文章编号:1004-0609(2007)03-0465-06
常温酸性条件下黄铜矿的电化学行为
卢毅屏,蒋小辉,冯其明,欧乐明,张国范
(中南大学 资源加工与生物工程学院,长沙 410083)
摘 要:在温度为25 ℃及pH=2的条件下,通过循环伏安法和恒电位I—t曲线研究了黄铜矿特殊的电化学分解行为。通过循环伏安曲线发现:电位在400~800 mV(vs SHE)范围内,黄铜矿电极表面的阳极氧化反应电流很小;主要是由于生成的中间产物很难被进一步氧化分解,从而产生了钝化;当电位小于-400 mV (vs SHE)时,黄铜矿阴极还原反应电流较大,晶格中的Fe3+能较快地溶解出来,产生的中间产物(铜的硫化物)在氧化电位下发生较强的阳极氧化分解反应,但是随后反应进一步被钝化。黄铜矿的阴极还原反应较强烈,且对黄铜矿氧化浸出具有重要意义。此外,恒电位I—t曲线也证实了以上结论。
关键词:黄铜矿;分解;阳极氧化;阴极还原
中图分类号:TD 952 文献标识码:A
Electrochemical behavior of chalcopyrite at normal temperature in acidic solution
LU Yi-ping, JIANG Xiao-hui, FENG Qi-ming, OU Le-ming, ZHANG Guo-fan
(School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The electrochemical reaction behaviors on chalcopyrite were investigated using cycle voltammetry and potentiostatic I—t curve at 20 ℃ and pH=2. The voltammograms show that the oxidization of chalcopyrite occurs at slow rate in potential range from 400 to 800 mV(vs SHE). The reason is that the intermediate products are very difficult to be further dissolved unceasingly if Fe3+ ion in lattice has not been dissolved. Fe3+ ion in lattice can be dissolved effectively for the strong deoxidization when the negative potential is lower than -400 mV(vs SHE). Meanwhile, the products can be oxidized easily under higher positive potential, but this reactions are also passivated later. The cathodic reduction is stronger and plays an important role for the dissolution of chalcopyrite. The potentialstatic I—t curve also identify the above conclusion.
Key words: chalcopyrite; decomposition; anodic oxidization; cathodic reduction
黄铜矿是一种很难被氧化分解的硫化矿物,其分子式为CuFeS2, 属四方晶系,晶体格子内每个硫离子被4个金属(Cu、Fe)离子所围绕。每个铁或铜离子被4个硫离子所包围,其中Cu为+1价,Fe为+3价。晶体中各质点连接的方向性都很强,且共价键的键能比较大,解离后化学键的不饱和能较大[1]。黄铜矿的分解反应通过电子转移或空穴转移分步进行,且分解的机理较复杂[2]。
在实际浸出过程中,黄铜矿的分解也是分步进行的,且反应十分缓慢。很多研究者认为有钝化层阻止了反应的进一步进行。在硫酸浸出黄铜矿的过程中,钝化物被认为是具有半导体性质的中间物质,如Cu1-xFe1-yS2-z[3]、CuS2[4-5]、Cu0.8S2 [6]和CuSn[7]等。对于细菌浸出黄铜矿的钝化原因一直存在争议,钝化物一般被认为是覆盖在矿物表面的元素单质硫[8]或黄铁矾类[9]物质。但假设上述这些物质都起钝化作用,比较黄铜矿和其他硫化矿的细菌浸出,仅仅只有黄铜矿等极少数矿的浸出率很低,这说明黄铜矿浸出的钝化和它自身特有的分解过程是分不开的, 因此研究黄铜矿电化学分解行为,对探索中温细菌浸出的钝化原因很有意义。
