文章编号:1004-0609(2008)05-0794-05
Ti/ZrO2和TiAl/ZrO2反应的差热分析
刘爱辉,李邦盛,隋艳伟,于 杰,郭景杰,傅恒志
(哈尔滨工业大学 材料科学与工程学院,哈尔滨150001)
摘 要:借助SEM和差热分析研究Ti-ZrO2(CaO)和TiAl-ZrO2(CaO)体系在不同加热速率时的界面显微组织和反应温度,并利用Kissinger方程对反应动力学参数进行计算。结果表明:随着加热速率的提高,Ti-ZrO2(CaO)和TiAl-ZrO2(CaO)体系的化学反应逐渐减弱,初始反应温度逐渐升高;Al元素的加入使Ti和ZrO2(CaO)的初始反应温度提高近170 ℃,从而弱化Ti与ZrO2(CaO)的界面反应;Ti-ZrO2(CaO)体系和TiAl-ZrO2(CaO)体系反应活化能分别为1 348 kJ/mol和1 675 kJ/mol,反应级数分别为1.51和1.59。
关键词:氧化锆;差热分析;反应;显微组织
中图分类号:TG 146.2 文献标识码:A
Differential thermal analysis of reactions in Ti/ZrO2 and TiAl/ZrO2 systems
LIU Ai-hui, LI Bang-sheng, SUI Yan-wei, YU Jie, GUO Jing-jie, FU Heng-zhi
(School of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150001, China)
Abstract: The microstructures and the reaction temperatures of Ti-ZrO2(CaO stabilized) and TiAl-ZrO2(CaO stabilized) systems were researched at different heating rates by SEM and differential thermal analysis(DTA), and the reaction kinetics parameters in the two systems were calculated by means of Kissinger equation. The results show that, with the increase of heating rates, the chemical reactions of the two systems are weakened gradually, and the initial reaction temperatures increase. Compared to Ti-ZrO2(CaO stabilized) system, the initial reaction temperature in the TiAl-ZrO2(CaO stabilized) system increases about 170 ℃ and the interfacial reaction between Ti and ZrO2(CaO stabilized) is weakened. The activation energies of the two systems are 1 348 kJ/mol and 1 675 kJ/mol, and the reaction orders are 1.51 and 1.59, respectively.
Key words: zirconia; differential thermal analysis; reaction; microstructure
钛合金具有优异的比强度、比刚度、耐蚀性能、高温力学性能和抗氧化性能,是极具潜力的轻质高温高强结构材料,已经在航空航天、舰船制造及汽车等领域崭露头角
[1-5]。在钛合金的众多成形工艺中,精密铸造技术以其材料利用率高,生产成本低,能够成形复杂构件而受到国内外的广泛关注
[6-8]。然而,由于钛合金在高温熔融状态下具有极高的化学反应活性,在熔模铸造过程中几乎与所有的耐火材料发生界面化学反应,导致铸件表面产生α污染层
