DOI: 10.11817/j.ysxb.1004.0609.2020-35783
湿法炼锌危废铁矾渣水热分解及铁物相转化行为
楚 铭,李存兄,张 鹏,吉文斌,魏 昶,邓志敢,李兴彬,樊 刚,李旻廷
(昆明理工大学 冶金与能源工程学院,昆明 650093)
摘 要:湿法炼锌过程产出的铁矾渣含有大量的有价金属锌、铅以及伴生金属铁,在水热条件下,危废铁矾渣将发生高效分解与转化,有价金属转入溶液,伴生铁转化为赤铁矿。本文以湿法炼锌企业产出的铁矾渣为研究对象,研究了反应温度、反应时间、液固比、初始酸度、晶种浓度等宏观技术参数对铁矾渣分解与转化的影响规律。理论计算和实验结果均表明在高温水热体系中,铁矾渣中的黄钾铁矾、黄铵铁矾和铁酸锌物相均可有效转化为赤铁矿,而铅铁矾性质稳定不易转化。升高温度并延长反应时间有利于黄钾铁矾、黄铵铁矾和铁酸锌物相的水热分解与转化。在220 ℃下反应1 h后,铁矾物相转化基本完成,其转化率达94%;反应4 h后铁酸锌物相衍射峰完全消失,锌浸出率达87%,转化渣中赤铁矿含量达68%。适当提高初始酸度有利于铁酸锌的转化,但当体系初始酸度高于15 g/L时将抑制铁矾物相转化。在反应温度220 ℃、反应时间4 h、液固比(mL/g) 10:1、初始酸度0.01 g/L的条件下,锌浸出率为89%,铁矾物相的转化率可达95%,铁矾转化渣中主要物相为赤铁矿,其含量为68%。
关键词:铁矾渣;水热分解;赤铁矿;转化行为
文章编号:1004-0609(2020)-05-1119-12 中图分类号:TF813 文献标志码:A
我国80%以上的锌采用湿法炼锌工艺生产,锌铁分离是湿法炼锌中重要的环节。目前,从含铁硫酸锌浸出液中分离锌铁并进行工业化应用的方法有黄钾铁矾法、针铁矿法及赤铁矿法[1-3]。其中,黄钾铁矾法因操作成本低、有价金属回收率高、利于体系酸平衡等优点占据了主导地位[4-5]。在黄钾铁矾法沉铁工艺过程中,需加入锌焙砂作中和剂,焙砂中大量锌、铜、铟等有价金属及镉、铅、砷等有毒元素与铁共沉淀一起进入铁矾渣中,导致其物相成分极其复杂,在自然堆存条件下重金属及有毒元素不断溶出污染地下水和土壤,引起二次污染,现今铁矾渣已被列入危险固体废弃物行列。据统计,在湿法炼锌过程中每生产1 t电锌将产生0.3~0.5 t铁矾渣,目前国内每年将产生超过100万t的危废铁矾渣[6-11]。铁矾渣的无害化及资源化处理关乎湿法炼锌行业的生存与发展。
近年来,国内外科研工作者采用火法、火法湿法联合或湿法等工艺进行了铁矾渣的无害化固定处理和回收铁及有价金属的研究工作。韩国釜山冶炼厂采用的两段Ausemelt炉回收处理工艺是唯一实现工业化的处理工艺[12],有学者[13-14]利用火法工艺对铁矾渣中铁及有价金属铅、锌等进行资源化利用;采用火法工艺处理铁矾渣具有工艺简单、可操作性强的优点,但过程能耗高、工作环境差且易造成二氧化硫的低空污染。巨少华等[15]、张魁芳等[16]分别采用低温焙烧-NH4Cl浸出-碱浸法和焙烧-水浸法对铁矾渣中铁及锌、铅、铜、铟等有价金属进行回收利用,分别得到含铁53.84%的碱浸渣和银、铅富集的浸出渣;利用火法湿法联合在处理铁矾渣时具有有价金属回收率高、有价金属回收种类全等优点,但存在工艺复杂、成本高等不足。因此,有人提出采用湿法工艺提取铁矾渣中的有价金属,采用苛性碱浸出或硫酸浸出时,可将铁矾渣中的铁及铟、锌等有价金属分别富集于浸出渣和浸出液中[17-19]。湿法处理工艺相对能耗低、金属浸出率高但易产生废液、废渣,造成二次污染。谭宏斌等[20]、ASOKAN等[21]将铁矾渣进行固化处理,固化物符合国家标准要求。固化法处理危废铁矾渣成本低,适用性强,但对于渣中有价金属的回收不够完善,造成了渣中有价金属的浪费。
李存兄等[22-25]的研究表明,在水热赤铁矿沉铁过程中亚稳态铁矾物相可以进一步向赤铁矿转化,通过升高温度、添加晶种、延长反应时间等手段,可促进大部分亚稳态铁矾转化为赤铁矿。本文利用该研究思路,以湿法炼锌过程产出的铁矾渣为研究对象,在水热条件下使其发生分解与转化,有价金属转入溶液,伴生铁转化为具有潜在利用价值的赤铁矿。通过研究反应温度、反应时间、液固比、初始酸度、晶种浓度等宏观技术参数对铁矾渣中铁矾及铁酸锌物相分解与转化的影响规律,获得优化技术参数,为下一步铁矾渣中有价金属的高效提取提供参数。
1 实验原理
湿法炼锌工业产生的危废铁矾渣物相组成极其复杂,通常铁矾渣中的铁矾物相是黄铵铁矾(NH4Fe3(SO4)2(OH)6)、黄钠铁矾(NaFe3(SO4)2(OH)6)、黄钾铁矾(KFe3(SO4)2(OH)6)、铅铁矾(PbFe6(SO4)4-(OH)12)中的一种或几种的混合物。铁矾物相在水热条件下按反应式(1)发生反应,随着铁矾物相转化为赤铁矿,转化渣中硫残留率将减小,同时赋存于铁矾渣中的锌、铜等有价金属也随之溶解进入溶液。
2AFe3(SO4)2(OH)6=A2SO4+3Fe2O3+3H2SO4+3H2O
(A=Na, K, NH4, 0.5Pb) (1)
铁矾渣中铁矾物相种类及组成不同其反应活性也不同,利用Material Studio软件对黄铵铁矾、黄钠铁矾、黄钾铁矾、铅铁矾进行几何优化,获得稳定结构并得出以上4种铁矾的结构总能量,结果如图1所示,4种铁矾依次收敛于:黄铵铁矾(-9833 eV)、黄钠铁矾(-10241 eV)、黄钾铁矾(-10651 eV)、铅铁矾(-29574 eV)。由此可知,在铁矾渣水热分解与转化过程中其稳定性由大到小依次为铅铁矾、黄钾铁矾、黄钠铁矾和黄铵铁矾,表明黄铵铁矾最易分解,铅铁矾最难分解。

