文章编号:1004-0609(2013)05-1388-08
铜经MBT和HQ钝化处理后在3.5%NaCl溶液中的电化学行为
贺 甜1, 2,谭澄宇1, 2,唐 娟1,郑 勇1
(1. 中南大学 材料科学与工程学院,长沙 410083;
2. 中南大学 有色金属材料科学与工程教育部重点实验室,长沙 410083)
摘 要:采用循环伏安曲线、极化曲线和交流阻抗谱研究铜经2-巯基苯并噻唑(MBT)和8-羟基喹啉(HQ)钝化处理后在3.5%NaCl溶液中的电化学行为,利用扫描电镜观察铜经缓蚀溶液处理前后在3.5%NaCl盐水中的腐蚀形貌。结果表明,MBT或HQ在铜表面形成的络合物膜能明显改善铜在3.5%NaCl溶液中的耐蚀能力;经0.5 mmol/L MBT+0.5 mmol/L HQ复配溶液处理后,其缓蚀率达90.3%;缓蚀剂的缓蚀效果由大到小的顺序为:MBT+HQ,MBT,HQ,Blank。分析了MBT与HQ两者具有缓蚀协同作用的机理。
关键词:铜;缓蚀剂;2-巯基苯并噻唑;8-羟基喹啉;协同作用
中图分类号:O646.6 文献标志码:A
Electrochemical behavior of copper passivated by MBT and HQ in 3.5%NaCl solution
HE Tian1, 2, TAN Cheng-yu1, 2, TANG Juan1, ZHENG Yong1
(1. College of Material Science and Engineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Non-Ferrous Metal Materials Science and Engineering, Ministry of Education,
Central South University, Changsha 410083, China)
Abstract: The performance of benzotriazole(BTA) and 2-mercaptobenzothiazole(MBT) as corrosion inhibitors for copper was investigated in 3.5% NaCl solution. Potentiodynamic polarization, cyclic voltammogram and AC impedance spectroscopy were used to study the electrochemical behaviors of copper electrodes passivated by inhibitors and their complex. Corrosion morphologies of copper electrodes in 3.5%NaCl solution were observed directly by scanning electron microscopy (SEM). The results show that the complex compound films formatted by MBT or HQ on copper surface apparently increase the copper electrode anticorrosion in 3.5%NaCl solution. The anticorrosive efficiency of 0.5 mmol/LMBT+0.5 mmol/L HQ combined inhibitor is 90.3%. The anticorrosive effect order of those corrosion inhibitors is MBT+HQ>MBT>HQ>Blank. The mechanism of the synergistic effect for MBT and HQ has also been discussed in detail.