标准电化学方法对研究黄铜矿的分解电化学行为和探讨浸出钝化机理很有帮助。在浸出和浮选等各种的矿物加工领域,前人对黄铜矿的电化学行为做过一些研究 [10-14]。由于大部分硫化矿的浸出过程是氧化溶解过程,因此大多数研究都是关于在阳极氧化反应方面的研究[1-7],而对黄铜矿阴极还原反应及其意义的研究很少。本研究中黄铜矿电极采用静止电极和旋转电极的循环伏安图进行比较分析,并根据需要进行多次重复扫描,在不同初始方向扫描以及不同电位范围扫描,分析黄铜矿在常温酸性条件下的电化学行为。
1 实验
1.1 仪器与试剂
M273恒电位仪,恒电流系统(美国EG&G公司);采用三电极系统: 工作电极为黄铜矿旋转圆盘电极和静止电极,铂电极作为辅助电极,Ag/AgCl电极作为参比电极;用带鲁金毛细管的盐桥与工作电极室相连;实验用含量为99.9%氮气;恒温仪。
1.2 实验方法和条件
电极矿物为厚5 mm,直径为10 mm的圆柱体块状黄铜矿。工作面打磨抛光,用丙酮清洗,真空保存用于制作电极。将制作好的块矿插入以聚四氟乙烯为材料的柱形塑料套中,并用环氧树脂密封固定。矿物电极外露的工作面面积为0.785 cm2, 另一面用弹簧铜导线向外连接到旋转轴上,做成旋转圆盘电极。静止电极表示旋转轴处于静止状态。
电化学实验在恒温(298 K), pH=2的条件下进行,扫描速度为20 mV/s, 电极旋转速度为900 r/min,传导电解质为0.5 mol/L分析纯Na2SO4溶液。实验中所有电位都采用相对氢标电位(SHE),以便与其他文献中关于黄铜矿的静电位表示方法保持一致。
2 结果和讨论
图1和2所示为电位扫描范围相同初始电位不同的循环伏安曲线。图1中曲线初始扫描电位从400 mV开始(黄铜矿的标准静电位为φ=400 mV)。图2中初始电位为-600 mV。两图都比较了旋转电极和静止电极的电化学行为,实线、虚线部分分别代表旋转、静止电极的循环伏安图。通过对比看出,两种电极的电流峰都不可逆,曲线上大体有两种峰:存在静止电极曲线而不存在旋转电极曲线的峰C1′、A3′、A4′;两者曲线都存在,但是旋转电极曲线比静止电极曲线大的峰A1、C2、C3,两者差别不大的峰A0。

图1 扫描范围为-600~1 000 mV和初始电位为400 mV时的循环伏安曲线
Fig.1 Cyclic voltammograms obtained with chalcopyrite under condition of potential from -600 mV to 1 000 mV and scan potential of 400 mV
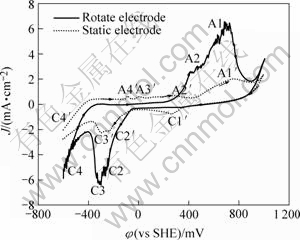
图2 扫描范围为-600~1 000 mV和初始电位为-600 mV时的循环伏安曲线
Fig.2 Cyclic voltammograms obtained with chalcopyrite under condition of potential range from -600 mV to 1 000 mV and scan potential of -600 mV
1) 阳极氧化反应
在一般酸浸和细菌浸出的过程中,浸出液的氧化还原电位约为400~800 mV,且在此电位区,黄铜矿发生氧化分解反应,因此在黄铜矿电化学行为中,对此电位区的电化学反应的研究尤其重要。图1中扫描电位区为 400~800 mV时,旋转电极和静止电极曲线上的电流峰A0、A0′峰很相似,且表示电流密度都很微小。A0峰和A0′的情况与Price and Warren[15]的发现大致相同。在开路电位扫描到550 mV的电位时,循环伏安图上出现了阳极峰,认为发生了如下反应:
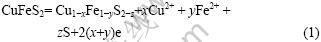
其中氧化产物Cu1-xFe1-yS2-z是一层中间产物,离子部分分解后形成的具有黄铜矿结构的缺铁硫化物,它对矿物表面继续分解起钝化作用。Parker等[16]认为这层物质Cu1-xFe1-yS2-z具有半导体属性。
阳极峰A2′中电流峰随重复扫描次数增加而减少,说明它是黄铜矿阳极氧化形成的电流峰。峰A2和A2′同样也可以用反应(1)表示,只不过这时式中的x或y较小而已。