图1 铁矾物相最终能量收敛图
Fig.1 Final energy convergence of jarosite
湿法炼锌黄钾铁矾法除铁过程中,需加入大量锌焙砂作为中和剂,导致锌焙砂中大量的锌以铁酸锌形式进入铁矾渣,在水热条件下,随着铁矾的转化,反应体系酸度升高,铁酸锌发生分解。利用HSC数据库绘制出220℃下Fe-Zn-S-H2O系电位-pH图,如图2所示。由图2可知,在高温水热条件下铁酸锌按反应式(2)发生分解,在高温水热条件下,硫酸盐体系中的Zn2+与 结合形成ZnSO4·H2O结晶,随着体系温度降低ZnSO4·H2O结晶将溶解于转化液中,最终有价金属锌浸出富集于转化液。
结合形成ZnSO4·H2O结晶,随着体系温度降低ZnSO4·H2O结晶将溶解于转化液中,最终有价金属锌浸出富集于转化液。
ZnFe2O4(s)+ (aq)+H+(aq)=ZnSO4·H2O(s)+Fe2O3(s) (2)
(aq)+H+(aq)=ZnSO4·H2O(s)+Fe2O3(s) (2)

图2 220 ℃ Fe-Zn-S-H2O系电位-pH图
Fig. 2 Potential-pH patterns for Fe-Zn-S-H2O system at 220 ℃ (a=1)
在铁矾渣水热分解及转化过程中,体系酸度逐步增大,生成的赤铁矿将部分反溶,按反应(3)溶解于转化液中[26]。
Fe2O3(s)+6H+(aq)=2Fe3+(aq)+3H2O(l) (3)
2 实验
2.1 原料
本实验所用原料为国内某湿法炼锌企业产出的铁矾渣,铁矾渣经水洗后过滤,置于70 ℃烘箱烘干至恒量,研磨过100目筛(孔径150 μm),装袋以备分析。其化学成分、XRD检测结果如表1、图3所示。采用分析纯Fe2O3作为实验晶种,D50为0.691 μm,其粒度分析如图4所示。
由表1可知,铁矾渣中除含有大量的铁(29.37%)外还含有锌(6.37%)、铅(1.36%)等有价金属。结合图3可知,铁矾渣中物相主要为黄铵铁矾、黄钾铁矾、铁酸锌和二水硫酸钙。黄钾铁矾法除铁过程中,锌焙砂、氨水等中和剂的使用导致铁矾物相的复杂多变并含有一定量的铁酸锌。
2.2 实验方法
实验按图5所示流程进行,取一定量铁矾渣,按预设晶种浓度和液固比加入一定量晶种(Fe2O3)和水后调浆至1 L并加入至压力釜内。固定搅拌转速为500 r/min,预设实验温度,达到预设温度后开始计时。到达预设反应时间后,快速冷却并采用抽虑设备对浸出矿浆进行液固分离,得到转化液和转化渣,量取转化液体积,称量转化渣湿重并置于70 ℃真空干燥烘箱中,烘干至恒量称重并取样送检。
表1 铁矾渣的主要化学成分
Table 1 Major chemical composition of jarosite residue