Key words: copper; corrosion inhibitor; 2-mercaptobenzothiazole; 8-oxyquinoline; synergistic effect
采用缓蚀剂处理是提高铜及其合金在实际环境中防腐能力的重要途径。目前,工业上使用最广泛的铜缓蚀剂主要是苯骈三氮唑(BTA)及其衍生物,其次是巯基苯骈噻唑(MBT)。BTA对铜的缓蚀作用效果十分显著,已得到公认[1-3],但是BTA价格贵,毒性较高。巯基苯并噻唑价格低廉,毒性低,是人们较早就发现的对铜系金属具有较好缓蚀效果的缓蚀剂;BRUSIC等[4]曾报道,在冷却水系统中加入1~2 μg/L的 MBT即可实现铜的良好缓蚀。与BTA对铜的缓蚀作用不同,MBT是通过直接吸附在铜表面阻止铜的腐蚀,吸附速度快。钟文英等[5]研究表明,MBT能与铜表面的Cu2O作用形成一层致密的络合膜,从而能阻止铜的腐蚀。旷亚非等[6]认为,MBT是通过其硫原子上的孤对电子与铜表面的CuO和Cu2O形成稳定的配位键而牢牢地吸附在铜/溶液界面。然而,李风亭等[7]认为MBT是以硫醇盐的形式吸附在铜的表面,这种化学吸附虽然能有效地阻滞铜的腐蚀,但所形成的钝化膜很薄,对铜的缓蚀效果有限。另外,MBT的水溶解性较差,只溶于强碱,抗氧化性和抗氯化性较差,都影响到它的缓蚀效果,也使它在实际中的应用受到限制。
8-羟基喹啉(HQ)在中性介质中对铜也有明显的保护作用。HQ能与Cu2+发生配位络合作用,有利于在金属表面上形成更加致密的保护膜。
为了提高巯基苯并噻唑(MBT)的缓蚀效果,同时也减少单一使用MBT的用量;可考虑将MBT与HQ两者复合使用对铜表面进行钝化处理。从已有的文献资料可以看出,MBT能与铜表面的Cu+作用形成配合物,形成一层致密的Cu(Ⅰ)-MBT膜,而HQ是与Cu2+生成 [Cu(Ⅱ)HQ]络合膜作为缺陷处的补添剂,这样MBT和HQ复配有望实现互补,保证金属表面的聚合钝化层的致密性和完整性, 从而增加了对金属铜的保护能力。
目前,国内外大多数文献报道还主要是围绕在腐蚀溶液中添加单独缓蚀剂或复配缓蚀剂时,铜及铜合金的电化学行为与耐蚀性能;例如,EL-TAIB HEAKAL F等[8]就曾在多元醇冷却液中添加MBT与磷酸钠作为铜及黄铜的复合缓蚀剂。张大全等[9]研究了 BTA和8-羟基喹啉(HQ)在3%NaCl 溶液中的缓蚀协同作用。KARPAGAVALLI等[10]研究了黄铜在含有MBT和聚氧乙烯失水山梨醇的NaCl溶液中的电化学行为,发现MBT和聚氧乙烯失水山梨醇有明显的协同效应,两者复配后缓蚀效率达94.0 %。但是,关于铜表面先经MBT和HQ复配缓蚀溶液处理,随后利用电化学手段研究其浸泡在NaCl盐水溶液(此时溶液中无缓蚀剂存在)中电化学行为的尚未见报道。
本文作者利用电化学工作站研究纯铜表面经MBT和HQ复配溶液处理后在3.5%NaCl溶液中的电化学行为,借助扫描电镜观察铜表面经缓蚀剂处理前后的腐蚀形貌,以评估复配缓蚀溶液处理效果,分析MBT与HQ在纯铜表面协同成膜与耐蚀机理,为今后深入研究提供参考。
1 实验
1.1 电极试样处理
