LiBH4/Mg17Al12-氢化物复合体系的放氢行为改善及其机制
来源期刊:中国有色金属学报(英文版)2014年第1期
论文作者:韩乐园 肖学章 范修林 李 芸 李寿权 葛红卫 王启东 陈立新
文章页码:152 - 157
关键词:配位氢化物;LiBH4;Mg17Al12;吸放氢行为;可逆性
Key words:complex hydride; LiBH4; Mg17Al12; de/rehydrogenation behaviors; reversibility
摘 要:选择Mg17Al12-氢化物作为失稳剂与LiBH4进行球磨以改善LiBH4体系的吸放氢性能。研究表明,LiBH4/Mg17Al12-氢化物复合体系发生两步放氢过程。复合体系在300 °C开始放氢并在500 °C下产生9.8%的放氢量。通过添加Mg17Al12-氢化物,LiBH4的放氢动力学得到有效改善,并且其放氢温度降低20 °C。复合体系的放氢产物在450 °C的首次再加氢容量可高达8.3%。XRD分析表明,复合体系在放氢过程中所形成的MgB2和AlB2可降低LiBH4的热力学稳定性,进而有效改善LiBH4/Mg17Al12-氢化物复合体系的可逆储氢行为。
Abstract: Mg17Al12-hydride (abbreviated as MAH) was selected as a destabilization agent to improve de/rehydrogenation properties of LiBH4. 58LiBH4+Mg17Al12-hydride composite was prepared by ball-milling. It is found that the dehydrogenation of ball-milled LiBH4/MAH composite presents a two-step reaction for hydrogen release. The composite starts desorbing hydrogen at about 300 °C and yields 9.8% of hydrogen (mass fraction) below 500 °C. By adding MAH, the dehydrogenation kinetics of LiBH4 is improved and the dehydrogenation temperature of LiBH4 is also lowered by 20 °C. High rehydriding capacity of 8.3% was obtained for the dehydrogenated composite in the first cycle at 450 °C. The XRD analysis shows the formation of MgB2 and AlB2 in the dehydrogenation process, which reduces the thermodynamics stability of LiBH4 system and is beneficial to the reversible hydrogen storage behaviors of LiBH4/MAH composite.
Trans. Nonferrous Met. Soc. China 24(2014) 152-157
Le-yuan HAN, Xue-zhang XIAO, Xiu-lin FAN, Yun LI, Shou-quan LI, Hong-wei GE, Qi-dong WANG, Li-xin CHEN
Key Laboratory of Advanced Materials and Applications for Batteries of Zhejiang Province, Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China
Received 6 November 2012; accepted 1 April 2013
Abstract: Mg17Al12-hydride (abbreviated as MAH) was selected as a destabilization agent to improve de/rehydrogenation properties of LiBH4. 58LiBH4+Mg17Al12-hydride composite was prepared by ball-milling. It is found that the dehydrogenation of ball-milled LiBH4/MAH composite presents a two-step reaction for hydrogen release. The composite starts desorbing hydrogen at about 300 °C and yields 9.8% of hydrogen (mass fraction) below 500 °C. By adding MAH, the dehydrogenation kinetics of LiBH4 is improved and the dehydrogenation temperature of LiBH4 is also lowered by 20 °C. High rehydriding capacity of 8.3% was obtained for the dehydrogenated composite in the first cycle at 450 °C. The XRD analysis shows the formation of MgB2 and AlB2 in the dehydrogenation process, which reduces the thermodynamics stability of LiBH4 system and is beneficial to the reversible hydrogen storage behaviors of LiBH4/MAH composite.
Key words: complex hydride; LiBH4; Mg17Al12; de/rehydrogenation behaviors; reversibility
1 Introduction
Lightweight hydrogen storage materials, such as alanates [1,2] and borohydrides [3,4], are regarded as promising energy carrier in mobile applications due to their large hydrogen capacities compared with traditional metal hydrides [5-7]. Of all lightweight hydrogen storage materials, LiBH4 has a high theoretical gravimetric capacity beyond 18.5% in mass fraction [3]. Unfortunately, its practical application is limited for both thermodynamic and kinetic deficiencies. Under moderate conditions, reversibility of LiBH4 is rather limited [8].
