
Kinetics of oxidation-precipitation of cobalt(Ⅱ) from solution by ozone
TIAN Qing-hua(田庆华), GUO Xue-yi(郭学益), YI Yu(易 宇), LI Zhi-hai(李治海)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract: In order to precipitate cobalt(Ⅱ) in cobalt chloride solution, a novel method using ozone as the precipitant for its strong oxidability was proposed. The results show that the precipitation reaction is diffusion-controlled. The main factors affecting the oxidation rate such as the stirring speed, solution temperature, ozone partial pressure, initial concentration and flow rate were investigated. The kinetics equation of each condition was established. The results indicate that the oxidation rate is independent of the initial concentration or solution temperature. The oxidation rate increases obviously with increasing the stirring speed. The linear relationship between ozone partial pressure or flow rate and oxidation rate is found.
Key words: ozone; diffusion control; cobalt chloride; oxidation; kinetics
____________________________________________________________________________________________
1 Introduction
Ozone (O3) is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope (O2)[1]. Ozone, with a standard redox potential of 1.24 V in alkaline solutions, is one of the most powerful oxidizing agents, far stronger than O2, which has been extensively used in several fields including environmental protection[2-3], food preservation[4], pharmaceutical industry[5] and chemical industry[6], etc.
Now, the application of ozone in every field of people’s life and the oxidation kinetics of organic compounds with ozone has been reported. HE et al[7] studied the degradation of fluorobenzene by using ozonation process, and investigated the influence on the reaction kinetics. JIA et al[8] investigated the kinetics of the reaction of ozone with propylene under real atmospheric environmental conditions. WU et al[9] studied the kinetics of decolorization of aqueous textile reactive dye by ozone. Some researchers[10-11] investigated the kinetics of the oxidation of cyanide to cyanate by ozone. But few reports introduced its usage in forming a precipitate for its strong oxidative especially in oxidation of cobalt(Ⅱ).
In this study, the oxidation-precipitation of cobalt (Ⅱ) in cobalt chloride solution by ozone is reported. Various factors affecting the oxidation rate are investigated comprehensively, and the kinetics equation is established.
2 Experimental
The experimental apparatus, shown in Fig.1, has two main parts: ozone generator and sedimentation reactor. The ozone generator (OZOMJB-10B, OZOMAX, Inc) has a maximum ozone generation of 10 g/h. Ozone was produced from pure oxygen, and the volumetric flow rate at the outlet of the generator, controlled by regulation of electric current, can be measured by a flowmeter. Ozone was supplied to the bottom of 1 000 mL glass sedimentation reactor, which contained 500 mL of cobalt chloride solution. A replaceable porous glass ring pipe gas diffuser was located at the bottom. On the sides are ports to insert pH electrodes and thermometer. All sensors were connected to an analog-digital system to record data continuously. The dissolved ozone concentration was measured by the indigo method[12]. The concentration of cobalt ion was determined by potentiometric titration. The pH value was adjusted to the desired value with sodium hydroxide solution (1 mol/L) because a large amount of hydrogen ions were generated in the process.
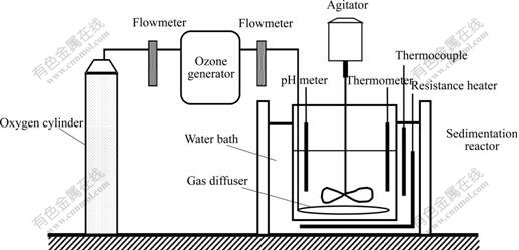
Fig.1 Experimental apparatus
The oxidation reaction using ozone as the precipitant is as follows:
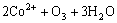 →
→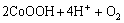 (1)
(1)
3 Results and discussion
3.1 Effect of stirring speed
The stirring speed has an obvious effect on the oxidation rate by ozone. In this experiment, the conditions of 45 ℃, ozone flow rate of 400 mL/min, ozone partial pressure of 4 435 Pa and pH 5.0 were kept constant. As shown in Fig.2, Co2+ concentration in solution decreases more quickly with the increase of stirring speed. That is to say, increasing stirring speed can enhance the oxidation rate obviously. The Co2+ concentration in the solution shows a linear decrease with reaction time increasing. It is indicated that the precipitation reaction is diffusion-controlled, and the reaction rate of cobalt precipitation is faster than the ozone mass transfer rate in the solution. With high agitation intensity, the reaction rate becomes fast with the acceleration of ozone diffusion rate, and the reaction time decreases. Simultaneously, as the ozone diffusion rate keeps constant at a constant stirring speed, the concentration of Co2+ shows a linear decrease with the reaction time inevitably.
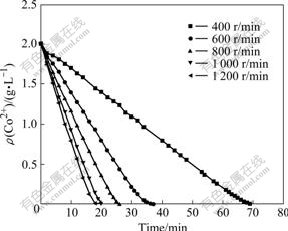
Fig.2 Co2+ concentration in solution with time at different stirring speeds
The relationship between the change rate of cobalt concentration in the solution and the stirring speed is obtained by calculating the slopes of all the reaction lines in Fig.2. It can be seen that the change rate of cobalt concentration in the solution tends to be steady with the increases of the stirring speed. The kinetic equation can be established as follows:
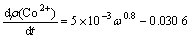 (2)
(2)
where ω is the stirring speed.