[9-7]和气孔等铸造缺陷,显著降低了铸件的表面质量和力学性能
[9-12],成为阻碍钛合金铸造工艺发展的重要原因。因此,如何提高界面反应温度和控制界面反应进程对获得高质量的钛铝基合金铸件具有重要意义。
本文作者对Ti-ZrO2(CaO)体系和TiAl-ZrO2(CaO)体系在不同加热速率下的界面反应组织形貌和初始 反应温度进行了研究,考察Al对Ti和ZrO2(CaO)界面反应进程和初始反应温度的影响,并测定Ti-ZrO2(CaO)体系和TiAl-ZrO2(CaO)体系的相关动力学参数,以期进一步控制钛合金与氧化锆陶瓷铸型间的界面反应,制造优质的钛合金铸件。
1 实验
选用粒度均为45 μm的高纯度钛粉、铝粉和氧化钙稳定的氧化锆(ZrO2(CaO))粉,纯钛粉和钛铝粉分别与氧化钙稳定的氧化锆(ZrO2(CaO),45 μm)陶瓷粉按预定的质量比充分混合,在60 t万能材料实验机上压制成直径为20 mm,高为10 mm的预制块,再将预制块加工成直径为3 mm,高为6 mm的圆柱试样,在法国SETARAM公司的SETSYS Evolution 16/18热分析仪上进行热分析实验,测得不同加热速率下的DTA曲线。Ti-ZrO2(CaO)试样的加热速率分别为30、40和 50 ℃/min,加热温度为1 570 ℃;TiAl-ZrO2(CaO)试样的加热速率分别为20、30和40 ℃/min,加热温度为1 440 ℃。将差热分析后的试样经手工磨制、机械抛光和V(HF)?V(HNO3)?V(H2O)=1?3?5腐蚀液腐蚀后,借助S-570型扫描电镜观察其组织形貌。
2 结果与分析
2.1 反应产物的形貌
图1和2所示分别是钛粉、钛铝粉和氧化锆粉在不同加热速率下进行DTA测试后所得试样的SEM像(图中的黑色部分是钛与氧化锆的界面反应产物,白色的为氧化锆,灰色的为未反应的钛或钛铝与氧化锆的混合物)。由图1可见,当加热速率为30 ℃/min时,试样中的黑色反应产物最多,说明此时Ti与ZrO2(CaO)的反应较充分;当加热速率为40 ℃/min时,试样中的反应产物与加热速率为30 ℃/min时的相比明显减少,白色氧化锆和灰色钛明显增多,界面反应明显减弱;当加热速率为50 ℃/min 时,试样中遍布了未反应的钛和氧化锆的固相混合物,界面反应明显弱于前两种情况,这说明,加热速率越高,钛与氧化锆的界面反应越不充分。在上述3种加热速率情况下,加热速率为30 ℃/min时,界面反应最充分,反应产物也最多遍布整个试样;而当加热速率为50 ℃/min时,界面反应产物最少。
由图2中可看出,当加热速率为20和30 ℃/min时,界面反应的激烈程度相近,反应产物为块状,局部反应产物已经结合在一起;而当加热速率为40 ℃/min时,界面反应与前两者相比最弱,反应产物细小均匀,弥散分布在整个TiAl-ZrO2(CaO)体系中;而TiAl- ZrO2(CaO)的反应产物较少,说明Al元素的加入在一定程度上缓和了Ti和ZrO2(CaO)间的化学反应。
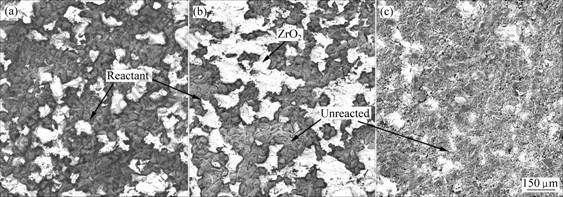
图1 Ti-ZrO2(CaO)试样以不同升温速率加热至1 570 ℃时的微观组织
Fig.1 Microstructures of Ti-ZrO2 (CaO stabilized) specimens heated to 1 570 ℃ at different heating rates: (a) 30 ℃/min; (b) 40 ℃/min; (c) 50 ℃/min
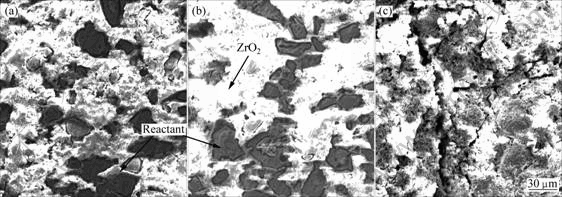
图2 TiAl-ZrO2(CaO)试样以不同升温速率加热至1 440 ℃时的微观组织
Fig.2 Microstructures of TiAl-ZrO2(CaO stabilized) specimens heated to 1 440 ℃ at different heating rates: (a) 20 ℃/min; (b) 30 ℃/min; (c) 40 ℃/min
2.2 Ti-ZrO
2(CaO)体系界面反应DTA分析