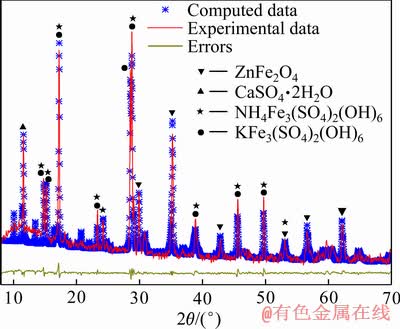
图3 铁矾渣的XRD谱
Fig. 3 XRD patterns of jarosite residue
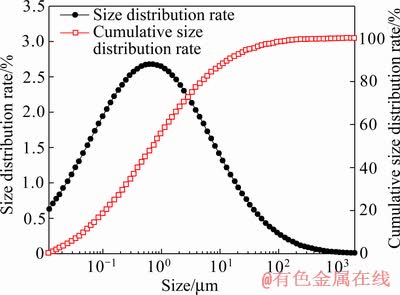
图4 Fe2O3晶种的粒径分布图
Fig. 4 Particle size distribution of hematite seed crystal
2.3 分析检测与计算
本实验研究,转化渣中铁、锌、硫等元素检测委托昆明冶金研究院分析测定,利用渣中硫残留率、锌浸出率进行表征,并利用XPS、红外光谱、XRD的精修结果即铁矾转化率和转化渣中Fe2O3的含量进行验证,铁矾转化率利用式(4)进行计算[27]。
 (4)
(4)
式中: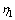 为铁矾转化率,%;m1为原料的质量,g;m2为转化渣的质量,g;w1为原料中铁矾物相的Fe含量,%;w2为XRD精修所得转化渣中铁矾物相的Fe含量,%。
为铁矾转化率,%;m1为原料的质量,g;m2为转化渣的质量,g;w1为原料中铁矾物相的Fe含量,%;w2为XRD精修所得转化渣中铁矾物相的Fe含量,%。

图5 流程示意图
Fig. 5 Flow diagram
3 结果与讨论
3.1 反应温度对铁矾渣水热矿物相转化的影响
在反应时间4 h、液固比(mL/g)10:1、初始酸度0.01 g/L的条件下,考察了反应温度对铁矾渣水热转化的影响。转化渣的XRD谱如图6和7所示,转化渣中硫残留率、铁矾转化率、锌浸出率、转化渣中Fe2O3含量和冷却前后转化液中Fe3+浓度如图8所示。

图6 不同反应温度的转化渣XRD谱
Fig. 6 XRD patterns of precipitates for various temperatures
由图6和7可知,当反应温度由160 ℃升高至180 ℃时,在47°~53°衍射角内出现赤铁矿衍射峰;随着温度升高至200 ℃时,黄钾铁矾和黄铵铁矾物相衍射峰消失,赤铁矿衍射峰明显增强;在220 ℃时,铁矾的转化率为95%,硫残留率由80%降低为17%,其转化反应基本完成,残留硫主要为硫酸钙和硫酸铅中的硫。此时转化渣的主要物相组成为赤铁矿、硫酸钙和硫酸铅,并含有微量铅铁矾。这是因为在铁矾渣水热转化分解过程中,铅铁矾性质稳定较难转化,这与铁矾物相最终能量收敛图(见图1)分析结果相符。

图7 160、170和200 ℃转化渣的XRD谱放大图
Fig. 7 XRD enlarge pattern of precipitates at 160, 180 and 200 ℃
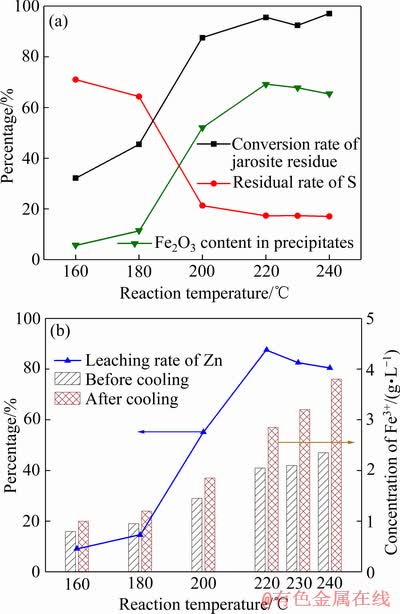
图8 反应温度对铁矾渣转化的影响
Fig. 8 Effect of reaction temperature on transformation of jarosite residue
在反应温度由160 ℃升高至220 ℃的过程中,随着铁矾物相的转化,反应体系酸度升高,铁酸锌不断分解,其衍射峰逐步降低,锌浸出率也由9%提高至87%。
升高温度有利于铁矾和铁酸锌物相向赤铁矿转变,使得转化渣中三氧化二铁含量由160 ℃的6%升高至220 ℃的69%。但由图8(b)可知,反应结束后随着反应温度的降低,部分赤铁矿返溶,在反应温度由160 ℃升高至240 ℃后,冷却前后转化液中Fe3+浓度差由0.2 g/L升高至1.45 g/L,返溶现象加剧,通过前期降温实验结果表明,通过提高降温速率可有效阻止转化渣中新生赤铁矿物相的返溶。
3.2 反应时间对铁矾渣水热矿物相转化的影响
在反应温度220 ℃、液固比10:1、初始酸度0.01 g/L的条件下,考察反应时间对铁矾渣转化的影响。转化渣的XRD谱如图9和10所示,转化渣中硫残留率、铁矾转化率、锌浸出率和转化渣中Fe2O3含量如图11所示。
由图9、10和11可知,在220 ℃下反应1 h后,黄钾铁矾和黄铵铁矾物相衍射峰基本消失,铁矾物相转化率达94%;继续延长反应时间至5 h,铁矾转化率约为95%。由此可见,反应1 h后铁矾转化基本完成,继续延长时间对铁矾转化率和转化渣中硫含量影响不大。铁酸锌的衍射峰随反应时间的延长而逐步减弱,反应4 h后铁酸锌物相衍射峰完全消失,锌浸出率由68%升高至87%,转化渣中赤铁矿含量由56%升高至69%,表明转化渣中赤铁矿由铁矾物相转化和铁酸锌物相分解而来。