Doping additives is one of the most effective methods to improve the performance of hydrogen storage materials [9-11]. By doping additives, hydrogen storage properties of traditional metal-hydrogen systems are becoming favorable [12]. Similarly, metals, metal halides and metal hydrides are added to LiBH4 [13-15]. Among these composite systems, LiBH4-MgH2 system exhibits greatly improved hydrogen storage performance [16]. The enthalpy change is decreased by overall 27 kJ per mol H2 for reaction (1) in comparison with neat LiBH4 [16]. Meanwhile, the lower kinetic barrier of reaction (1) favors the re-formation of LiBH4 [17]. This novel pathway stimulates extensive explorations on the reactions of LiBH4 with additives to obtain metal borides, such as, AlB2 [13,18] and CaB6 [19,20].
2LiBH4+MgH2 2LiH+MgB2+4H2 (1)
2LiH+MgB2+4H2 (1)
Among metal-boride systems, MgB2 and AlB2 exert an important influence on both reversibility and improved kinetics [16,18]. MgH2-Al mixture showed enhanced destabilization in LiBH4-MgH2-Al system compared with LiBH4-MgH2 and LiBH4-Al systems [21]. A synergistic effect of MgH2 and Al generated from Mg(AlH4)2 was observed in modifying LiBH4 [22]. However, above studies did not consider the function of Mg-Al composition in destabilizing LiBH4. Melting effect of Mg17Al12 was rarely taken into account on the formation of MgB2 and AlB2 before. Additionally, the previous work indicated that alloy or compounds could act as catalyst in NaAlH4 system [23,24]. Here we present that LiBH4 can be reversibly dehydrogenated and rehydrogenated with a reduced temperature by adding MAH. The dehydrogenation reactions are put forward as well.
In this study, the dehydrogenation and rehydrogenation were experimentally investigated by differential scanning calorimetry/mass spectrometry (DSC-MS), temperature programmed desorption (TPD) measurements in a Sievert-type apparatus. The microstructures were characterized by X-ray diffraction (XRD) and Fourier transform infrared spectrometry (FT-IR).
2 Experimental
LiBH4 powder (95% purity) was purchased from Acros and used without further purification. Magnesium (ingot from Goodfellow, China) and aluminum (foil from Sigma-Aldrich, China) had 99% purity and were not further purified. Mg17Al12 was prepared under the stoichiometric amount of Mg17Al12 by magnetic levitation melting. Considering the losses during melting, extra 6% of Mg in mass fraction was added. The Mg17Al12 was firstly remelted thrice to ensure homogeneity. Then, the sample was ground to powder size less than 75 μm and loaded into an autoclave for hydriding for 12 h under 4 MPa H2 (99.99% purity) at 350 °C. Hereinafter, MAH is referred to the Mg17Al12-hydride (17MgH2+12Al). Mixture of LiBH4 and MAH was ball-milled on a planetary mill at a speed of 400 r/min under argon atmosphere for 4 h. Mass ratio of ball to powder was 30:1. The mole ratio of LiBH4 to MAH was 58:1 according to the reaction (2), assuming that MAH reacts with LiBH4 to generate MgB2 and AlB2 completely. The prepared LiBH4/MAH mixture in this work has a maximum hydrogen capacity of 10.3%. For comparison, LiBH4 was ball-milled and investigated under the identical conditions.
58LiBH4+17MgH2+12Al→17MgB2+12AlB2+58LiH+H2 (2)
TPD and rehydrogenation were measured in a Sieverts-type apparatus. About 200 mg sample was used each time. The TPD measurements were carried out under initial pressure of 1 Pa, and temperature was ramped at a heating rate of 5 °C/min from ambient to a given temperatures, such as 400 °C. The rehydrogenation measurements were performed isothermally under 8 MPa H2 at 450 °C.