钛合金与陶瓷铸型间的界面反应是放热反应,其反应的激烈程度,可以用二者之间的反应热效应作为衡量指标,即反应越剧烈,放热量越大。在钛合金与陶瓷耐火材料的研究中,通常采用座滴法,将钛合金块状试样置于陶瓷基片上保持面接触,然后加热至高温,研究金属与陶瓷反应后界面处及金属侧的组织形貌、相组成和元素分布等。但对于反应热效应的测量来说,由于钛合金和陶瓷基片的接触面积过小,因而反应放热量也很小,以致无法进行精确测量,从而不能真实反映钛合金与陶瓷型壳间的界面反应进程。
本文作者采用将金属粉和陶瓷粉均匀混合的方法,借助DTA差热分析技术测试钛合金与氧化锆陶瓷耐火材料间的反应热效应。由于钛合金与陶瓷耐火材料为粉状混合,大幅度增加了反应接触面积,使反应进行得更充分,反应放热量也显著增加,从而得以精确测定,并可准确反映Ti、TiAl与ZrO2(CaO) 陶瓷耐火材料间的反应动力学过程,并确定出Ti和TiAl合金与ZrO2(CaO)陶瓷耐火材料间发生强烈界面反应的温度以及相关动力学参数,为理论和数值模拟研究提供了理论依据。
图3所示为Ti-ZrO2(CaO)体系加热速率分别为30、40和50 ℃/min的DTA曲线。从图3中可以看出,当加热速率为30 ℃/min时,DTA曲线变化比较平缓,只在1 233 ℃附近有放热峰B,该峰是Ti与ZrO2(CaO)发生强烈化学反应而引起的放热所致;当加热速率为40 ℃/min时,在800~1 100 ℃间存在一个峰值约为 1 000 ℃的缓慢放热峰A,该峰为Ti与ZrO2(CaO)发生少量固态扩散而引起的微弱固相放热反应所致,在1 237.40 ℃产生的放热峰B为Ti与ZrO2(CaO)发生 反应所对应的峰顶温度;当加热速率为50 ℃/min时,Ti-ZrO2(CaO)体系的DTA曲线的峰形变化与加热速率为40 ℃/min时的相同,只是Ti与ZrO2(CaO)发生反应的峰顶温度升至1 240.26 ℃,对应峰为峰B。
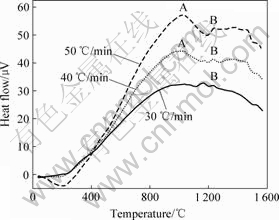
图3 不同升温速率下Ti-ZrO2(CaO)体系的DTA曲线
Fig.3 DTA curves of Ti-ZrO2 (CaO stabilized) system at different heating rates
2.3 TiAl-ZrO2(CaO)体系界面反应DTA分析
图4所示为TiAl-ZrO2(CaO)体系加热速率分别为20、30和40 ℃/min的DTA曲线。图4与图3相比,多出了一个放热峰C和一个吸热峰D。在500 ℃以下低温区出现的不规则小峰值放热峰是由于体系中所吸附的有机物杂质发生分解和挥发所致,与Ti和ZrO2(CaO)间的反应无关;670 ℃附近出现的吸热峰D是由于铝熔化吸热,铝熔化以后发生的Ti和液态Al的反应为液固反应,形成了强烈的放热峰C,并生成TiAl相[13],由于Ti与ZrO2(CaO)的初始反应温度远高于 670 ℃,因此Ti和Al间的反应对TiAl与ZrO2(CaO)间的差热结果影响可以忽略;再继续加热至1 100 ℃时,出现缓慢放热峰A,是由于Ti与ZrO2的固相放热反应所致;随着温度的进一步升高,Ti与ZrO2(CaO)才发生反应,对应于图4中的放热峰B。当加热速率为20 ℃/min时,Ti与ZrO2(CaO)的峰顶温度为1 400.93 ℃;加热速率为30 ℃/min时,峰顶温度为 1 406.64 ℃;当加热速率为达到40 ℃/min时,Ti与ZrO2(CaO)发生反应的峰顶温度为1 410.47 ℃。从图4中可以看出,由于Al元素的加入,Ti与ZrO2(CaO)的反应温度升高,与图3相比,温度提高了大约 170 ℃。这主要是由于Al元素的大量加入,一方面是Al与Ti形成金属间化合物,减小了Ti与ZrO2(CaO)的接触面积;另一方面是由于Al降低了Ti的高温化学反应活性,提高了Ti进入ZrO2(CaO)晶格内的能量,从而使反应温度升高,界面反应减弱。
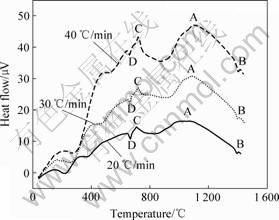
图4 不同升温速率下TiAl-ZrO2(CaO)体系的DTA曲线
Fig.4 DTA curves of TiAl-ZrO2 (CaO stabilized) system at different heating rates
2.4 反应动力学参数的确定