图9 不同反应时间的转化渣XRD谱
Fig. 9 XRD patterns of precipitates for various time

图10 不同反应时间转化渣的XRD谱放大图
Fig. 10 XRD enlarge patterns of precipitates for various time

图11 反应时间对铁矾渣转化的影响
Fig. 11 Effect of reaction time on transformation of jarosite residue
3.3 液固比对铁矾渣水热矿物相转化的影响
在反应温度220 ℃、反应时间4 h、初始酸度0.01 g/L的条件下,考察液固比对铁矾渣转化的影响,转化渣的XRD谱如图12所示,转化渣中硫残留率、铁矾转化率、锌浸出率和转化渣中Fe2O3含量如图13所示。

图12 不同液固比下转化渣的XRD谱
Fig. 12 XRD patterns of precipitates for various liquid-solid ratios

图13 液固比对铁矾渣转化的影响
Fig. 13 Effect of liquid-solid ratio on transformation of jarosite residue
由图12和13可知,当液固比由5:1升高至30:1时,XRD谱显示黄钾铁矾和黄铵铁矾物相衍射峰逐渐减弱直至消失,铁矾转化率由90%升高至96%;但转化渣中赤铁矿含量及锌浸出率由体系液固比5:1升高至20:1后先逐渐增大,之后进一步增大液固比后降低,这是由于体系酸度是影响铁酸锌分解与转化的重要因素之一[28],由图12证明,随着液固比的增大铁酸锌物相的衍射峰先逐步减弱并消失,之后在液固比为25:1时又重现,导致锌浸出率的降低和转化渣中赤铁矿含量的减少。
3.4 初始酸度对铁矾水热矿物相转化的影响
在反应温度220 ℃、反应时间4 h、液固比10:1的条件下,考察了初始酸度对铁矾渣转化的影响。转化渣的XRD谱如图14所示,转化渣中硫残留率、铁矾转化率、锌浸出率和转化渣中Fe2O3含量如图15所示。
由图14和15可知,初始酸度由0.01 g/L增加至5 g/L,锌浸出率由87%升高至92%,铁矾转化率、转化渣中赤铁矿及硫残留率几乎保持不变;初始酸度达15g/L后,继续升高酸度,转化渣中残留有大量未转化的铁矾及铁酸锌物相,硫残留率升高,转化渣中赤铁矿含量急剧降低。可见,初始酸度对铁矾水热转化影响显著,由于铁矾转化为产酸反应,过高的酸度将抑制铁矾转化。

图14 不同初始酸度的转化渣XRD谱
Fig. 14 XRD patterns of precipitates for various acidities
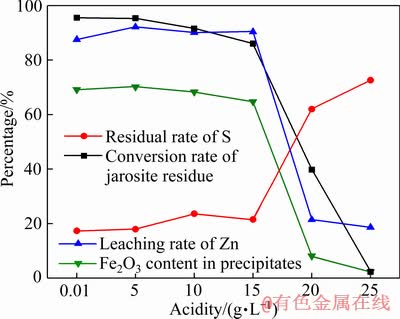
图15 初始酸度对铁矾渣转化的影响
Fig. 15 Effect of acidity on transformation of jarosite residue
3.5 晶种对铁矾渣水热矿物相转化的影响
在反应温度220 ℃、反应时间4 h、液固比10:1、初始酸度0.01 g/L的条件下,以Fe2O3作为晶种,对比0、5和10 g/L晶种浓度对铁矾渣转化的影响,并对反应0 h(温度达到220 ℃时开始计时)和加热1 h(加热至(183±2) ℃)进行取样,转化渣的宏观图如图16所示,不同时间、不同晶种浓度的转化渣XRD谱如图17所示,不同晶种浓度对铁矾渣转化的分析结果如图18和19所示。

图16 不同晶种浓度的转化渣宏观图
Fig. 16 Macro graphs of precipitates for various crystalline concentrations