实验采用纯铜片为工作电极。仅暴露10 mm×10 mm的工作面积,其余部位用环氧树脂封装;用金相砂纸逐级打磨,抛光后,用丙酮、无水乙醇、二次蒸馏水清洗,浸入6%H2SO4溶液中进行1 min酸洗,随后分别浸入1 mmol/L MBT、1 mmol/L HQ 或 0.5 mmol/L MBT+0.5 mmol/L HQ溶液中0.5 h,待用。
1.2 电化学测量方法
采用CHI660C型电化学工作站进行循环伏安曲线,极化曲线和电化学交流阻抗谱的测量。所有电化学测试都采用三电极体系:即以纯铜电极为工作电极,采用大面积光亮铂片做辅助电极,232型饱和甘汞电极(SCE)为参比电极。在3.5%NaCl腐蚀溶液中进行电化学测试。在测定循环伏安曲线,选取的扫描速度为100 mV/s;测定极化曲线时,取扫描速度为1 mV/s。在开路电位下进行交流阻抗谱的测试,取频率0.01~100 kHz范围,取激励信号峰值为 5 mV;测试溶液温度控制在(25±1) ℃。
铜经HQ、MBT和MBT+HQ溶液浸泡处理后在3.5%NaCl溶液中的抗腐蚀能力,可通过与没有经MBT与HQ浸泡处理的比较,以自腐蚀电流所发生的变化率(η)来描述。
2 结果与讨论
2.1 循环伏安曲线
图1所示为经MBT、HQ或MBT+HQ复配液处理后铜电极在3.5%NaCl溶液中的循环伏安曲线。由图1可以看出,未经处理的铜电极,在约0.15和-0.2 V左右处分别出现一个明显的阳极氧化峰与阴极还原峰(见图1中虚线);当铜电极经缓蚀剂处理后,其阳极氧化峰明显减弱,特别是经MBT处理或MBT+HQ复合处理后,阳极氧化峰减弱更为明显(见图1箭头A所示),这说明MBT缓蚀剂或MBT+HQ复配溶液处理后,铜表面上所生成的吸附膜或络合膜会对电极阳极反应过程有显著的阻碍作用;相比之下,阴极峰值变化不大,阴极峰位略有些负移(见图1箭头B所示),这可能表明MBT或HQ缓蚀剂对铜电极的阴极电化学过程未产生明显的影响。

图1 铜电极经不同缓蚀剂处理后在3.5%NaCl溶液中的循环伏安曲线
Fig. 1 Cyclic voltammograms of Cu electrodes in 3.5%NaCl solution
2.2 动电位极化曲线
铜电极经MBT、HQ及其复配溶液处理0.5 h后在3.5%NaCl溶液中的极化曲线如图2所示。从图2可以看出,未经缓蚀剂处理的铜电极在3.5%NaCl溶液中的自腐蚀电位为-342 mV左右,经MBT和HQ处理后的铜电极其自腐蚀电位分别负移至-407和-371 mV,而经MBT和HQ复配液处理后,铜电极自腐蚀电位又进一步负移至-420 mV;仔细观察可以发现,经含MBT和MBT+HQ溶液处理后的铜电极在NaCl溶液中的极化曲线的“倒喇叭口”明显向下移动,即在自腐蚀电位附近的弱极化区,阴极极化曲线与阳极极化曲线均下移;这说明在本实验条件下,MBT与MBT+HQ对铜电极浸在NaCl溶液中的阴、阳极电化学过程起到了抑制作用。
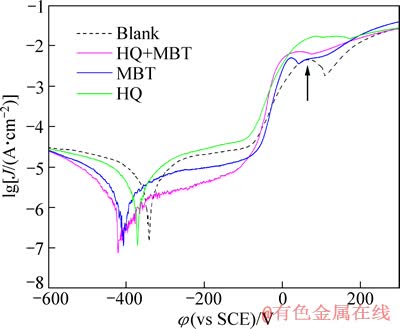
图2 经不同缓蚀剂处理后铜电极在3.5%NaCl溶液中的极化曲线
Fig. 2 Potentiodynamic polarization curves of Cu electrodes in 3.5%NaCl solution
由图2还可以看出,未经缓蚀剂处理的铜电极在0.06 mV左右明显出现了铜的氧化峰(如箭头所示),经缓蚀剂处理后该处的氧化峰已明显降低。表1所列为根据图2采用塔菲尔线性外推得到的数据。
由表1可以清楚看到,经HQ与MBT处理均可降低铜在3.5%NaCl盐水溶液中的腐蚀速度,但MBT缓蚀效果明显优于HQ的作用;单独使用MBT其缓蚀效果达79%,而经HQ和MBT复配液处理后,铜电极的自腐蚀电流密度又进一步降低,其缓蚀效果可达90.3%。