钛合金与铸型界面反应动力学直接影响铸钛工艺的拟定和钛铸件的质量,而反应动力学参数的确定是动力学研究的基础。本文作者采用Kissinger方法[14-16]对Ti和TiAl与ZrO2(CaO)间的化学反应进行了研究,反应的动力学方程为

将式(2)代入式(1),得
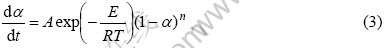
对式(3)两边微分,得
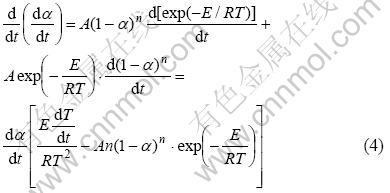
当T=Tm时,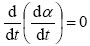 ,且n(1-α)n-1近似等于1,此时式(4)可表示为
,且n(1-α)n-1近似等于1,此时式(4)可表示为

对式(5)两边取对数,得Kissinger方程,即

确定出Ti-ZrO2(CaO)体系和Ti-ZrO2(CaO)体系在不同升温速率 时所对应的峰顶温度Tm,并计算得出1/Tm和
时所对应的峰顶温度Tm,并计算得出1/Tm和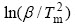 各数值,然后绘制出
各数值,然后绘制出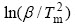 ~1/Tm的关系曲线(见图5和6)。根据直线的斜率计算出反应体系的活化能E,再根据Ti-ZrO2(CaO)体系和TiAl- ZrO2(CaO)体系中Ti与ZrO2发生化学反应时放热峰的形状,即峰形指数δ,由式(7)得出反应级数n,并进一步计算出频率因子A和反应速率常数k。表1列出了Ti-ZrO2(CaO)体系和TiAl-ZrO2(CaO)体系发生界面反应的动力学参数。
~1/Tm的关系曲线(见图5和6)。根据直线的斜率计算出反应体系的活化能E,再根据Ti-ZrO2(CaO)体系和TiAl- ZrO2(CaO)体系中Ti与ZrO2发生化学反应时放热峰的形状,即峰形指数δ,由式(7)得出反应级数n,并进一步计算出频率因子A和反应速率常数k。表1列出了Ti-ZrO2(CaO)体系和TiAl-ZrO2(CaO)体系发生界面反应的动力学参数。

表1 反应动力学数据
Table 1 Data of reaction kinetics
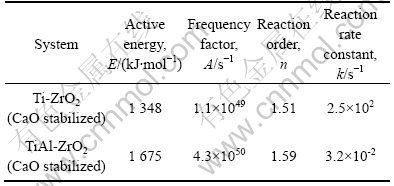
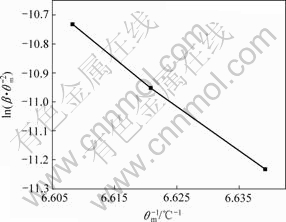
图5 Ti-ZrO2(CaO)的Kissinger曲线
Fig.5 Kissinger curve of Ti-ZrO2(CaO stabilized)
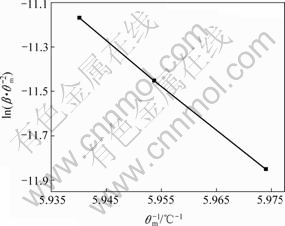
图6 TiAl-ZrO2(CaO)的Kissinger曲线
Fig.6 Kissinger curve of TiAl-ZrO2(CaO stabilized)
综上所述,在钛和氧化锆反应体系中加入Al能够显著地提高Ti与ZrO2(CaO)界面反应的活化能和初始反应温度,降低了反应速率常数,从而增大了ZrO2(CaO)型壳耐火材料对Ti的高温化学稳定性,这与Ti-ZrO2(CaO)体系和TiAl-ZrO2(CaO)体系扫描电镜的观察结果一致。
3 结论
1) 加入大量Al、Ti和ZrO2(CaO)的初始反应温度为1 390 ℃,比Ti-ZrO2(CaO)体系的Ti和ZrO2(CaO)的初始反应温度提高了近170 ℃,因而TiAl合金与ZrO2(CaO)型壳材料的反应稳定性要远高于Ti和ZrO2(CaO)间的反应稳定性。
2) 利用Kissinger方程对Ti-ZrO2(CaO)体系和TiAl-ZrO2(CaO)体系的动力学参数计算得出,Ti-ZrO2(CaO)体系的活化能为1 348 kJ/mol,反应速率常数为2.5×102,反应级数为1.51;TiAl-ZrO2(CaO)体系的活化能为1 675 kJ/mol,反应速率常数为3.2×10-2,反应级数为1.59。
REFERENCES
[1] NISHIKIORI S, TAKAHASHI S, YUNOKI N, OHKITA A. Manufacturability of TiAl alloys for turbocharger applications[J]. JOM, 2004, 56(11): 227-228.