图17 不同晶种浓度在反应0 h和4 h的转化渣XRD谱
Fig. 17 XRD patterns of precipitates after reactions of 0 h and 4 h for various crystalline concentrations
由图16可知,加入晶种达到反应温度时,转化渣的颜色呈桔红色,较未加入晶种时的黄色相比颜色加深,其XRD检测结果如图16所示,随着晶种浓度提升至5 g/L,转化渣中铁矾物相含量由46%降低至30%,晶种的加入加快了铁矾物相的转化。
由反应4 h的转化渣XRD和元素检测可知,0、5和10 g/L晶种浓度下的铁矾转化率为94.8%±0.7%,硫残留率和锌浸出率基本不变,转化渣中赤铁矿增加的质量与加入的晶种质量相等,且粒度D50分别为1.84、2.06和1.82 μm,可知加入晶种仅是提升反应速度,使反应加快进行,对反应进行的程度、转化渣粒度均无影响。

图18 晶种浓度对反应0 h和4 h的铁矾渣转化的影响
Fig. 18 Effect of crystalline concentration on transformation of jarosite residue after reactions of 0 h(a) and 4 h(b)
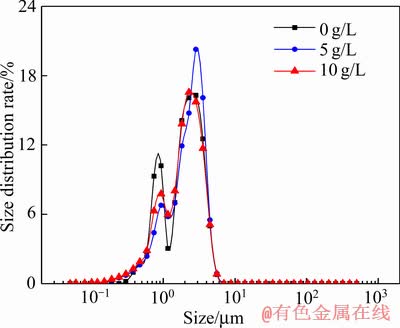
图19 不同晶种浓度下反应4 h的转化渣的粒径分布图
Fig. 19 Particle size distribution of precipitates after reaction of 4 h for various crystalline concentrations
4 综合条件实验
上述单因素条件实验结果表明:铁矾渣可在水热条件下转化为赤铁矿渣,在反应温度220 ℃、反应时间4 h、液固比10:1、初始酸度0.01 g/L且不加晶种的条件下进一步开展了综合验证研究。
对综合条件实验转化渣进行红外光谱检测,如图20所示;对比铁矾渣与转化渣在900~1300 cm-1范围内的 吸收峰,铁矾物相导致
吸收峰,铁矾物相导致 形成配位化合物使离子的对称性降低,简并震动发生分裂,在谱中出现具有拉曼活性的震动模式。而随着铁矾物相转化为赤铁矿,渣中含
形成配位化合物使离子的对称性降低,简并震动发生分裂,在谱中出现具有拉曼活性的震动模式。而随着铁矾物相转化为赤铁矿,渣中含 物相仅剩硫酸钙和硫酸铅,
物相仅剩硫酸钙和硫酸铅,  的对称性增强,只出现1095.50 cm-1处的吸收峰[29];同时由图21和22可知,铁矾渣的O 1s特征峰为双峰,铁矾渣中531.59 eV处为CaSO4·2H2O结晶水的特征峰,经水热处理转化为转化渣中530.06 eV处Fe2O3的特征峰[30];结合转化渣的XRD谱(见图23)可知,经水热处理后,渣中残留的
的对称性增强,只出现1095.50 cm-1处的吸收峰[29];同时由图21和22可知,铁矾渣的O 1s特征峰为双峰,铁矾渣中531.59 eV处为CaSO4·2H2O结晶水的特征峰,经水热处理转化为转化渣中530.06 eV处Fe2O3的特征峰[30];结合转化渣的XRD谱(见图23)可知,经水热处理后,渣中残留的 为硫酸钙、硫酸铅等物相,转化渣中主要物相为赤铁矿,且已无铁矾物相衍射峰。转化渣主要化学成分如表2所示。
为硫酸钙、硫酸铅等物相,转化渣中主要物相为赤铁矿,且已无铁矾物相衍射峰。转化渣主要化学成分如表2所示。

图20 铁矾渣和转化渣的红外光谱图
Fig. 20 FT-IR spectra of jarosite residue and precipitates

图21 铁矾渣和转化渣的全谱扫描谱
Fig. 21 Full spectrum scanning spectrogram of jarosite residue and precipitates

图22 铁矾渣和转化渣中O元素的窄区扫描图谱
Fig. 22 Resolved narrow scan spectra of element O in jarosite residue (a) and precipitates (b)
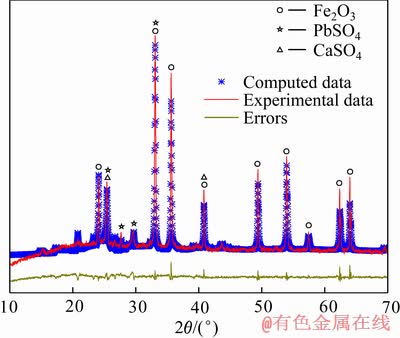
图23 综合实验转化渣的XRD谱
Fig. 23 XRD patterns of precipitates of comprehensive experiment
综合上述分析结果,经水热处理后,铁矾渣中铁矾物相转化率可达95%,有价金属锌浸出率可达89%,金属铁在渣中富集后含量可达48%。转化渣赤铁矿含量较高,后续处理回收铅、铟等有价金属后可作为炼铁原料,或作为着色剂[31]与硅酸盐水泥固化制备烧结砖[18],实现危废铁矾渣中伴生铁的资源化利用。
表2 转化渣主要化学成分
Table 2 Major chemical composition of precipitates (mass fraction, %)