表1 铜电极在3.5%NaCl溶液中的电化学参数
Table 1 Electrochemical parameter of Cu electrodes immersed in 3.5%NaCl solution

2.3 交流阻抗谱
图3和4所示分别为铜电极经HQ和MBT及其复配溶液处理后在3.5%NaCl溶液中的Nyquist和Bode图。由图3可以看到,所有曲线在高-中频端都呈现为一个压偏的不规则容抗弧;这表明在本实验的NaCl溶液体系中铜的腐蚀过程应主要受电荷传递控制[11]。与未经缓蚀剂处理的相比,经HQ、MBT或MBT+HQ缓蚀液处理后的铜电极在NaCl溶液中所表现出的容抗弧半径明显增大,尤其是经MBT或MBT+HQ处理后,容抗弧半径增加的更大;这意味着经缓蚀溶液处理后,电荷转移电阻显著增大。
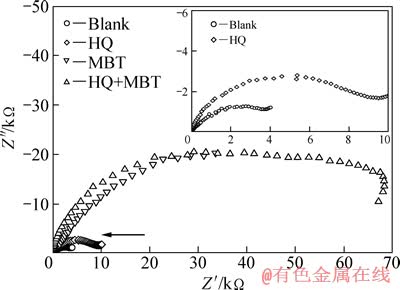
图3 铜电极在3.5%NaCl溶液中的Nyquist图
Fig. 3 Nyquist plots for Cu electrodes in 3.5%NaCl solution
曲线在低频端尚未出现明显的感抗弧或Warburg阻抗。由图4(a)可以看出,随着频率的增加,阻抗值Z呈单调下降趋势。低频端的阻抗值|Z|大致可以反映材料的耐蚀能力;阻抗值|Z|越大,则铜电极在3.5%NaCl溶液中的耐蚀能力越好[12]。由图4(a) 中lgZ—lg f关系曲线可以看出,经不同缓蚀剂处理后,铜电极的|Z|值(在频率低于100 Hz时对应的阻抗膜值)明显增大,其中以MBT+HQ复配溶液处理所表现出的缓蚀效果最好,这与前面由极化曲线所获得的结果是完全吻合的。
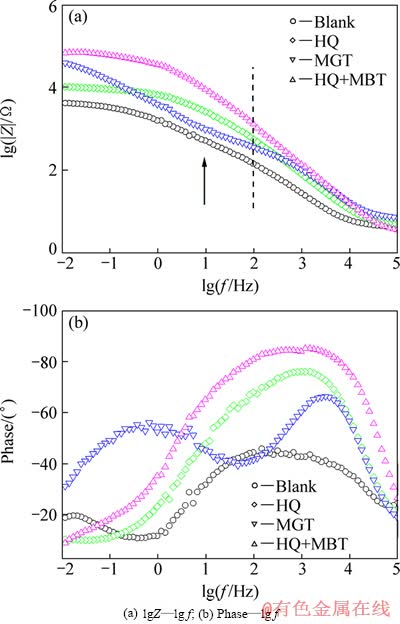
图4 铜电极在3.5%NaCl溶液中的Bode图(对应图3)
Fig. 4 Bode plots for Cu electrodes in 3.5%NaCl solution
铜经MBT、HQ或MBT+HQ缓蚀溶液处理后在3.5% NaCl溶液中的等效电路如表2所示。依据阻抗谱特性以及溶液/界面的特征在表2中采用了不同的等效电路图:未经缓蚀处理的铜电极在3.5% NaCl溶液中,主要表现出两个容抗特性CPE1和CPE2,在低频电流下表现出Rt和C1的串联。经HQ处理的铜电极在溶液中的等效电路与未经缓蚀处理的(空白)相类似,但考虑到吸附物所引起的电感L1起一定作用,故用电感L1替代了吸附物电阻Rp;经HQ+MBT处理的铜电极的等效电路也相应采用L1替代Rp电阻。其中,Rs为溶液电阻,Rp为电极表面吸附(如缓蚀剂)物电阻,Rt为电荷转移电阻,C1为电荷转移电容(水合电容),L1为缓蚀剂分子吸附表现出的电感;CPE1为外界电容(双电层电容),CPE2为吸附物(缓蚀剂等)电容,在这里采用常相位角元件CPE代替电容元件[13]。按照等效电路借助Zview软件进行拟合,试验曲线与拟合曲线非常吻合,所得各参数值列于表3。
表2 铜电极在3.5% NaCl溶液中的等效电路
Table 2 Equivalent circuit for Cu electrodes in 3.5%NaCl solution
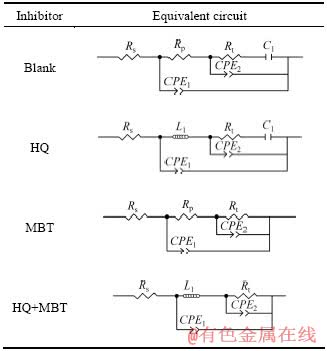
表3 铜电极在3.5% NaCl溶液中等效电路的拟合参数值
Table 3 Fitting data on equivalent circuit for Cu electrodes in 3.5%NaCl solution