X-ray diffraction (XRD) measurements were performed using an X’Pert-PRO diffractometer with Cu Kα radiation at 40 kV. Samples were sealed in a unique carrier to keep from oxygen and water vapor. Samples were also analyzed by the DSC-MS analyses on a Netzsch STA449F3 equipped with a Netzsch Q430 mass spectrometer, programming the heating system from room temperature to 500 °C and employing argon as gas carrier with a purge rate of 50 mL/min. The Fourier transform infrared spectrometry (FT-IR) analyses were using a Bruker Tensor 27. The mass ratio of sample-to-KBr was 1:100 and the wave numbers range was 4000-400 cm-1. All samples were prepared and handled under a continuously purified argon atmosphere in an MBraun glovebox maintained with O2 and H2O vapor levels below 1×10-6.
3 Results and discussion
The X-ray diffraction measurements were performed to examine the phase evolution of the as-prepared LiBH4/MAH composite. Figure 1 displays the XRD patterns of as-cast Mg17Al12, MAH and ball-milled LiBH4/MAH samples. The result shows that the various diffraction peaks of as-cast Mg17Al12 are sharp. After hydrogenation under 4 MPa H2, Mg17Al12 phase has completely transformed into MgH2 and Al phases. After ball-milling, the diffraction peaks of MgH2, Al and LiBH4 are still visible, but the corresponding peaks are broadened, indicating that the grain size is decreased during ball-milling.

Fig. 1 XRD patterns of as-cast Mg17Al12 (a), MAH (b) and ball-milled LiBH4/MAH composite (c)
The thermal properties of ball-milled LiBH4/MAH composite were investigated by DSC and MS to analyze the effect of MAH on structural transition temperature, melting temperature and dehydrogenation temperature of LiBH4. For comparison, the ball-milled LiBH4 was also measured under the identical conditions. The DSC and MS curves of ball-milled LiBH4 and ball-milled LiBH4/MAH composite are shown in Fig. 2. For the ball-milled LiBH4, the first endothermic peak around 113 °C is due to the phase transformation from orthorhombic to hexagonal system, the second one at 285 °C is ascribed to the melting of LiBH4 and the third endothermic peak around 470 °C indicates the dehydrogenation reaction of LiBH4 [25]. After adding MAH, the temperatures of phase transformation and melting point are slightly lowered to 110 °C and 282 °C. For the LiBH4/MAH composite, the third DSC endothermic peak (329 °C) is due to the decomposition of MgH2. As the temperature is further escalated, LiBH4 starts to decompose at temperature higher than 400 °C. Figure 2 shows that both the starting temperature and peak temperature of dehydrogenation of the LiBH4/MAH composite are decreased. The possible decomposition process will be discussed later. Moreover, it can be found that no other volatile boron-containing gases (like B2H6, m/e=27) were detected throughout the dehydrogenation process. In brief, the major dehydrogenation peak of LiBH4 shifts to lower temperature by 20 °C after adding MAH in the LiBH4/MAH system. This result suggests that MAH could lower the decomposition temperature of LiBH4.

Fig. 2 DSC (a) and MS (b, c) profiles of ball-milled LiBH4 and LiBH4/MAH composite
TPD curves of the ball-milled LiBH4 and LiBH4/MAH composite are shown in Fig. 3. The TPD measurements were carried out under an initial hydrogen pressure of 10-6 MPa with different temperatures. For the ball-milled LiBH4, its dehydrogenation temperature is above 400 °C and it desorbs about 9.0% of H2 after 2.5 h with slow kinetics. For the ball-milled LiBH4/MAH composite, when the composite is heated from room temperature to 400 °C and kept at this temperature, it begins to release hydrogen at 320 °C and slowly desorbs about 2% of H2 in 10 h. When the composite is heated to 450 °C, the dehydrogenation performance is improved and it yields about 4% of hydrogen. As the composite heated to 500 °C, the release of hydrogen becomes faster and the composite desorbs about 7% of H2 before performing holding at 500 °C. After keeping the reactor at 500 °C for 30 min, the composite gives off more than 9.8% of H2, which is close to the theoretical value (10.3%) of the LiBH4/MAH composite. As shown in Fig. 3(c), there are two evident dehydrogenation steps at 310-360 °C and 400-500 °C for the ball-milled LiBH4/MAH composite, which agrees well with the DSC-MS results. Moreover, no incubation period is detected between these two dehydrogenation steps. The first one is very fast and related to the decomposition of MgH2, and the second one is the decomposition of LiBH4 and relatively slow.