5 结论
1) 理论计算和实验结果表明,铁矾渣水热转化分解过程铁矾物相的分解次序依次为黄铵铁矾、黄钠铁矾、黄钾铁矾、铅铁矾。危废铁矾渣经水热处理可转化为赤铁矿渣,转化渣中赤铁矿由铁矾物相转化和铁酸锌物相分解组成。
2) 温度是影响铁矾渣转化的关键因素,升高温度可促进铁矾物相和铁酸锌物相向赤铁矿的转化;在220℃的水热体系中,94%的铁矾物相在反应1 h完成转化,锌浸出率在反应4 h后达87%;提高液固比可促进铁矾物相向赤铁矿的转化,但过高的液固比将阻碍铁酸锌的分解;酸度大于15 g/L将阻碍铁矾物相转化,导致铁矾物相转化率的降低;添加晶种可以加速铁矾物相转化;高温水热条件下形成的赤铁矿渣粒径较小,在降温过程中赤铁矿存在返溶现象,提高降温速率是阻止转化渣中新生赤铁矿返溶的有效方法。
3) 在反应温度220 ℃、反应时间4 h、液固比10:1、初始酸度0.01 g/L且不加入晶种的条件下,铁矾物相的转化率可达95%,锌浸出率达89%,铁矾转化渣中主要物相为赤铁矿,其含量为68%。
REFERENCES
[1] KAKSONEN A H, MORRIS C, HILARIO F, REA S M, LI J, USHER K M, WYLIE J, GINIGE M P, CHENG K Y, DU PLESSIS C. Iron oxidation and jarosite precipitation in a two-stage airlift bioreactor[J]. Hydrometallurgy, 2014, 150: 227-235.
[2] 梅光贵, 王德润, 周敬元, 王 辉. 湿法炼锌学[M]. 长沙: 中南大学出版社, 2001: 197-198.
MEI Guang-gui, WANG De-run, ZHOU Jing-yuan, WANG Hui. Zinc hydrometallurgy[M]. Changsha: Central South University Press, 2001: 197-198.
[3] 杨 凡, 邓志敢, 魏 昶, 李存兄, 李兴彬. 采用赤铁矿去除高铁闪锌矿浸出液中的铁[J]. 中国有色金属学报, 2014, 24(9): 2387-2391.
YANG Fan, DENG Zhi-gan, WEI Chang, LI Cun-xiong, LI Xing-bin. Iron-removal by hematite from leaching liquor of high iron sphalerite[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2387-2391.
[4] 翟秀静. 重金属冶金学[M]. 北京: 冶金工业出版社, 2013: 197-198.
ZHAI Xiu-jing. Metallurgy of heavy metals[M]. Beijing: Metallurgical Industry Press, 2013: 197-198.
[5] CRABBE H, FERMANDEZ N, JONES F. Crystallization of jarosite in the presence of amino acids[J]. Journal of Crystal Growth, 2015, 416: 28-33.
[6] KENDALL M R, MADDEN A S, MADDEN M E E, HU Qin-hong. Effects of arsenic incorporation on jarosite dissolution rates and reaction products[J]. Geochimica et Cosmochimica Acta, 2013, 112(3): 192-207.
[7] 刘三平, 王海北, 蒋开喜, 冯亚平, 黄 胜. 含锌铁钒渣的回收利用[J]. 矿冶, 2009, 18(1): 23-28.
LIU San-ping, WANG Hai-bei, JIANG Kai-xi, FENG Ya-ping, HUANG Sheng. Utilization of jarosite containing zinc[J]. Mining & Metallurgy, 2009, 18(1): 23-28.
[8] MEHRA P, GUPTA R C, THOMAS B S. Properties of concrete containing jarosite as a partial substitute for fine aggregate[J]. Journal of Cleaner Production, 2016, 120: 241-248.
[9] DUTRIZAC J E, JAMBOR J L. Jarosites and their application in hydrometallurgy[J]. Reviews in Mineralogy and Geochemistry, 2000, 40(1): 405-452.
[10] KAKSONEN A H, MORRIS C, REA S M JIANLI, USHER K M, MCDONALD R G, HILARIO F, HOSKEN T, JACKSON M, PLESSIS C A. Biohydrometallurgical iron oxidation and precipitation: Part Ⅱ—Jarosite precipitate characterisation and acid recovery by conversion to hematite[J]. Hydrometallurgy, 2014, 147/148: 264-272.
[11] 阳征会, 龚竹青, 李宏煦, 陈文汨, 龚 胜. 用黄钠铁矾渣制备复合镍锌铁氧体[J]. 中南大学学报(自然科学版), 2006, 37(4): 685-691.
YANG Zheng-hui, GONG Zhu-qing, LI Hong-xu, CHEN Wen-mi, GONG Sheng. Preparation of Ni-Zn ferrite from sodium jarosite residue[J]. Journal of Central South University (Science and Technology), 2006, 37(4): 685-691.
[12] 薛佩毅, 巨少华, 张亦飞, 王文新. 焙烧-浸出黄钾铁矾渣中多种有价金属[J]. 过程工程学报, 2011, 11(1): 56-60.
XUE Pei-yi, JU Shao-hua, ZHANG Yi-fei, WANG Xin-wen. Recovery of valuable metals by leaching of roasted jarosite residue[J]. The Chinese Journal of Process Engineering, 2011, 11(1): 56-60.
[13] 王亚运. 基于直接还原法回收铁矾渣中铅锌铁及同步固硫基础研究[D]. 北京: 北京科技大学, 2018: 24-30.
WANG Yun-xue. Fundamental study on recovery of lead,zinc an iron and simultaneous sulfur from jarosite residues based on direct reduction[D]. Beijing: Civil and Resource Engineering University of Science and Technology Beijing, 2018: 24-30.
[14] 路殿坤, 金哲男, 谢 峰, 王 萌. 铁矾渣还原焙烧制备磁铁矿的研究[J]. 铜业工程, 2013(1): 6-11.
LU Dian-kun, JIN Zhe-nan, XIE Feng, WANG Meng. Research on magnetite preparation by reductive baking of jarosite residue[J]. Copper Engineering, 2013(1): 6-11.