图1和2显示静止电极在电位扫描到50 mV和-100 mV处都出现了两个微小的阳极电流峰A3′及A4′。在-600~400 mV电位范围的多次重复扫描图3也显示,A3′和A4′电流峰随着重复扫描次数的增加而增大。这说明它们可能不是直接阳极氧化反应电流峰。由于阳极氧化反应在重复扫描后,其产物会阻碍下一次扫描的化学反应进行, 电流峰逐渐减弱。另外,图1和2显示A3′峰及A4′电流峰只存在于静止电极的曲线中,而旋转电极曲线并没有出现,由此说明导致反应发生的这种阴极还原产物容易脱附。结合文献[16],可以认为峰A3′和峰A4′表示的反应如式(2)所示:


图3 黄铜矿静止电极在-600~400 mV时的循环伏安曲线
Fig.3 Cyclic voltammograms obtained with static chalcopyrite electrode at potential of -600 mV to 400 mV (1,2,3 are series numbers of scan, scan potential is initiated from -600 mV)
2) 阴极还原反应
在所有循环伏安图中,小于-400 mV电位区都存在峰C4和峰C4′。Elsherief[17]提出在阴极电位扫描到-700 mV出现了较大还原波峰,此时黄铜矿分解产物中Fe3+已经全部溶解出来,发生如下反应:
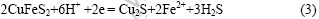
实验还观测到,电位越向负方向,峰C4表示的电流就越大,可以认为峰C4和C4′所示的是式(3)表示的化学反应。阴极还原反应使黄铜矿晶格中的Fe3+得到电子为Fe2+分解出来的。在这个过程中,新的中间产物不断形成,直到Fe3+全部溶解出来,最终产物为铜的硫化物。
图2中的阴极还原峰C2和峰C3值远远大于图1中的相应峰。图1中的初始扫描电位为400 mV,而图2的初始扫描电位-600 mV。由于图2中的峰C2和峰C3形成之前,扫描电位经过了较负电位区,由此说明,较负电位下的阴极还原对峰C2和峰C3的形成具有重要作用。
Elsherief[17]在电解质中加Fe2+和Cu2+, 考查了阳离子对黄铜矿阴极极化的影响。通过对黄铜矿电极进行伏安扫描发现,当阴极扫描到-275 mV时出现明显的还原波峰,发生的化学反应如方程式(4)和(5)所示。
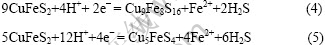
Elsherief[17]提出的上述反应方程式(4)和(5)表示黄铜矿的直接阴极分解反应,旋转电极应该有利于反应峰的形成。虽然上述图中出现的阴极峰C2′和C3′与他提出的反应电位相近,但是电极的旋转也并没有提高电流峰值,反而使该电流峰消失了(见图4),所以可以判断峰C2′和C3′并不表示方程(4)和(5)所发生的反应。
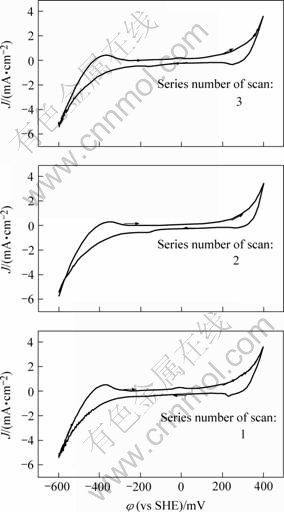
图4 黄铜矿旋转圆盘电极在-600~400 mV时的循环伏安曲线
Fig.4 Cyclic voltammograms obtained with rotate chalcopyrite electrode at potential of -600 mV to 400 mV (Arrows indicate potential scan direction. Scan potential is initiated from -600 mV)
图3所示为阴极电位为-300~0 mV时出现了明显的阴极还原峰C2′和C3′,而在图4中此电位区没有出现波峰。另外,图3中第一次扫描的循环伏安图的峰C2′和C3′基本重叠成一个波峰,电流峰值较大,且随扫描次数增加而逐渐减少,由此说明此反应的产物没有及时迁移到溶液中,累积在电极表面对下一次反应起钝化作用。再比较图2和4,图2中正电位极限为1 000 mV,图4中为400 mV。图2中的峰C2和C3很明显,而图4中这一电位区没有观测到峰C2和C3,这说明阳极氧化反应对该峰作用也很大。由此可以推测峰C2和C3为阳极氧化生成容易脱落的产物,发生阴极还原反应产生的电流峰。