3.2 Effect of temperature
The temperature is an important factor to the oxidation process. The mass transfer rate of ozone increases with the increase of temperature. In this experiment, the condition of 800 r/min, ozone flow rate of 400 mL/min, ozone partial pressure of 4 435 Pa, pH=5 were kept constant. As shown in Fig.3, Co2+ concentration in solution kept constant with the temperature increasing at different time. That is to say, increasing temperature has no effect on the oxidation rate.
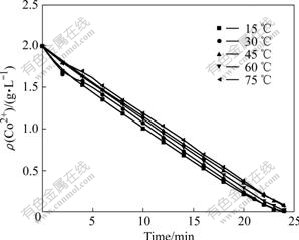
Fig.3 Co2+ concentration in solution with time at different temperatures
If the precipitation reaction is chemically controlled, the reaction rate will increase with increasing temperature obviously. It is suggested that the precipitation reaction is diffusion controlled. This can be explained by the ozone dissolution characteristics in water solution[13] as
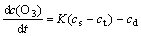 (3)
(3)
where K is the mass transfer coefficient of ozone in the solution, cs is the saturated solubility of ozone, ct is the solubility of ozone at the specified time, and cd is the concentration of ozone which is decomposed.
Under the diffusion controlled reaction, the ozone dissolved in the solution reacts with the cobalt immediately. So, ct and cd can be considered as 0. And the equation can be obtained as follows:
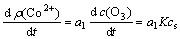 (4)
(4)
where a1 is a constant. From Eq.(4), it is found that the oxidation rate depends on not only the mass transfer coefficient of ozone in the solution but also the solubility of ozone in the solution. As we know, the solubility of ozone in the water decreases with the temperature increasing, and the mass transfer coefficient of ozone in the water is reverse[14-15]. The reaction rate does not change with the temperature by the co-action of these two factors, and the change rate of cobalt concentration in the solution is also kept constant. The kinetic equation can be calculated and established as follows:
 (5)
(5)
where a2 is a constant.
3.3 Effect of ozone partial pressure
The concentration of ozone in the mixed gas will be higher if the ozone partial pressure becomes higher. In diffusion controlled reaction, if more ozone can contact with cobalt ions, the reaction rate will increase. In this experiment, the condition of 45 ℃, ozone flow rate of 400 mL/min, stirring speed of 800 r/min, pH 5 were kept constant. As shown in Fig.4, Co2+ concentration in solution decreases more quickly with the increase of ozone partial pressure.
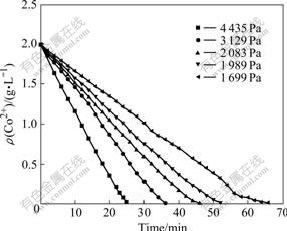
Fig.4 Co2+ concentration in solution with time at different ozone partial pressures
The relationship between the change rate of cobalt concentration in the solution and the ozone partial pressure can be obtained by calculating the slopes of all the reaction lines in Fig.4.
It is suggested that there is a direct proportion relation between the change rate of cobalt concentration and ozone partial pressure, and the kinetic equation can be established as follows:
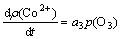 (6)
(6)
where a3 is a constant; 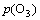 is the ozone partial pressure.
is the ozone partial pressure.
3.4 Effect of gas-flow rate
In this experiment, the condition of 45 ℃, pH 5, ozone partial pressure of 4 435 Pa, the stirring speed of 800 r/min were kept constant. As shown in Fig.5, Co2+ concentration in solution changes with the time. It is easy to find that it takes about 110 min for cobalt to precipitate completely when the O2 flow rate is 100 mL/min. When the O2 flow rate increases to 400 mL/min, it takes 25 min for cobalt to react completely. It is indicated that more ozone molecules can diffuse into the solution to react with cobalt ions at a high flow rate of O2, and the oxidation rate increases.
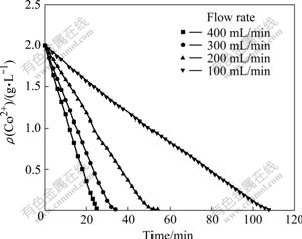
Fig.5 Co2+ concentration in solution with time at different flow rates
By the slopes in Fig.5, it can be seen that there is also a direct proportion relation between the change rate of cobalt concentration and O2 flow rate. The kinetic equation can be established as:
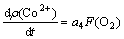 (7)
(7)
where a4 is a constant, 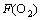 is the oxygen flow rate.
is the oxygen flow rate.
3.5 Effect of initial Co2+ concentration
In this experiment, the conditions of 45 ℃, pH 5, ozone partial pressure of 4 435 Pa, the O2 flow rate of 400 mL/min, and the stirring speed of 800 r/min were kept constant. The cobalt chloride solution with initial Co2+ concentration of 0.5, 1.0, 2.0 and 4.0 g/L was used. It can be seen from Fig.6 that the curves of the Co2+ concentration in solution with time are parallel at different initial Co2+ concentration. It is indicated that the changes of initial Co2+ concentration have no effect on the precipitation rate of cobalt by the oxidation of ozone.