After complete dehydrogenation, the isothermal rehydrogenation was executed at 450 °C under 8 MPa H2. The LiBH4/MAH composite exhibits a much better reversibility as shown in Fig. 4. For the ball-milled LiBH4/MAH composite, approximately 8.3% and 7.4% of hydrogen are achieved in 5 h for the second and the third rehydrogenation, respectively, while the ball-milled LiBH4 shows very poor reversibility (below 1%) under the same condition. In addition, the FT-IR spectra of different states shown in Fig. 5 confirm the rehydrogenation of LiBH4 under the established condition of 450 °C and 8 MPa H2 for the LiBH4/MAH system.

Fig. 3 Temperature programmed desorption curves of ball-milled LiBH4/MAH composite at 400 °C (a), 450 °C (b), 500 °C (c), and ball-milled LiBH4 at 500 °C (d) for comparison (initial hydrogen pressures 1 Pa; final pressures 0.01-0.015 MPa)
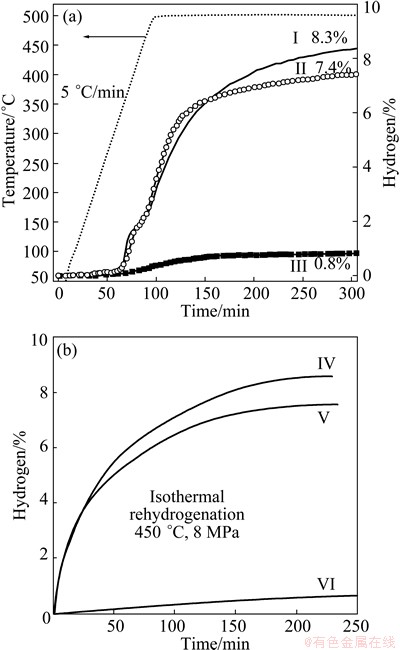
Fig. 4 TPD curves (a) of rehydrogenated LiBH4/MAH sample in the second cycle (I), third cycle (II), and rehydrogenated LiBH4 sample in the second cycle (III), isothermal rehydrogenation curves (b) of dehydrogenated LiBH4/MAH sample in the first cycle (IV), second cycle (V), and rehydrogenated LiBH4 sample in the first cycle (VI) (The samples were hydrogenated at 450 °C under 8 MPa H2 pressure)

Fig. 5 FT-IR spectra of LiBH4/MAH sample in different states
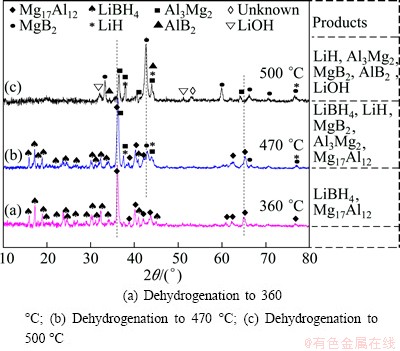
Fig. 6 XRD patterns of ball-milled LiBH4/MAH sample in different dehydrogenation stages
Figure 6 displays the XRD patterns of LiBH4/MAH composite dehydrogenated at different temperatures. Before dehydrogenation, the LiBH4, MgH2 and Al are all well present with strong diffraction peaks shown in Fig. 1(c). After dehydrogenation at 360 °C, the intensity of LiBH4 phase becomes weakened, while MgH2 phase and Al phase are disappeared completely. Meanwhile, Mg17Al12 phase is markedly present in the products at 360 °C. This result indicates that the MgH2 decomposes and reacts with Al to form Mg17Al12. The reaction pathway of the MgH2 and Al could be described as follows:
17MgH2+12Al→Mg17Al12+17H2(g) (3)
When the temperature is further increased from 360 °C to 470 °C, the LiBH4 phase becomes evidently weakened. At the same time, some diffraction peaks of Mg17Al12 disappear and the first main peak of Mg17Al12 phase is overlapped by the ones of Al3Mg2 phase. The diffraction peaks of MgB2 and LiH become visible in the products. This result suggests that Mg17Al12 can react with LiBH4 to form new phases of MgB2 and Al3Mg2. Figure 6(c) indicates that MgB2, AlB2, LiH and Al3Mg2 are the main phases after dehydrogenation at 500 °C. The Mg17Al12 and LiBH4 completely disappear. The trace amount of LiOH is due to oxidation during transport in the XRD test procedure.