由表3可以看出,经过MBT或HQ溶液处理后,能明显提高铜在3.5%NaCl溶液中电极反应的电荷转移电阻Rt值,这说明MBT、HQ和MBT+HQ溶液处理所形成的吸附膜或络合产物膜对铜在3.5% NaCl溶液中的腐蚀有明显的抑制作用。分别经MBT、HQ或MBT+HQ溶液处理后,其Rt值由大到小的变化顺序为:MBT+HQ,MBT,HQ,Blank;同时也可以看到,相应的CPE1和CPE2值也大致依次减小。笔者认为,当经MBT和HQ溶液处理后,有机化合物分子在铜电极表面上吸附,甚至形成Cu(Ⅰ)-MBT膜与 [Cu(Ⅱ)HQ]膜;由于有机化合物的介电常数小于水的,导致CPE1和CPE2值明显减小。经MBT+HQ复配溶液处理后电荷转移电阻Rt值最大,CPE1和CPE2值最小;表明MBT与HQ之间存在明显的协同作用,从而可以有效地改善了铜在3.5%NaCl溶液耐蚀性能。
2.4 电极表面腐蚀形貌
图5所示为纯铜电极经HQ和MBT溶液处理后在3.5%NaCl溶液中浸泡14 d后的表面腐蚀形貌。由图5 (a)可以看出,未经处理的铜电极经过14 d浸泡后,表面出现大量深浅不一的蚀坑,呈现较为严重的腐蚀;经HQ处理后(见图5 (b))蚀坑数量明显减少,蚀坑变小,说明HQ对铜起到了一定的缓蚀作用。经MBT处理后(见图5 (c)),铜表面的腐蚀区域减少,腐蚀坑的尺度与不均匀性增加,值得注意的是其形成的部分腐蚀坑呈现为不规则方块蚀坑,这可能是腐蚀沿特定晶面择优发展的结果。经MBT+ HQ复配溶液处理后,铜电极在3.5%NaCl溶液中浸泡14 d其表面只有少量小尺度的轻微腐蚀点的痕迹(见图5 (d)),表明经HQ+MBT复配缓蚀溶液处理,的确可以显著提高铜在3.5%NaCl溶液中的抗蚀能力。MBT和HQ两者之间存在明显的协同效应。
2.5 缓蚀机理分析
本研究是把铜电极置于含有MBT、HQ或MBT+HQ不同缓蚀剂的水溶液中进行0.5 h浸泡处理后,再将铜电极浸在没有缓蚀剂的3.5%NaCl溶液中腐蚀;这区别于通常直接在腐蚀介质中添加缓蚀剂的做法。因此,在本实验条件下讨论缓蚀剂的作用,实际上就是分析经缓蚀溶液处理后在铜表面生成的络合膜对铜电极随后在盐水溶液中腐蚀行为的影响。
MBT或HQ的缓蚀效果实际上与其在铜表面上形成的吸附或与铜成键形成络合物膜的性质有密切关系。而MBT或HQ分子在不同状态下的结构特征及原子上的电荷分布直接影响到它们在铜表面的吸附或成键形成络合物膜的行为。

图5 铜电极经不同缓蚀剂处理后在3.5% NaCl溶液中腐蚀14 d后表面形貌
Fig. 5 Morphologies of Cu electrodes after immersed with various inhibitors in 3.5%NaCl solution for 14 d
表4 各种状态下MBT的部分结构数据及原子电荷分布数据[14]
Table 4 Structural parameter and charge distribution on atoms of MBT under various conditions[14]
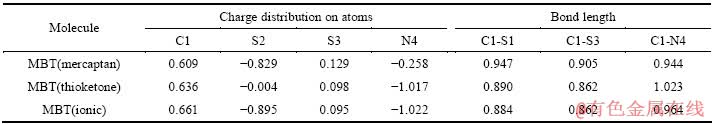
表4所列为引用文献所得的MBT在不同状态下部分结构数据及部分原子上的电荷分布。MBT主要以硫醇或硫酮状态存在。由表4可知,MBT在硫醇状态下,S2上负电荷分布最多,为-0.829,此时主要由S2提供电子与铜原子作用,而S2采用的是不等量的sp3杂化,各轨道夹角近似为108°28′,S2与铜原子的成键垂直于铜表面,故MBT分子平面与铜表面成个很小的锐角(见图6)。而MBT在硫酮状态下,N4上的负电荷最多,为-1.017,此时N4采用的则是sp3杂化,故分子平面亦与铜表面成一很小锐角;因此,即无论MBT以硫醇或硫酮状态存在,MBT在铜表面上都将近乎平铺的方式存在,覆盖面积较大,在随后的腐蚀溶液中有效地阻碍电荷与铜表面进行的交换,也妨碍了H2O、Cl-离子等在铜表面上直接吸附,因此,使得铜电极在盐水溶液中的腐蚀速度明显降低。尽管MBT分子平面近乎平行于铜的表面,但是其偶极矩的方向并不是有序一致,这可能就是在阻抗谱上没有表现出电感特性的缘故。
