,其可能反应见反应式(13)~(28),其相应的反应吉布斯自由能随温度的变化曲线如图5所示。
(3)CaO·2Al2O3+SiO2=3CaO·SiO2+6Al2O3 (13)
(3)CaO?Al2O3+SiO2=3CaO?SiO2+3Al2O3 (14)
(1/4)12CaO·7Al2O3+SiO2=3CaO?SiO2+7/4Al2O3 (15)
3CaO·Al2O3+SiO2=3CaO?SiO2+Al2O3 (16)
(2)CaO·2Al2O3+SiO2=2CaO·SiO2+4Al2O3 (17)
(2)CaO·Al2O3+SiO2=2CaO·SiO2+2Al2O3 (18)
(1/6)12CaO·7Al2O3+SiO2=2CaO·SiO2+7/6Al2O3 (19)
(2/3)3CaO·Al2O3+SiO2=2CaO·SiO2+2/3Al2O3 (20)
(3/2)CaO·2Al2O3+SiO2=(1/2)3CaO·2SiO2+3Al2O3 (21)
(3/2)CaO·Al2O3+SiO2=
(1/2)3CaO·2SiO2+(3/2)Al2O3 (22)
(1/8)12CaO·7Al2O3+SiO2=
(1/2)3CaO·2SiO2+7/8Al2O3 (23)
(1/2)3CaO·Al2O3+SiO2=(1/2)3CaO·2SiO2+1/2Al2O3 (24)
CaO·2Al2O3+SiO2=CaO·SiO2+2Al2O3 (25)
CaO·Al2O3+SiO2=CaO·SiO2+Al2O3 (26)
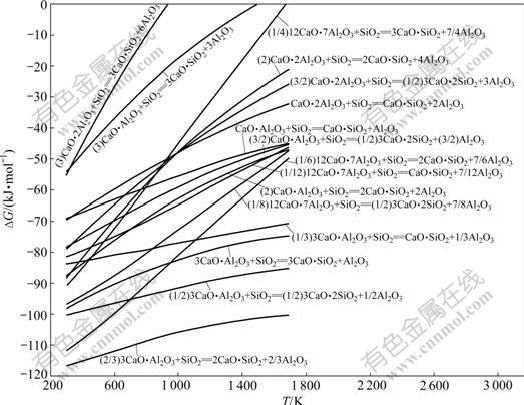
图5 反应式(13)~(28)的反应吉布斯自由能与温度的关系
Fig.5 Relationship between Gibbs free energy changes of reactions (13)-(28) and temperature
(1/12)12CaO·7Al2O3+SiO2=CaO·SiO2+7/12Al2O3 (27)
(1/3)3CaO·Al2O3+SiO2=CaO·SiO2+1/3Al2O3 (28)
由图5可知:反应的吉布斯自由能均随着温度的升高而增加;在正常烧结温度下,反应式(13)不可能发生,也即钙铝比最低的铝酸钙不能向钙硅比(CaO与SiO2摩尔比)最高的硅酸钙转变,当温度在1 500 K以上时,反应式(14)的吉布斯自由能也大于0,反应也不会向右进行,其余铝酸钙均能与SiO2生成硅酸钙;在热力学上,在正常烧结温度下,SiO2与铝酸钙反应 的顺序是先与C3A,然后与C12A7、CA和CA2反应,也即先与高钙铝比铝酸钙反应再与低钙铝比铝酸钙 反应。
由图2~5可知,在热力学上,当有CaO、SiO2和Al2O3同时存在时,在不考虑形成三元化合物的情况下,更容易先生成C2S,然后是C12A7。
由图1可知,反应式(1)比(2)的吉布斯自由能更负,因此,从热力学上分析,Fe2O3与CaCO3反应只能在Al2O3与CaCO3反应后有剩余CaCO3的条件下才能进行。当CaCO3不足时,新生成的铁酸钙有可能向铝酸钙转变,其反应如下:
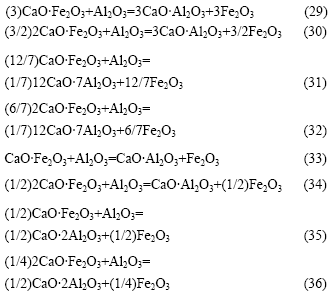