It is noteworthy that the thermodynamics stability of LiBH4/MAH system could be reduced for the formation of MgB2 and AlB2. Meanwhile,LiBH4/MAH system could achieve good reversibility because of MgB2 and AlB2 [26,27], which was confirmed by Figs. 4 and 5. It was reported that hydrogen pressure during dehydrogenation is important for the formation of MgB2 [28]. However, the experiments were carried out under initial vacuum and final pressures were still low ranging from 0.01 MPa to 0.015 MPa. This means that the pressure has little effect on the formation of MgB2 and AlB2 in LiBH4/MAH system. Hence, the study of the formation mechanism of MgB2 and AlB2 is very significant to in-depth understand the reversible reaction process in the LiBH4/MAH system. The theoretical melting point of Mg17Al12 is 460 °C according to the Mg-Al binary diagram. It was also reported that the Mg17Al12 compound presents a melting peak at 460 °C [29]. Therefore, it is considered that Mg17Al12 is melting at approximately 460 °C in our study. The melting Mg17Al12 provides favorable condition for the generation of MgB2 and AlB2 as the following reactions:
Mg17Al12(l)+45LiBH4(l)→1.3Al3Mg2(s)+14.4MgB2(s)+8.1AlB2(s)+45LiH(s)+67.5H2(g) (4)
13LiBH4(l)→13LiH(s)+13B(s)+19.5H2(g) (5)
4 Conclusions
The dehydrogenation and rehydrogenation behaviors of LiBH4/MAH system were investigated.
1) It is found that 9.8% of hydrogen is released below 500 °C in this system. More than 8.3% of hydrogen can be reabsorbed for the dehydrogenated sample at 450 °C.
2) The LiBH4/MAH composite goes through two dehydrogenation steps, corresponding to dehydrogenation of MgH2 and decomposition of LiBH4, respectively.
3) The dehydrogenation temperature of LiBH4 can be lowered by 20 °C after adding MAH. The thermodynamics stability of LiBH4/MAH system could be reduced due to the formation of MgB2 and AlB2.
References
[1] SCHLAPBACH L,  A. Hydrogen-storage materials for mobile applications [J]. Nature, 2001, 414: 353-358.
A. Hydrogen-storage materials for mobile applications [J]. Nature, 2001, 414: 353-358.
[2]  B, FELDERHOFF M, POMMERIN A, SCHUETH F, SPIELKAMP N. Advanced hydrogen-storage materials based on Sc-, Ce-, and Pr-doped NaAlH4 [J]. Advanced Materials, 2006, 18: 1198-1201.
B, FELDERHOFF M, POMMERIN A, SCHUETH F, SPIELKAMP N. Advanced hydrogen-storage materials based on Sc-, Ce-, and Pr-doped NaAlH4 [J]. Advanced Materials, 2006, 18: 1198-1201.
[3]  A, RENTSCH S, FISCHER P, WENGER P, SUDAN P, MAURON P, EMMENEGGER C. Hydrogen storage properties of LiBH4 [J]. Journal of Alloys and Compounds, 2003, 356: 515-520.
A, RENTSCH S, FISCHER P, WENGER P, SUDAN P, MAURON P, EMMENEGGER C. Hydrogen storage properties of LiBH4 [J]. Journal of Alloys and Compounds, 2003, 356: 515-520.
[4] AU M, WALTERS R T. Reversibility aspect of lithium borohydrides [J]. International Journal of Hydrogen Energy, 2010, 35: 10311-10316.
[5] CHI H Z, CHEN C P, CHEN L X, WANG Q D. Hydriding properties of La2Mg16Ni alloy prepared by mechanical milling in benzene [J]. Journal of Alloys and Compounds, 2003, 360: 312-315.
[6] GAO L H, CHEN C P, CHEN L X, WANG X H, ZHANG J W, XIAO X Z, WANG Q D. Hydriding/dehydriding behaviors of La1.8Ca0.2Mg14Ni3 alloy modified by mechanical ball-milling under argon [J]. Journal of Alloys and Compounds, 2005, 399: 178-182.