表5所列为引用文献[15]得到HQ部分原子上的电荷分布。由表5可以看出,在中性环境下,N10和O11原子上带的负电荷最多,也就是说在反应中,主要由N10和O11提供电子。根据前线轨道理论,HQ的HOMO与Cu的LUMO作用,形成配位键;Cu的HOMO与HQ的LUMO作用,形成反馈键,从而使得HQ吸附在金属表面。

图6 2-巯基苯并噻唑在铜电极表面的吸附示意图
Fig. 6 Proposed structures for 2-mercaptobenzothiazole adsorb on copper electrodes

图7 8-羟基喹啉在铜电极表面的吸附示意图
Fig. 7 Proposed structures for 8-hydroxyl decoquinate adsorb on copper electrodes
表5 HQ分子中原子上的电荷分布[15]
Table 5 Charge distributions on various atoms of HQ molecule

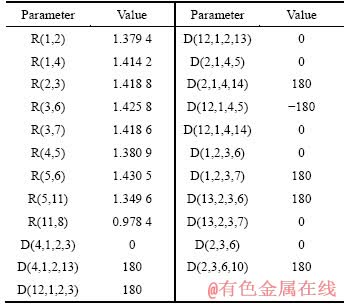
表6所列为引用文献[15]得到HQ的结构数据参数。由表6可知,HQ分子上除H18以外,其他原子均处于同一平面上。由于原子轨道的能量决定了分子轨道的能量,在中性溶液中,成键则主要由N10、O11原子完成,即整个分子的HOMO主要表现为N10和O11的前线轨道。由于碳原子均采用sp2杂化,N10采用sp2杂化,O11采用的是sp3杂化,故两者与铜原子的成键平面垂直于铜表面,整个HQ分子平面垂直于铜表面,示意图如图7所示,这使铜电极随后在盐水溶液中的腐蚀速度有所降低;但是由于HQ分子在铜表面的有效覆盖面积较小,致使HQ的缓蚀效果明显不如MBT的缓蚀效果。
由于HQ分子垂直吸附在铜的表面,分子的偶极矩是有序的,导致反应过程中电荷在传输过程中会受到分子偶极矩的作用,其作用相当于在阻抗谱上存在一个电感L1,这也是表2在含HQ缓蚀剂溶液的等效电路中给出了一个电感元件的原因。电荷的转移主要通过吸附在HQ分子之间并与铜表面接触的氯离子进行,致使电荷转移电阻较小,影响了HQ的缓蚀效果。
表6 HQ的结构数值[15]
Table 6 Structural parameter of HQ molecule
当铜经MBT与HQ缓蚀剂复配溶液处理后,由于MBT以平铺的方式吸附在铜电极的表面,并与铜表面的Cu+作用形成一层致密的Cu(Ⅰ)-MBT膜;HQ则以垂直的方式吸附在铜表面上,并与Cu2+生成 [Cu(Ⅱ)HQ]络合膜;HQ与MBT分子在铜表面交错排列,平行吸附与垂直吸附相结合,相互协同作用,在铜表面上形成了紧密结合粘附良好的络合膜,使铜电极随后在3.5%NaCl溶液中的耐蚀效果明显增强,HQ和MBT之间有明显的缓蚀协同作用,其缓蚀率可达90.3%;图8所示为经HQ+MBT复配处理在铜表面生成的络合膜后抵抗NaCl溶液腐蚀的示意图。
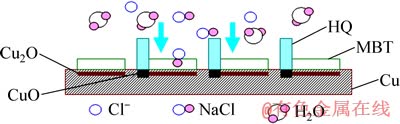
图8 铜电极经HQ+MBT复配液处理后在3.5%NaCl溶液中的腐蚀行为示意图