在以上静止电极的循环伏安图中,在阴极方向扫描到300 mV时出现阴极电流峰C1′。旋转电极曲线没有出现这些峰, 说明此时电极旋转对反应发生起了决定性作用。图5所示为初始扫描方向为阴极方向,扫描范围为0~1 000 mV时的静止电极重复循环伏安曲线。通过比较图1、2、和5可看出,峰C1′有如下特点:首先,此峰在所有的静止电极上都很明显,表示反应电流比较大;其次,此峰只出现于静止电极的曲线上,旋转电极曲线上没有出现,这说明致使该峰发生的反应物很容易从电极表面脱落;再次,它与初始扫描方向没有关系,由于初始阴极方向扫描也可以形成(见图5),说明它可以是以阳极氧化反应的产物为反应物的电流峰。值得注意的是,黄铜矿阳极氧化反应有元素硫单质的生成,而单质硫的特性也符合上述反应的现象。综合以上几点,可以认为C1′峰是单质元素硫发生阴极还原反应的结果。参考热力学计算结果,其反应如方程式(6)所示:


图5 黄铜矿静止电极在0~1 000 mV时的初始阴极方向的循环伏安曲线
Fig.5 Cyclic voltammograms obtained with static chalcopyrite electrode at potential of 0~1 000 mV (Arrows indicate scan potential direction, scan potential is initiated from 1 000 mV)
3) 阴极还原产物的阳极氧化
由图1可看出,在与A0和A0′峰的同一电位区,循环回扫后则形成很大的峰A1和峰A1′,旋转电极曲线中的A1峰远大于静止电极的A1′峰,达到4 mA/cm2。这说明一方面电极旋转使电化学反应浓差极化的作用减少,另一方面经过较负电位下扫描反应对它们的形成有重要的作用。
不同初始扫描电位的循环伏安图说明了以上问题。由图6可知,初始扫描电位在300 mV以上的曲线上显示的阳极氧化峰特别微小。且初始扫描电位越向负方向, 处于400~800 mV范围的波峰A1就越大。由此可以推测,A1峰表示停留在电极表面的阴极还原产物被阳极氧化而形成的电流峰。黄铜矿晶格中的Fe3+被完全还原出来以后,产物为Cu2S, 结合反应方程式(6),峰A1和A1′代表的反应可表示为


图6 黄铜矿旋转圆盘电极不同初始扫描电位的循环伏安曲线
Fig.6 Cyclic voltammograms obtained with rotate electrode over different application potential range (Arrows indicate potential scan direction)
为了进一步证实峰A1和A1′与阴极还原反应的关系以及它们对黄铜矿分解的重要意义,在以上相同的实验条件下,比较了新鲜表面电极和恒电位φ=-600 mV还原后的电极同在恒电位φ=550 mV的I—t图,极化时间为100 s。黄铜矿在φ=550 mV恒电位下发生阳极氧化,电流从115 μA降至20 μA左右(见图7(a))。φ=-600 mV还原以后的电极在恒电位φ=550 mV阳极氧化,电流从4.7 mA降至0.2 mA左右,比直接阳极氧化增大了几十倍(见图7(b))。以上说明黄铜矿在φ=550 mV阳极氧化很微弱,而阴极还原后形成的表面中间产物则很容易被阳极氧化。这些结果与以上循环伏安法得到的结论是一致的。黄铜矿电极在电位φ=-600 mV下发生的阴极还原反应也比较强烈,电流从-6.3 MA升至-2.8 mA左右(见图8)。

图7 不同黄铜矿电极在外加恒电位为500 mV时的I—t曲线
Fig.7 I—t curves representing deoxidized chalcopyrite at fixed potential of 550 mV: (a) Fresh electrode; (b) Reducted electrodes over potential -600 mV
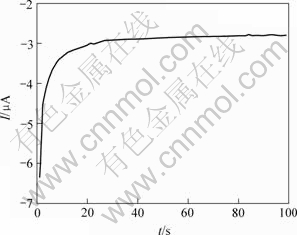
图8 黄铜矿在外加恒电位为-600 mV的I—t曲线
Fig.8 I—t curve representing reduction of chalcopyrite at fixed potential of -600 mV
3 结论
1) 在常温酸性条件下,黄铜矿的阳极氧化很微弱,分析认为分解过程有中间钝化产物和单质元素硫生成。
2) 在一定外加负电位下,黄铜矿阴极还原反应比阳极氧化反应要强烈得多,尤其是在电位小于-400 mV的情况下。当阴极负电位达到一定值, Fe3+被完全还原出来并形成稳定的固体产物Cu2S。这种中间物质不容易脱落,且在氧化电位下能发生较快的阳极氧化反应,但是随后又形成钝化层CuxS。
REFERENCES
[1] 许志华. 铜工艺矿物学[J]. 广州有色金属学报, 1999, 9(1): 1-8.
