Trans. Nonferrous Met. Soc. China 23(2013) 1179-1183
Thermodynamics of leaching roasted jarosite residue from zinc hydrometallurgy in NH4Cl system
Shao-hua JU1,2,3, Li-bo ZHANG1,2,3, Jin-hui PENG1,2,3, Zhe SHI3, Sheng-hui GUO1,2,3, Bin-guo LIU1,2,3, Ya-jian WANG1
1. Key Laboratory of Intensification Metallurgy in Yunnan Province, Kunming 650093, China;
2. Key Laboratory of Unconventional Metallurgy, Ministry of Education, Kunming University of Science and Technology, Kunming 650093, China;
3. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China
Received 6 January 2012; accepted 3 December 2012
Abstract: The thermal decomposition process of jarosite residue and the solubility of various oxides presented in the decomposed residue in NH4Cl-H2O system were studied. The results of heat decomposition of jarosite residue show that the insoluble ZnFe2O4 phase in the residue can be decomposed at temperatures ranging from 500 °C to 650 °C for 1 h. The OLI Systems software was used to study the thermodynamics of the solubility of various metal oxides existing in the decomposed residue in NH4Cl-H2O system. The results show that the solubility of ZnO, PbO, CdO, CuO and Ag2O is high, while the solubility of Fe2O3 is less than 10-4 mol/L in the pH range from 4.0 to 9.0. The calculated data are in accordance with the experimental results.
Key words: jarosite residue; zinc hydrometallurgy; thermodynamics; NH4Cl system
1 Introduction
In China, the production capacity of zinc was reported to be more than 4.4 million tons in 2009, and it was expected to be more because a lot of new plants were built up in 2009-2012. About 70% of the zinc production was yielded by hydrometallurgy process, which will averagely produce 0.3 t of waste jarosite residue for yielding 1 t of zinc cathode. Thus, about one million tons of such jarosite can be produced in China every year.
This kind of residue contains not only some valuable metals such as Fe, Zn, Cu and Ag, but also some toxic elements such as Pb, Cd and As. Thus, it is very hard to treat the residue efficiently and profitably.
In our earlier study [1], a process of roasting, leaching and reduction using NH4Cl media was reported. The merits of the process are that: 1) ZnO·Fe2O3 can be decomposed during roasting; 2) Cl- ion or/and NH3 can form stable complexes with Zn, Pb, Ag, Cd and Cu; 3) Fe and As will not be dissolved due to the near neutral pH value of the solution.
FROST et al [2] studied the thermal decomposition of jarosites of potassium, sodium and lead, and found that mass loss of K-jarosite occurs in the temperature range of 130-330 °C and 500-622 °C and is attributed to dehydroxylation and desulphation.  and FRIEDRICH [3] studied the thermal decomposition of leach residue after neutral leaching process from zinc winning in a nitrogen atmosphere using DTA and TGA techniques, and found that ZnO·Fe2O3 would be decomposed to ZnO and Fe3O4 when the temperature is higher than 1100 °C.
and FRIEDRICH [3] studied the thermal decomposition of leach residue after neutral leaching process from zinc winning in a nitrogen atmosphere using DTA and TGA techniques, and found that ZnO·Fe2O3 would be decomposed to ZnO and Fe3O4 when the temperature is higher than 1100 °C.
Chemical modeling of aqueous electrolyte solutions is of great importance to the development, analysis, design, and control of hydrometallurgical processes. Thermodynamic model is extremely useful since it can accurately describe the behavior of electrolyte solutions over wide ranges of temperature, pressure, concentration and predict the solution properties up to saturation levels. There are a number of tools available for thermodynamic modeling of aqueous systems.
In general, Debye–Hückel model and its extensions [4], which have been presented by the Davis equation and the B-dot equation, are the most widely applied models. But these models are applicable only to dilute solutions. For the concentrated solutions, Bromley model and its extensions, Bromley–Zemaitis model [5,6], PITZER model [7], Electrolyte NRTL model [8], and UNIQUAC model [9-11], are applied successfully. The Bromley–Zemaitis model embedded within OLI Systems software [6], and the Electrolyte NRTL model in AspenPlusTM [9] are being utilized for modeling in industry.