Fig. 8 Corrosion behaviors of Cu electrodes after immersed HQ+MBT liquor in 3.5%NaCl solution
3 结论
1) 经MBT或HQ缓蚀液处理后,铜在3.5% NaCl溶液中的自腐蚀电位负移,腐蚀电流密度Jcorr明显降低;与HQ作用比较,MBT缓蚀效果较好;采用HQ+MBT复配液处理后,铜的自腐蚀电位进一步负移,腐蚀电流密度Jcorr也进一步降低,有明显的协同效应,复配液的缓蚀率可达90.3%。
2) MBT或HQ在铜表面形成的络合物膜,使电荷转移电阻增大,而界面电容与膜孔电容减小,能明显改善铜在3.5%NaCl溶液中的耐蚀能力。HQ与MBT之间由于协同作用,经HQ+MBT复配液处理,电荷转移电阻显著增大,铜的耐蚀能力进一步提高。
3) 经MBT处理后,MBT分子在铜电极表面近乎平铺方式吸附,并与Cu+作用在Cu/溶液界面上快速形成Cu(Ⅰ)-MBT膜;经HQ处理后,HQ分子在铜表面近乎垂直方式吸附,并与Cu2+发生配位作用形成[Cu(Ⅱ)HQ]络合膜;MBT缓蚀效果较好与其MBT分子在铜表面以平铺方式吸附和成膜有关。
REFERENCES
[1] 董泉玉, 张 强, 李 锐, 杨从贵. 国内铜缓蚀剂的最新发展现状[J]. 全面腐蚀控制, 2003, 17(6): 19-23.
DONG Quan-yu, ZHANG Qiang, LI Rui, YANG Cong-gui. The latest domestic development condition of corrosion inhibitor for copper[J]. Total Corrosion, 2003, 17(6): 19-23.
[2] 张 娟, 李 周, 陈 畅, 唐 宁, 金 鹏. BTA及其复配铜缓蚀剂的研究进展[J]. 材料导报, 2008, 22(9): 83-86.
ZHAN Juan, LI Zhou, CHEN Chang, TANG Ning, JIN Peng. Research progress in BTA and it s combined copper cor rosion inhibitors[J]. Materials Review, 2008, 22(9): 83-86.
[3] RAVICHANDRAN R, NANJUNDAN S, RAJENDRAN N. Corrosion inhibition of brass by benzotriazole derivatives in NaCl solution[J]. Anti-Corrosion Methods and Materials, 2005, 52(4): 226-232.
[4] BRUSIC V, FRISCHM A, FRANKEL S G. Copper corrosion with and without inhibitors[J]. Journal of the Electrochemical Society, 1991, 138 (8): 253-259.
[5] 钟文英, 郑朝华. 光谱电化学研究α-巯基苯并噻唑对铜的缓蚀机理[J]. 无机化学学报, 1999, 15(6): 756-760.
ZHONG Wen-ying, ZHENG Zhao-hua. Spectro-electrochemical studies of the corrosion inhibition of copper by mercap to benzothiazole[J]. Chinese Journal of Inorganic Chemistry, 1999, 15(6): 756-760.