[7] CHI H Z, CHEN C P, CHEN L X, AN Y, WANG Q D. Hydriding/dehydriding properties of La2Mg16Ni alloy prepared by mechanical ball milling in benzene and under argon [J]. International Journal of Hydrogen Energy, 2004, 29: 737-741.
[8] ORIMO S, NAKAMORI Y, KITAHARA G, MIWA K, OHBA N, TOWATA S,  A. Dehydriding and rehydriding reactions of LiBH4 [J]. Journal of Alloys and Compounds, 2005, 404-406: 427-430.
A. Dehydriding and rehydriding reactions of LiBH4 [J]. Journal of Alloys and Compounds, 2005, 404-406: 427-430.
[9] FAN X L, XIAO X Z, CHEN L X, YU K R, WU Z, LI S Q, WANG Q D. Active species of CeAl4 in the CeCl3-doped sodium aluminium hydride and its enhancement on reversible hydrogen storage performance [J]. Chemical Communications, 2009: 6857-6859.
[10] XIAO X Z, CHEN L X, WANG X H, LI S Q, CHEN C P, WANG Q D. Reversible hydrogen storage properties and favorable co-doping mechanism of the metallic Ti and Zr co-doped sodium aluminum hydride [J]. International Journal of Hydrogen Energy, 2008, 33: 64-73.
[11] ZHANG Guo-ying, LIU Gui-li, ZHANG Hui. First-principles study of intrinsic defects, dopants and dopant-defect complexes in LiBH4 [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(7): 1717-1722.
[12] XIAO X Z, LIU G C, PENG S K, YU K R, LI S Q, CHEN C P, CHEN L X. Microstructure and hydrogen storage characteristics of nanocrystalline Mg+xwt% LaMg2Ni (x=0-30) composites [J]. International Journal of Hydrogen Energy, 2010, 35: 2786-2790.
[13] WU Xiao-cheng, WANG Xin-hua, LI Shou-quan, GE Hong-wei, CHEN Li-xin, YAN Mi, CHEN Chang-pin. Dehydrogenation properties and mechanism of LiBH4/Li3AlH6 composite [J]. Rare Metal Materials and Engineering, 2012, 41(8): 1405-1408.
[14] GUO Y H, YU X B, GAO L, XIA G L, GUO Z P, LIU H K. Significantly improved dehydrogenation of LiBH4 destabilized by TiF3 [J]. Energy & Environmental Science, 2010, 3: 464-469.
[15] DENG Shuai-shuai, XIAO Xue-zhang, CHEN Li-xin, HAN Le-yuan, LI Shou-quan, GE Hong-wei, WANG Qi-dong. Effects of stoichiometry and dehydrogenation back-pressure on the dehydrogenation behavior of LiBH4+xMg2NiH4 composites [J]. Chemical Journal of Chinese Universities, 2012, 33(9): 2030-2034. (in Chinese)
[16] VAJO J J, SKEITH S L, MERTENS F. Reversible storage of hydrogen in destabilized LiBH4 [J]. Journal of Physical Chemistry B, 2005, 109: 3719-3722.
[17] BARKHORDARIAN G, KLASSEN T, DORNHEIM M, BORMANN R. Unexpected kinetic effect of MgB2 in reactive hydride composites containing complex borohydrides [J]. Journal of Alloys and Compounds, 2007, 440: L18-L21.
[18] KANG X D, WANG P, MA L P, CHENG H M. Reversible hydrogen storage in LiBH4 destabilized by milling with Al [J]. Applied Physics A: Materials Science & Processing, 2007, 89: 963-966.
[19] JIN S A, LEE Y S, SHIM J H, CHO Y W. Reversible hydrogen storage in LiBH4-MH2 (M=Ce, Ca) composites [J]. Journal of Physical Chemistry C, 2008, 112: 9520-9524.
[20] JIANG K, XIAO X Z, DENG S S, ZHANG M, LI S Q, GE H W, CHEN L X. A novel Li-Ca-B-H complex borohydride: Its synthesis and hydrogen storage properties [J]. Journal of Physical Chemistry C, 2011, 115: 19986-19993.