[15] JU S H, ZHANG Y F, ZHANG Y, XUE P Y, WANG Y H. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy[J]. Journal of Hazardous Materials, 2011, 192(2): 554-558.
[16] 张魁芳, 刘志强, 戴子林, 高丽霞. 含铟铁矾渣焙烧水浸法回收锌和铟[J]. 中国有色金属学报, 2017, 27(5): 1045-1050.
ZHANG Kui-fang, LIU Zhi-qiang, DAI Zi-lin, GAO Li-xia. Recovery of Zn and In from ammonium jarosite residue bearing indium by roasting-water leaching method[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(5): 1045-1050.
[17] 陈永明, 唐谟堂, 杨声海, 何 静, 唐朝波, 杨建广, 鲁君乐. NaOH分解含铟铁矾渣新工艺[J]. 中国有色金属学报, 2009, 19(7): 1322-1331.
CHEN Yong-ming, TANG Mo-tang, YANG Sheng-hai, HE Jing, TANG Chao-bo, YANG Jian-guang, LU Jun-yue. Novel technique of decomposition of ammonium jarosite bearing indium in NaOH medium[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(7): 1322-1331.
[18] 刘鹏飞, 张亦飞, 游韶玮, 薄 境, 江小舵. 热酸浸出回收黄钾铁矾渣中有价元素[J]. 过程工程学报, 2016, 16(4): 584-589.
LIU Peng-fei, ZHANG Yi-fei, YOU Shao-wei, BO Jing, JIANG Xiao-duo. Recovery of valuable elements in jaroaite residue by hot acid leaching[J]. The Chinese Journal of Process Engineering, 2016, 16(4): 584-589.
[19] 何 静, 杨建平, 杨声海, 陈永明, 王夏阳. 铁矾渣热酸分解及硫脲提银[J]. 中国有色金属学报, 2017, 27(7): 1504-1512.
HE Jing, YANG Jian-ping, YANG Sheng-hai, CHEN Yong-ming, WANG Xia-yang. Decomposition of jarosite residue in sulfuric acid medium and recovery of silver with thiourea solution[J].The Chinese Journal of Nonferrous Metals, 2017, 27(7): 1504-1512.
[20] 谭宏斌, 侯小强, 郑旭涛, 郭从盛. 硅酸盐水泥与铁矾渣反应产物及固化[J]. 有色金属工程, 2015, 5(6): 74-77.
TAN Hong-bin, HOU Xiao-qiang, ZHENG Xu-tao, GUO Cong-sheng. Reaction product and solidification of portland cement and jarosite slag[J]. Nonferrous Metals Engineering, 2015, 5(6): 74-77.
[21] ASOKAN P, SAXENA M, ASOLEKAR S R. Hazardous jarosite use in developing non-hazardous product for engineering application[J]. Journal of Hazardous Materials, 2006, 137(3): 1589-1599.
[22] 李存兄, 魏 昶, 邓志敢, 李兴彬, 樊 刚, 王益昭, 易烁文, 李旻廷. FeSO4-H2O 体系中水热赤铁矿沉铁及亚稳态铁物相转变行为[J]. 中国有色金属学报, 2018, 28(3): 628-634.
LI Cun-xiong, WEI Chang, DENG Zhi-gan, LI Xing-bin, FAN Gang, WANG Yi-zhao, YI Shuo-wen, LI Min-ting. Hydrothermal hematite precipitation and conversion behavior of metastable iron phase in FeSO4-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(3): 628-634.
[23] DENG Zhi-gan, ZHU Bei-ping, ZENG Peng, WEI Chang, LI Xing-bin, LI Cun-xiong, FAN Gang. Behavior and characterization of hematite process for iron removal in hydrometallurgical production[J]. Canadian Metallurgical Quarterly, 2019, 60(2): 222-231.
[24] 王益昭, 李存兄, 魏 昶, 邓志敢, 李兴彬, 樊 刚, 易烁文. 湿法炼锌过程中赤铁矿生成及硫的吸附转化[J]. 中国有色金属学报, 2017, 27(10): 2145-2153.
WANG Yi-zhao, LI Cun-xiong, WEI Chang, DENG Zhi-gan, LI Xing-bin, FAN Gang, YI Shuo-wen. Production of hematite and conversion of adsorption S in zinc hydrometallurgy process[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(10): 2145-2153.
[25] LI Cun-xiong, DENG Zhi-gan, WEI Chang, FAN Gang, LI Xing-bin, LI Min-ting, WANG Yi-zhao. Production of low-sulfur hematite by hydrothermal oxydrolysis of ferrous sulfate[J]. Hydrometallurgy, 2018, 178: 294-300.
[26] DUTRIZAC J E, SUNYER A. Hematite formation from jarosite type compounds by hydrothermal conversion[J]. Canadian Metallurgical Quarterly, 2012, 51(1): 11-23.
[27] 黄继武, 李 周. 多晶材料X射线衍射[M]. 北京: 冶金工业出版社, 2012: 241-263.
HUANG Ji-wu, LI Zhou. Polycrystalline material X-ray diffraction[M]. Beijing: Metallurgical Industry Press, 2012: 241-263.
[28] 郑 宇, 邓志敢, 樊 刚, 魏 昶, 樊 光, 李兴彬, 李存兄, 李旻廷. 二氧化硫还原分解铁酸锌及锌浸渣工艺[J].中国有色金属学报, 2019, 29(1): 170-178.