反应式(29)~(36)的反应吉布斯自由能与温度的关系如图6所示。由图6可知:Al2O3不能置换铁酸钙中的Fe2O3生成C3A;也不能置换CaO?Fe2O3(CF)中的Fe2O3生成C12A7,但当温度大于1 000 K以上时,可以将2CaO?Fe2O3(C2F)中的Fe2O3置换生成C12A7;温度越高,反应的吉布斯自由能越负;Al2O3 可以与CF和C2F生成CA或CA2,且随着温度的升高,反应的吉布斯自由能更负。由于在热力学上,Fe2O3与CaO反应更容易生成C2F(见图7),因此,在正常烧结温度下,当原料中配CaO充足时,反应产物最终为C12A7。
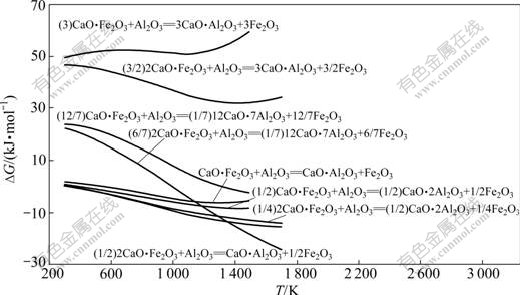
图6 反应式(29)~(36)的反应吉布斯自由能与温度的关系
Fig.6 Relationship between Gibbs free energy changes of reactions (29)-(36) and temperature
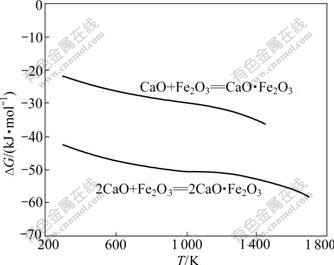
图7 CaO与Fe2O3的反应吉布斯自由能与温度的关系
Fig.7 Relationship between Gibbs free energy changes of reactions CaO and Fe2O3 and temperature
1.2 Al2O3与SiO2的反应
SiO2主要来自高铁铝土矿和焦粉灰分,Al2O3与SiO2反应可生成的硅酸盐有Al2O3?2SiO2(AS2)、Al2O3?SiO2(AS)红柱石、AS蓝晶石、AS硅线石、3Al2O3?2SiO2(A3S2)。热力学计算表明,在烧结条件下,Al2O3与SiO2反应不可能生成AS2。其余各反应的方程式如下:
Al2O3+SiO2=Al2O3?SiO2(蓝晶石) (37)
Al2O3+SiO2=Al2O3?SiO2(硅线石) (38)
Al2O3+SiO2=Al2O3?SiO2(红柱石) (39)
Al2O3+2/3SiO2=(1/3)3Al2O3?2SiO2 (40)
反应式(37)~(40)的反应吉布斯自由能与温度的关系如图8所示。由图8可知:在正常烧结温度下,反应式(37)的吉布斯自由能大于零,反应不可能发生;反应式(38)和(39)的吉布斯自由能受温度的变化很小;反应式(40)的吉布斯自由能随着温度的升高而降低。从热力学上分析,在正常烧结温度下,生成硅酸铝由难至易的顺序为A3S2, AS红柱石和AS硅线石。在有CaO存在时,由图2及4可知,CaO与SiO2和Al2O3反应更容易先生成C2S,然后是C12A7。因此,在烧结过程中,在热力学上硅酸铝形成的可能性较小。
1.3 Al2O3与FeO的反应
在高铁铝土矿的烧结过程中,Al2O3还有可能与烧结过程中产生的富氏体(FeO)发生反应,生成
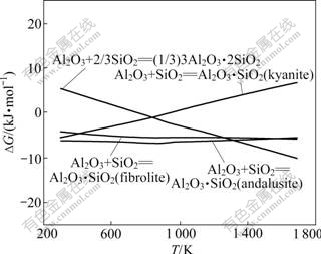
图8 反应式(37)~(40)的反应吉布斯自由能与温度的关系
Fig.8 Relationship between Gibbs free energy changes of reactions (37)-(40) and temperature
FeO?Al2O3,其化学反应方程式如下:
Al2O3+FeO=FeO?Al2O3 (41)
反应式(41)的反应吉布斯自由能与温度的关系如图9所示。由图9可知:在正常烧结温度下,吉布斯自由能为负,反应式(41)可以向右进行,生成FeO·Al2O3;反应式(41)的反应吉布斯自由能随着烧结温度的升高而升高,在热力学上,烧结温度越高,反应进行的趋势越低。