[21] ZHANG Y, TIAN Q F, CHU H L, ZHANG J, SUN L X, SUN J C, WEN Z S. Hydrogen de/resorption properties of the LiBH4- MgH2-Al system [J]. Journal of Physical Chemistry C, 2009, 113: 21964-21969.
[22] LIU D M, LIU Q Q, SI T Z, ZHANG Q A, FANG F, SUN D L, OUYANG L Z, ZHU M. Superior hydrogen storage properties of LiBH4 catalyzed by Mg(AlH4)2 [J]. Chemical Communications, 2011, 47: 5741-5743.
[23] FAN X L, XIAO X Z, CHEN L X, HAN L Y, LI S Q, GE H W, WANG Q D. Thermodynamics, kinetics and modeling investigation on the dehydrogenation of CeAl4-doped NaAlH4 hydrogen storage material [J]. Journal of Physical Chemistry C, 2011, 115(45): 22680-22687.
[24] CHEN Li-xin, FAN Xiu-lin, XIAO Xue-zhang, XUE Jing-wen, LI Shou-quan, GE Hong-wei, CHEN Chang-pin. Influence of TiC catalyst on absorption/desorption behaviors and microstructures of sodium aluminum hydride [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(6): 1297-1302.
[25] GOMES S, HAGEMANN H, YVON K. Lithium borohydride LiBH4: II. Raman spectroscopy [J]. Journal of Alloys and Compounds, 2002, 346: 206-210.
[26] DU A J, SMITH SEAN C, YAO X D, SUN C H, LI L, LU G Q. First principle study of hydrogenation of MgB2: An important step toward reversible hydrogen storage in the coupled LiBH4/MgH2 system [J]. Journal of Nanoscience and Nanotechnology, 2009, 9(7): 4388-4391.
[27] JIN S A, SHIM J H, CHO Y W, YI K W, ZABARA O, FICHTNER M. Reversible hydrogen storage in LiBH4-Al-LiH composite powder [J]. Scripta Materialia, 2008, 58: 963-965.
[28] KOU Hua-qin, XIAO Xue-zhang, CHEN Li-xin, LI Shou-quan, WANG Qi-dong. Formation mechanism of MgB2 in 2LiBH4+MgH2 system for reversible hydrogen storage [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 1040-1046.
[29] CRIVELLO J C, NOBUKI T, KATO S, ABE M, KUJI T. Hydrogen absorption properties of the γ-Mg17Al12 phase and its Al-richer domain [J]. Journal of Alloys and Compounds, 2007, 446-447: 157-161.
韩乐园,肖学章,范修林,李 芸,李寿权,葛红卫,王启东,陈立新
浙江大学 材料科学与工程学系,浙江省电池新材料与应用技术研究重点实验室,杭州 310027
摘 要:选择Mg17Al12-氢化物作为失稳剂与LiBH4进行球磨以改善LiBH4体系的吸放氢性能。研究表明,LiBH4/Mg17Al12-氢化物复合体系发生两步放氢过程。复合体系在300 °C开始放氢并在500 °C下产生9.8%的放氢量。通过添加Mg17Al12-氢化物,LiBH4的放氢动力学得到有效改善,并且其放氢温度降低20 °C。复合体系的放氢产物在450 °C的首次再加氢容量可高达8.3%。XRD分析表明,复合体系在放氢过程中所形成的MgB2和AlB2可降低LiBH4的热力学稳定性,进而有效改善LiBH4/Mg17Al12-氢化物复合体系的可逆储氢行为。
关键词:配位氢化物;LiBH4;Mg17Al12;吸放氢行为;可逆性
(Edited by Hua YANG)
Foundation item: Project (2010CB631304) supported by the National Basic Research Program of China; Projects (51001090, 51171173) supported by the National Natural Science Foundation of China; Project (20090101110050) supported by the University Doctoral Foundation of the Ministry of Education, China
Corresponding author: Xue-zhang XIAO; Tel: +86-571-87951876; Fax: +86-571-87951152; E-mail: xzxiao@zju.edu.cn
DOI: 10.1016/S1003-6326(14)63041-7