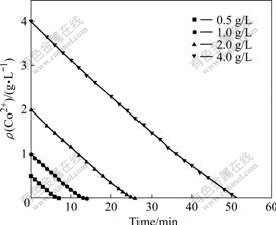
Fig.6 Co2+ concentration in solution with time at different initial Co2+ concentration
The relationship between the change rate of cobalt concentration in the solution and the initial Co2+ concentration is obtained by calculating the slopes of all the reaction lines in Fig.6. The kinetic equation can be established as:
 (8)
(8)
where a5 is a constant.
By integrating all the factors studied above, the kinetic equation of the precipitation rate of cobalt by ozone oxidation is established as
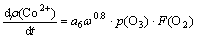 (9)
(9)
where a6 is a constant.
4 Conclusions
1) The use of ozone to precipitate cobalt(Ⅱ) from cobalt chloride solution was demonstrated. The results from reaction kinetics indicate that the precipitation reaction is diffusion-controlled.
2) The oxidation rate increases obviously with the increase of stirring speed but the oxidation rate is independent of the initial concentration or solution temperature. And there is a linear relationship between ozone partial pressure or flow rate and oxidation rate.
3) The kinetic equation of the precipitation rate of cobalt by ozone oxidation is calculated and established.
References
[1] LIU Jia-le, LUO Han-jin, WEI Chao-hai. Degradation of anthraquinone dyes by ozone[J]. Transactions of Nonferrous Metals Society of China, 2007, 17(4): 880-886.
[2] ZHENG Hong-ai, ZHANG Da-quan, PAN Li-li. New technology study on ozone methods applied to industrial wastewater treatment [J]. Journal of Shanghai University of Electric Power, 2006, 22(3): 249-253. (in Chinese)
[3] LI Jing, LIU Guo-rong. Application of advanced oxidation technologies with O3 to waste water treatment[J]. Pollution Control Technology, 2007, 20(6): 55-58. (in Chinese)
[4] WU Xiao-hong, LI Jian-ke, HUI Wei, NIU Le, ZHU Gui-qin, WEN Yan-xia. Application of ozone in the food industry[J]. China Dairy Industry, 2006, 34(4): 42-45. (in Chinese)
[5] WEN Yan-hua. Study on use of ozone in obtaining aseptic environment in pharmaceutical industry[J]. Pharmaceutical and Engineering Design, 2006, 27(4): 14-15. (in Chinese)
[6] CHEN Jia, LIU Ming-you. Application of ozone in pulp bleaching[J]. Paper Science and Technology, 2007, 26(6): 47-49. (in Chinese)
[7] HE Zhi-qiao, SONG Shuang, YANG Yue-ping, XU Xin-hua, CHEN Jian-meng. Kinetics of fluorobenzene degradation by ozone in aqueous solution[J]. Journal of Chemical Engineering of Chinese Universities, 2007, 21(2): 298-303. (in Chinese)
[8] JIA Long, XU Yong-fu, GE Mao-fa, DU Lin, WANG Geng-chen, ZHUANG Guo-shun. Kinetic study of the gas-phase ozonolysis of propylene[J]. Acta Physico-Chimica Sinica, 2006, 22(10): 1260-1265. (in Chinese)
[9] WU J, DOAN H, UPRETI S. Decolorization of aqueous textile reactive dye by ozone[J]. Chemical Engineering Journal, 2008, 142: 156-160.
[10] BARRIGA-ORDONEZ F, NAVA-ALONSO F, URIBE-SALAS A. Cyanide oxidation by ozone in a steady-state flow bubble column[J]. Minerals Engineering, 2006, 19: 117-122.
[11] CARRILLO-PEDROZA F R, NAVA-ALONSO F, URIBE-SALAS A. Cyanide oxidation by ozone in cyanidation tailings: Reaction kinetics [J]. Minerals Engineering, 2000, 13(5): 541-548.
[12] WILLIAMS M E, DARBY J L. Measuring ozone by indigo method: Interference of suspended material[J]. Journal of Environmental Engineering, 1992, 118(6): 988-992.
[13] KASKIALA T. Determination of oxygen solubility in aqueous sulphuric acid media[J]. Minerals Engineering, 2002, 15: 853-857.
[14] FLORINELLA M, CLEMENS V S. Determination of fast ozone reaction in aqueous solution by competition kinetics[J]. Journal of the Chemical Society, Perkin Transactions 2, 2000, 4: 661-664.
[15] RISCHBIETER E, STEIN H, SCHUMPE A. Ozone solubilities in water and aqueous salt solutions[J]. Journal of Chemical and Engineering Data, 2000, 45(2): 338-340.
_________________________
Foundation item: Projects(2008GK3031, 2009GK2010) supported by Science and Technology Planning Project of Hunan Province, China
Corresponding author: GUO Xue-yi; Tel: +86-731-88877863; Email: xyguo@mail.csu.edu.cn
(Edited by YUAN Sai-qian)