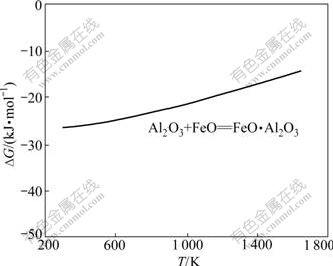
图9 反应式(41)的反应吉布斯自由能与温度的关系
Fig.9 Relationship between Gibbs free energy changes of reactions (41) and temperature
1.4 三元化合物
烧结过程CaO、Al2O3、SiO2生成的三元化合物 主要有C2AS、CAS2、CAS和C3AS3。其反应方程式如下:
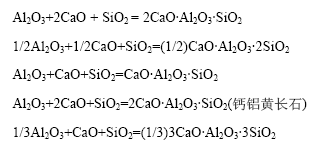
除此之外,CaO、Al2O3、Fe2O3还可以生成4CaO?Al2O3?Fe2O3(C4AF),其反应式如下:
4CaO +Al2O3+Fe2O3=4CaO?Al2O3?Fe2O3 (47)
反应式(42)~(47)的反应吉布斯自由能与温度的关系如图10所示。由图10可知:除反应式(46)外,反应式(42)~(47)的吉布斯自由能随温度的升高而更负;在热力学上,在1 473 K烧结温度下,三元化合物的生成由难至易的顺序为C2AS (钙铝黄长石)、C4AF、CAS、C3AS3、C2AS、CAS2。
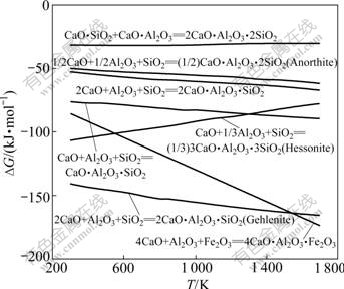
图10 反应式(42)~(46)的反应吉布斯自由能与温度的关系
Fig.10 Relationship between Gibbs free energy changes of reactions (42)-(46) and temperature
C2AS也可能由CA和CS反应生成,其吉布斯自由能与温度的关系曲线如图10所示。由图10可知,反应式(45)的吉布斯自由能比CA和CS反应生成C2AS的吉布斯自由能负得多,因此,从热力学角度分析,烧结矿中的C2AS不是由二元化合物CA和CS反应的结果,而是Al2O3、CaO、SiO2 三者直接反应的产物。周秋生等[14]认为,C4AF不能由铁酸钙和铝酸钠相互反应产生,而可能是CaO、Al2O3 和Fe2O3三者直接反应的产物。由图1~10中的热力学分析可知,Al2O3更容易与Fe2O3、SiO2、CaO生成C2AS和C4AF,因此,高铁铝土矿烧结矿有较好的冷强度,这在烧结实验中得到了验证[5]。因此,在添加CaO的氧化气氛烧结过程中,不经高炉冶炼而直接生成C12A7的可能性很小。假定高炉冶炼时不再添加石灰石熔剂,按照CaO-Al2O3-SiO2三元相图中2CaO?SiO2-12CaO?7Al2O3线上化学组分进行烧结配料,经高炉冶炼渣铁分离除铁后,从热力学角度可以使炉渣中SiO2全部转换成C2S,Al2O3以C12A7形式存在,从而使Al2O3得到尽可能高的回收率。李殷泰等[4]证实,经过“高炉冶炼─炉渣浸出提铝”工艺,通过严格控制烧结配料及高炉操作,可以使炉渣的最终成分为C2S和C12A7,从而实现铝铁综合回收利用。
2 实验结果