[2] HODQE A M, HSIUNG L M, NIEH T G. Creep of nearly lamellar TiAl alloy containing W[J]. Scripta Materialia, 2004, 51(5): 411-415.
[3] 李 梁, 孙健科, 孟祥军. 钛合金的应用现状及发展前景[J]. 钛工业进展, 2004, 21(5): 19-24.
LI Liang, SUN Jian-ke, MENG Xiang-jun. Application state and prospects for titanium alloys[J]. Titanium Industry Progress, 2004, 21(5): 19-24.
[4] LIU K, MA Y C, GAO M, RAO G B, LI Y Y, WEI K, WU Xin-hua, LORETTO M H. Single step centrifugal casting TiAl automotive valves[J]. Intermetallics, 2005, 13(9): 925-928.
[5] DIMCIC B, JOVANOVIC M T, BOBIC I, ZEC S, TADIC S. TiAl-A new high temperature material for turbocharger rotors[J]. Materials Science Forum, 2004, 453/454: 169-174.
[6] 胡桂珍, 邬彦如. 钛及钛合金铸造[J]. 稀有金属材料与工程, 1995, 24(3): 26-30.
HU Gui-zhen, WU Yan-ru. Titanium and titanium alloys casting[J]. Rare Metal Mater Eng, 1995, 24(3): 26-30.
[7] LARSEN D E, GOVERN C. Gamma titanium aluminides[J]. JOM, 1995, 47(7): 38.
[8] DONALD E, LARSEN D E Jr. Status of investment cast gamma titanium aluminides in the USA[J]. Mater Sci Eng A, 1996, A213(1/2): 128-133.
[9] BARBOSA J, PUGA H, RIBEIRO C S, TEODORO O M N D, MONTEIRO A C. Characterization of metal/mould interface on investment casting of γ-TiAl[J]. International Journal of Cast Metals Research, 2006, 19(6): 331-338.
[10] SUNG S Y, KIM Y J. Alpha-case formation mechanism on titanium investment castings[J]. Mater Sci Eng A, 2005, A405(1/2): 173-177.
[11] KUANG J P, HARDING R A, CAMPBELL J. Microstructures and properties of investment castings of γ-titanium aluminide[J]. Mater Sci Eng A, 2002, A329/331: 31-37.
[12] KIM M G, SUNG S Y, KIM Y J. Microstructure, metal-mold reaction and fluidity of investment cast-TiAl alloys[J]. Materials Transactions, 2004, 45(2): 536-541.
[13] 王衍行, 林均品, 贺跃辉, 王艳丽, 陈国良. 元素粉末Ti与Al反应激励的研究进展[J]. 材料导报, 2007, 21(1): 83-85.
WANG Yan-hang, LIN Jun-pin, HE Yue-hui, WANG Yan-li, CHEN Guo-liang. Progress in reactive mechanism of Ti with Al elemental powders[J]. Materials Review, 2007, 21(1): 83-85.
[14] 沈 兴. 差热、热重分析与非等温固相反应动力学[M]. 北京: 冶金工业出版社, 1995: 112.
SHEN Xing. DTA, TG and non-isothermal solid phase reaction kinetics[M]. Beijing: Metallurgical Industry Press, 1995: 112.
[15] 蔡正千. 热分析[M]. 北京: 高等教育出版社, 1993: 113.
CAI Zheng-qian. Thermal analysis[M]. Beijing: Higher Education Press, 1993: 113.
[16] 胡荣祖, 史启祯. 热分析动力学[M]. 北京: 科学出版社, 2001: 65.
HU Rong-zu, SHI Qi-zhen. Thermal analysis dynamics[M]. Beijing: Science Press, 2001: 65.
基金项目:国家自然科学基金资助项目(50434030)
收稿日期:2007-08-24;修订日期:2008-11-10
通讯作者:李邦盛,教授,博士;电话:0451-86412198;E-mail: hlglah@tom.com
(编辑 李艳红)