Among them, OLI has the most comprehensive database and solver engine available for simulation of aqueous chemistry. A simple interface gives power to the nonexperts, enabling quick evaluation of process chemistry issues and significant savings in laboratory testing. It has been successfully used to simulate a number of systems, including the dissolution behavior of Fe, Co, and Ni from nonferrous smelter slag in aqueous sulphur dioxide [12], thermodynamic equilibrium of the O2-ZnSO4- H2SO4-H2O system from 25 to 250 °C [13], and the thermodynamic equilibrium of monometallic sulphate solutions Al2(SO4)3-H2SO4, MgSO4-H2SO4, NiSO4-H2SO4, Fe2O3-H2SO4, ternary Al-Mg-H2SO4 solutions and real laterite leaching solutions at 230-270 °C [14].
The solubility of pure ZnO or pure CuO in NH4Cl solution has been studied separately [15]. However, no simulation of such a complicated system of jarosite residue in NH4Cl has been studied.
The present work attempts to investigate the basic theory of NH4Cl leaching through application of thermodynamic modeling. Firstly, the XRD patterns of the roasted jarosite residue at different temperatures, from 300 °C-850 °C with an interval of 50 °C, were obtained to analyze the phase transition at each temperature. Then, the OLI Systems software was utilized to estimate the thermodynamics of leaching jarosite in NH4Cl media.
2 Basic theory of roasting
The fresh sample of potassium jarosite residue was obtained from Baiyin Nonferrous Metals Group, China. After being dried for 12 h at 80 °C, the chemical composition of the sample was analyzed and the result is shown in Table 1. A series of roasting experiments were conducted to obtain the optimal roasting conditions.
Table 1 Chemical composition of jarosite residue (mass fraction, %)
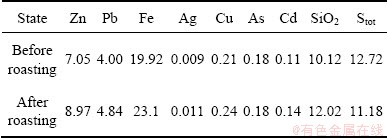
10 g of the dried jarosite sample was put into a ceramic crucible, and then was placed into a muffle furnace. The temperature of the muffle furnace was set at a desired temperature ranging from 300 to 850 °C, at an interval of 50 °C. XRD analyses for each roasted sample were performed to investigate the decomposition of the residue.
The XRD results of main phases in the original and roasted residues at each temperature are shown in Fig. 1.
It is shown in Fig. 1 that the main phases of the original residue are K2Fe6(SO4)4(OH)12 and ZnFe2O4. When the roasting temperature is below or equal to 350 °C, the main phases do not change, while the diffraction intensity of K2Fe6(SO4)4(OH)12 phase decreases quickly, indicating that the jarosite begins to decompose. In the temperature range of 400-450 °C, the main phases change to Fe2SO4 and ZnFe2O4. In the temperature range of 550-650 °C, the main phase is Fe2O3, showing that Fe2(SO4)3 and ZnFe2O4 have decomposed in this temperature range. In the temperature range of 700-850 °C, the main phases are ZnFe2O4 and Fe2O3, indicating the formation of ZnFe2O4 again at higher temperature.
This explains that the concentration of zinc leaching is high with NH4Cl solution for the residue roasted in the temperature range of 500-650 °C, while the Zn concentration is low at other temperatures.
3 Thermodynamic modeling using OLI Systems software
The equilibrium solubility of various metal oxides such as ZnO, CuO, PbO, Ag2O, CaO, MgO and Fe2O3 in NH4Cl media, is the main concern during the thermodynamic modeling of leaching.
In NH4Cl system, the reaction is very complicated because NH3 and Cl- are both strong ligands for metal ions such as Zn2+, Cu2+, Pb2+, Ag+ and Cd2+, as their coordination numbers are very high. Additionally, there are many insoluble solids produced during leaching procedures.
The data input to the OLI Systems for modeling and estimating the equilibrium conditions of leaching are listed in Table 2. The concentration of each species is got by the experiments and from the reported data in earlier work [1].