采用Al2O3含量26.35%,总铁含量 31.22%,铝硅比(A/S)3.17的广西某高铁铝土矿进行烧结试验。该铝土矿中铝的赋存矿物中主要为三水铝石、针赤铁矿中以类质同像存在的Al2O3、高岭石和一水硬铝石,分别占矿石中总Al2O3的45.02%、23.68%、23.14%和8.16%;铁矿物主要为针赤铁矿,占矿石中总铁物量的98.60%;硅矿物主要为铝硅酸盐,占96.51%。试验时将高铁三水铝石型铝土矿、焦粉、石灰石、返矿按烧结矿二元碱度4.0、焦粉用量8.0%组成混合料,混合料水分按照9.0%添加,经一次混合、二次圆筒混合机混合制粒3 min,然后在d200×640 mm烧结杯中进行烧结试验。试验条件为:点火温度1 050~1 150 ℃、点火时间1.5 min、点火负压5 kPa、烧结负压10 kPa。图11为烧结矿的XRD谱。由图11可知,烧结矿主要成分为铁铝酸钙、铝铁酸钙、铁氧富氏体、赤铁矿、磁铁矿和二氧化硅。XRD分析表明:烧结矿中的铁铝酸钙分子式为Ca2(Fe1.33Al0.67)2O5,铝铁酸钙分子式为Ca2(Al1.66Fe0.40Si0.94)3O7,其分子式为C2AS中的Al2O3和SiO2被一部分Fe2O3所取代的结果。因而,可以 认为烧结过程中形成C2AS,这与热力学分析结果相 一致。
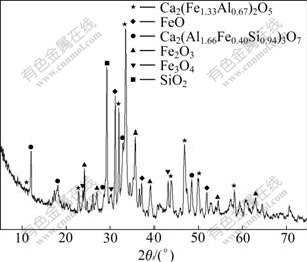
图11 烧结矿的XRD谱
Fig.11 XRD pattern of sintering ore
3 结论
1) 在烧结条件下,Al2O3比Fe2O3更易与CaCO3反应,当CaCO3不足时,先生成铝酸钙。Al2O3不能置换铁酸钙中的Fe2O3生成C3A,当烧结温度大于 1 000 K以上时,可以将C2F中的Fe2O3置换而形成C12A7,Al2O3可以与铁酸钙生成CA或CA2。
2) Al2O3与SiO2相比,SiO2更容易与CaO结合生成C2S,Al2O3与CaO优先生成C12A7。在1473~1673K烧结温度下,除CA2和CA不能向C3S转变外,其余铝酸钙均可在SiO2的作用下向硅酸钙转变,Al2O3与SiO2直接反应生成硅酸铝的可能性较小。
3) 在热力学上,C2AS不是CS和CA相互反应生成的,而是由CaO、Al2O3和SiO2直接反应生成的产物。CaO、Fe2O3、Al2O3和SiO2四元矿物存在时,烧结过程优先生成C2AS和C4AF。
REFERENCES
[1] 张家增. 中国铝工业发展历程与发展趋势[C]// 中国有色金属学会第五届学术年会论文集. 中国有色金属学会, 2003, 8: 40-42.
ZHANG Jia-zeng. The historical developmental course and future developmental trend of China’s aluminum industry[C]// The 5th Annual Conference of China, Institute of Nonferrous Metal of 2003, 8: 40-42.
[2] 顾松青. 我国的铝土矿资源和高效低耗的氧化铝生产技术[J]. 中国有色金属学报, 2004, 14(5): 91-97.
GU Song-qing. Alumina production technology with high efficiency and low consumption from Chinese bauxite resource[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(5): 91-97.