XU Zhi-hua. Technological mineralogy of copper[J]. Journal of Guangdong Non-Ferrous Metals, 1999, 9(1): 1-8.
[2] 李宏煦, 王淀佐. 硫化矿细菌浸出的半导体能带理论分析[J]. 有色金属, 2004, 56(3): 36-48.
LI Hong-xu, WANG Dian-zuo. Fundamental analysis of sulfide bioleaching process based on semiconductor electrochemistry[J]. Nonferrous Metals, 2004, 56(3): 36-48.
[3] Warren G W, Wadsworth M E, EI-Raghy S M. Anodic behavior of chalcopyrite in sulfuric acid[J]. Metallurgical Soc of AIME, 1982, 13(4): 261-275.
[4] Vaughan D J. Atmospheric and electrochemical oxidation of the surface of chalcopyrite[J]. Geochimica et Cosmochimica Acta, 1995, 59(1): 1091-1100.
[5] Yin Q, Vaughan D J, Kelsall G H, England K E R. Electrochemical oxidation of covellite(CuS) in alkaline solution[J]. Journal of Colloid and Interface Science, 1994, 59(6): 133-142.
[6] Klauber C, Parker A, Bronswijk W, Watling H R. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy[J]. Mineral Process, 2001, 62(1): 65-94.
[7] Hack R P, Dreisinger D B, Peters E, King J A. Passivation of chalcopyrite during oxidative leaching in sulfate media[J]. Hydrometallurgy, 1995, 39(3): 25-49.
[8] Dutrizac J E. Elemental sulphur formation during the ferric chloride leaching of chalcopyrite[J]. Hydrometallurgy, 1990, 23(2): 153-176.
[9] Stott M B, Watling H R, franzmann P D, Sutton D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching[J]. Mineral Engineering, 2000, 13(1): 1117-1127.
[10] Woods R, Hope G A. Spectroelectrochemical investigation of the intersection of o-isopropyl-nethylthionocarbomate with copper surfaces[J]. Colloids Surf, 1999, 146(1): 63-74.
[11] Buckley A N, Woods R. Under potential deposition of dithiophosphate on chalcocite[J]. Electroanal Chem, 1993, 357(1): 387-405.
[12] Koch D F A, McIntyre R. The application of reflectance spectroscopy to a study of the anodic oxidation of cuprous sulphide[J]. Electroanal Chem, 1976, 71(1): 285-296.
[13] 李宏煦, 邱冠周. 原电池效应对混合硫化矿细菌浸出的影响[J]. 中国有色金属学报, 2000, 13(5): 1284-1287.
LI Hong-xu, QIU Guan-zhou. Galvanic effect on mixed sulfide bioleaching[J]. The Chinese Journal of Nonferrous Metals, 2000, 13(5): 1284-1287.
[14] 欧乐明, 冯其明. 浮选过程中黄铜矿抑制的电化学研究[J]. 矿冶工程, 1999, 19(3): 68-86.
OU Le-ming, FENG Qi-ming. A study on electrochemistry of depressing chalcopyrite in flotation[J]. Mining and Metallurgical Engineering, 1999, 19(3): 68-86.
[15] Price D W, Warren G W. The influence of silver ion on the electrochemical response of chalcopyrite and other mineral sulfide electrodes in sulfuric acid[J]. Hydrometallurgy, 1986, 15(1): 303-324.
[16] Parker A J, Paul R L, Power G P. Electrochemistry of the oxidative leaching of copper from chalcopyrite[J]. Electroanal Chem, 1981, 118(1): 305-316.
[17] Elsherief A E. The influence of cathodic reduction Fe2+ and Cu2+ ions on the electrochemical dissolution of chalcopyrite in acidic solution[J]. Mineral Engineering, 2002, 15(1): 215-223.
基金项目:国家重点基础研究发展规划资助项目(2004 CB619204)
收稿日期:2006-04-10;修订日期:2006-06-26
通讯作者:卢毅屏,副教授,博士研究生;电话:0731-8830913;E-mail: Luyp309@sohu.com
(编辑 李艳红)