[6] 旷亚非, 陈 曙, 林志成. 2-巯基苯并噻唑对NaCl溶液中铜的缓蚀行为[J]. 中国腐蚀与防护学报, 1995, 15(2): 129-134.
KUANG Ya-fei, CHEN Shu, LIN Zhi-cheng. A study of inhibition behavior of 2-mercaptobenzothiazole on copper in sodium chloride solution[J]. Journal of Chinese Society for Corrosion and Protection, 1995, 15(2): 129-134.
[7] 李风亭, 张冰如. 2-巯基苯并噻唑在铜表面的吸附状态[J]. 腐蚀与防护, 2002, 23(8): 344-348.
LI Feng-ting, ZHANG Bing-ru. Adsorption of 2-mercaptobenzo- thiazole on copper surface[J]. Corrosion & Protection, 2002, 23(8): 344-348.
[8] EL TAIB HEAKAL F, HARUYAMA S. Impedance studies of the inhibitive effect of benzotriazole on the corrosion of copper in sodium chloride medium[J]. Corrosion Science, 1980, 20(7): 887-898.
[9] 张大全, 陆 柱. 苯并三唑和8-羟基喹啉对铜的缓蚀协同作用[J]. 物理化学学报, 2002, 18(1): 74-78.
ZHANG Da-quan, LU Zhu. Synergistic effects of benzotriazole and 8-hydroxyquinoline combined inhibitors on copper corrosion[J]. Acta Physico-Chimica Sinica, 2002, 18(1): 74-78.
[10] KARPAGAVALLI R, DARRAN R C, RAJESWARI S. Synergistic inhibition effect of 2-mercaptobenzothiazole and Tween-80 on the corrosion of brass in NaCl solution[J]. Applied Surface Science, 2008(254): 4483-4493.
[11] 王献群, 刘瑞泉, 朱丽琴, 宫建伟. 碱性介质中BIT, BIOHT和BIMMT对铜的缓蚀性能和吸附行为[J]. 物理化学学报, 2007, 23(1): 21-26.
WANG Xian-qun, LIU Rui-quan, ZHU Li-qin, GONG Jian-wei. Inhibition action and adsorption behaviors of BIT, BIOHT and BIMMT on copper in alkaline medium[J]. Acta Physico-Chimica Sinica, 2007, 23(1): 21-26.
[12] 曹楚南. 关于缓蚀剂研究的电化学方法[J]. 腐蚀科学与防护技术, 1990, 2(1): 1-9.
CAO Chu-nan. Electrochemical methods of the study on inhibitors[J]. Corrosion Science and Protection Technology, 1990, 2(1): 1-9.
[13] 曹楚南, 张鉴清. 电化学阻抗谱导论[M]. 北京: 科学出版社, 2004: 3-9.
CAO Chu-nan, ZHANG Jian-qing. An introduction to electrochemical impedance spectroscopy[M]. Beijing: Science press, 2004: 3-9.
[14] 杨 刚, 龙翔云. 巯基类浮选药剂电子结构及其与金属离子作用的量子化学研究[J]. 高等学校化学学报, 2001, 22(1): 86-89.
YANG Gang, LONG Xiang-Yun. A quantum chemistry study on the electronic structure of mercapto flotation reagents and its coordination with metal ions[J]. Chemical Journal of Chinese Universities, 2001, 22(1): 86-89.
[15] 谢 彦, 曾和平, 姚海波. 8-羟基喹啉衍生物泛函理论的研 究[J]. 计算机与应用化学, 2008, 25(5): 549-552.
XIE Yan, ZENG He-pin, YAO Hai-bo. A density functional theory study of 8-hydroxyquinoline derivatives[J]. Computers and Applied Chemistry, 2008, 25(5): 549-552.
(编辑 何学锋)
基金项目:民口配套项目(MKPT-98-106)
收稿日期:2012-04-24;修订日期:2013-04-01
通信作者:谭澄宇,教授,博士;电话:13786163431;E-Mail:tanchengyu@yahoo.com.cn