ZHENG Yu, DENG Zhi-gan, FAN Gang, WEI Chang, FAN Guang, LI Xing-bin, LI Cun-xiong, LI Min-ting. Reductive decomposition of zinc ferrite and zinc residues by sulfur dioxide[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(1): 170-178.
[29] 中本一雄, 黄德如. 无机和配位化合物的红外和拉曼光谱[M]. 北京: 化学工业出版社, 1986: 244-248.
NAKAMOTO Kazuo, HUANG De-ru. Infrared and Raman spectra of inorganic and coordination compounds[M]. Beijing: Chemical Industry Press,1986: 244-248.
[30] MOULDER J F, STICKLE W F, SOBOL P E, BOMBEN K D. Handbook of X-ray photoelectron spectroscopy[M]. Minnesota, USA: Physical Electronics Inc, 1995: 44-45.
[31] 陈 剑, 李碧雄, 梁鑫晓. 从化学成分探讨三种固废物掺入页岩烧结砖可行性[J]. 中国建材科技, 2017(6): 69-70.
CHEN Jian, LI Bi-xiong, LIANG Xin-xiao. The feasibility of the fired brick incorporating three kinds of solid waste based on chemical components[J]. China Academic Journal Electronic Publishing House, 2017(6): 69-70.
Hydrothermal decomposition of hazardous jarosite residue produced in zinc hydrometallurgy and transformation behavior of iron containing phase
CHU Ming, LI Cun-xiong, ZHANG Peng, JI Wen-bin, WEI Chang, DENG Zhi-gan, LI Xing-bin, FAN Gang, LI Min-ting
(Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: The jarosite produced in the process of zinc hydrometallurgy contains a large amount of valuable metal Zn, Pb and associated metal Fe. Under hydrothermal conditions, the jarosite will decompose and transform efficiently, the valuable metal will be transferred into solution, and the associated Fe will be transformed into hematite. In this paper, the jarosite produced by the zinc-iron separation process in the wet zinc smelting enterprise was taken as the research object, the effects of macroscopic technical parameters such as reaction temperature, reaction time, liquid-solid ratio, acidity and crystalline concentration on the decomposition and transformation of jarosite and zinc ferrite phases in precipitates were studied. Theoretical calculation and experimental results show that in high-temperature hydrothermal system, jarosite, ammoniojarosite and zinc ferrite phase in jarosite slag can be effectively transformed into hematite, while the plumbojarosite are stable and difficult to be transformed. Increasing the temperature and prolonging the reaction time are beneficial to the hydrothermal decomposition and transformation of the phase of jarosite, ammoniojarosite and zinc ferrite. After reaction of 1 h at 220 ℃, jarosite phase transformation is basically completed, the conversion rate is 94%; and after the reaction of 4 h, the diffraction peak of zinc ferrite phase disappears completely, zinc leaching rate is 87%, and the transformation of precipitates in hematite content is 68%. Appropriately increasing the acidity of the system is conducive to the transformation of zinc ferrite, but when the initial acidity of the system is higher than 15 g/L, the phase transformation of ferrite will be inhibited. Under the conditions of reaction temperature of 220 ℃, reaction time of 4 h, liquid-solid ratio(mL/g) of 10:1, and initial acidity of 0.01 g/L, the zinc leaching rate is 89%, the conversion rate of jarosite phase can reach 95%. Hematite is the main phase in precipitates, and its content is 68%.
Key words: jarosite; hydrothermal decomposition; hematite; transformation behavior
Foundation item: Project(51664038, 51474117, 51804146) supported by the National Natural Science Foundation of China
Received date: 2019-04-23; Accepted date: 2019-07-05
Corresponding author: LI Cun-xiong; Tel: +86-13518764748; E-mail: licunxiong@126.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51664038,51474117,51804146)
收稿日期:2019-04-23;修订日期:2019-07-05
通信作者:李存兄,教授,博士;电话:13518764748;E-mail:licunxiong@126.com