[3] 候宗林, 蔡立军. 中国铁矿资源与钢铁工业可持续发展[J]. 天津冶金. 2005, 131(6): 14-16.
HOU Zong-lin, CAI Li-jun. Everlasting development of Chinese iron ore resources and iron and steel industry[J]. Tianjin Metallurgy, 2005, 131(6): 14-16.
[4] 李殷泰, 毕诗文, 段振瀛, 杨毅宏, 张敬东. 关于广西贵港三水铝石型铝土矿综合利用工艺方案的探讨[J]. 轻金属, 1992(9): 6-14.
LI Yin-tai, BI Shi-wen, DUAN Zheng-yin, YANG Yi-hong, ZHANG Jin-Dong. Study on multipurpose utilization technology of gibbsite-bauxite in Guangxi[J]. Light Metals, 1992(9): 6-14.
[5] 朱忠平, 黄柱成, 姜 涛, 李光辉, 庄剑鸣. 高铁三水铝石型铝土矿烧结特性[J]. 中国有色金属学报, 2007, 17(8): 1360-1366.
ZHU Zhong-ping, HUANG Zhu-cheng, JIANG Tao, LI Guang-hui, ZHUANG Jian-ming. Sintering properties of high iron gibbsite-type bauxite ores[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(8): 1360-1366.
[6] 傅菊英, 姜 涛, 朱德庆. 烧结球团学[M]. 长沙: 中南工业大学出版社, 1996: 2.
FU Ju-ying, JIANG Tao, ZHU De-qing. Sintering and palletizing[M]. Changsha: Central South University Technology Press, 1996: 2.
[7] PRESNALL D C. Phase diagrams of earth-forming minerals[J]. Minerals Physics and Crystallography, 1995, 2: 248-268.
[8] 叶大伦, 胡建华. 实用无机物热力学数据手册[M]. 北京: 冶金工业出版社, 2002: 2.
YE Da-lun, HU Jian-hua. Practical thermodynamics manual of inorganic substances[M]. Beijing: Metallurgical Industry Press, 2002: 2.
[9] GUDFINNSSON G H, PRESNALL D C. Melting behavior of model lherzolite in the system CaO-MgO-Al2O3-SiO2-FeO at 0.7 to 2.8 GPa[J]. Journal of Petrology, 2000, 41: 1241-1269.
[10] BARIN I, KNACKE O. Thermochemical properties of inorganic substances[M]. Berlin: Supplement, 1997.
[11] 杨重愚. 氧化铝生产工艺学[M]. 北京: 冶金工业出版社, 1993.
YANG Chong-yu. Alumina production technology[M]. Beijing: Metallurgical Industry Press, 1993.
[12] 任万能. 氧化铝生产热力学数据库的优化与应用[D]. 长沙: 中南大学, 2005.
REN Wan-neng. Optimization and application of thermodynamic database for alumina production[D]. Changsha: Central South University, 2005.
[13] AKAOGI M, HARAGUCHI M, YAGUCHI M, KOJITANI H. High-pressure phase relations and thermodynamic properties of CaAl4Si2O11 CAS phase[J]. Physics of the Earth and Planetary Interiors, 2009, 173: 1-6.
[14] 周秋生, 齐天贵, 彭志宏, 刘桂华, 李小斌. 熟料烧结过程中氧化铁反应行为的热力学分析[J]. 中国有色金属学报, 2007, 17(6): 973-978.
ZHOU Qiu-sheng, QI Tian-gui, PENG Zhi-hong, LIU Gui-hua, LI Xiao-bin. Thermodynamics of reaction behavior of ferric oxide during sinter-preparing process[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(6): 973-978.
基金项目:国家杰出青年科学基金资助项目(50725416)
收稿日期:2009-02-16;修订日期:2009-06-24
通信作者:朱忠平,讲师,博士研究生;电话:0731-88879622;E-mail: zhuzp@mail.csu.edu.cn
(编辑 龙怀中)