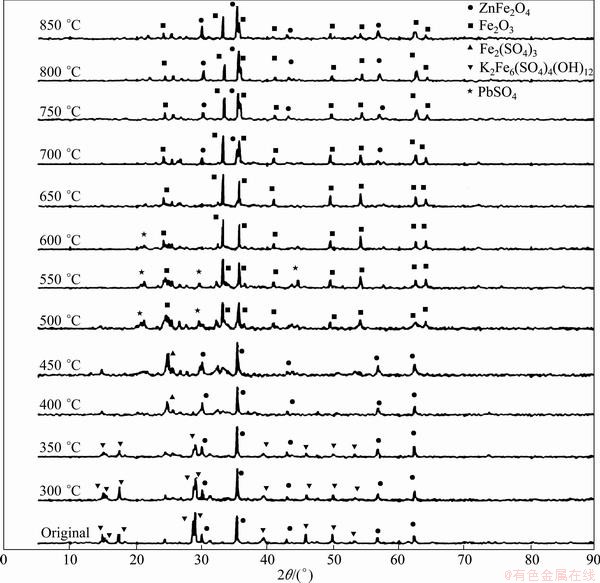
Fig. 1 XRD patterns of jarosite residue after roasting at different temperatures for 1 h
Table 2 Initial data input to OLI Systems for modeling of leaching

4 Modeling result of leaching procedure
The solubility results of various metal oxides at different pH values estimated by the OLI Systems are shown in Fig. 2.
Figure 2 shows that the solubilities of oxides of Zn, Cu, Ag and Pb are very high in this system in all pH ranges, and the variation rule of the concentration of their main complexes are also presented. However, iron oxide has a very low solubility in the pH range from 4.0 to 9.0; the oxides of Ca and Mg have a relatively high solubility in the pH lower than 10.0, and a relative low solubility when the pH is increased to more than 10.0.
5 Conclusions
The roasting of jarosite residue showed that ZnFe2O4 in the residue can be decomposed in the temperature range of 500 °C-650 °C for duration of 1 h.
OLI Systems was used to model the leaching process of valuable metal oxide in NH4Cl system. The results showed that the solubility of oxides of Zn, Cu, Ag and Pb is very high, while the solubility of Fe2O3 is less than 10-4 mol/L in the pH range from 4.0 to 9.0. These data are in accordance with the experimental results.

Fig. 2 Solubility of oxides of Fe, Zn, Pb, Cu, Ag, Ca and Mg in NH4Cl solution
References
[1] JU S, ZHANG Y, ZHANG Y, XUE P, WANG Y. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy [J]. Journal of Hazardous Materials, 2011, 192: 554-558.
[2] FROST R L, WEIER M L, MARTENS W. Thermal decomposition of jarosites of potassium, sodium and lead [J]. Journal of Thermal Analysis and Calorimetry, 2005, 82(1): 115-118.
[3]  S, FRIEDRICH B. Kinetics and mechanism of thermal zinc-ferrite phase decomposition [C]//Europe Metallurgical Conference. Innsbruck, Austria, 2009: 1167-1181.
S, FRIEDRICH B. Kinetics and mechanism of thermal zinc-ferrite phase decomposition [C]//Europe Metallurgical Conference. Innsbruck, Austria, 2009: 1167-1181.
[4] HELGESON H C. Thermodynamics of hydrothermal systems at elevated temperatures and pressures [J]. American Journal of Science, 1969, 267: 729-804.
[5] BROMLEY L A. The thermodynamic properties of strong electrolytes in aqueous solutions [J]. AiChE Journal, 1973, 19: 313-320.
[6] CASAS J M, PAPANGELAKIS V G, LIU H. Performance of three chemical models on the high-temperature aqueous Al2(SO4)3- MgSO4-H2SO4-H2O system [J]. Industrial and Engineering Chemistry Research, 2005, 44: 2931-2941.
[7] PITZER K S, SILVESTER L F. Thermodynamics of electrolytes. VI. Weak electrolytes including H3PO4 [J]. Journal of Solution Chemistry, 1976, 5: 269-278.
[8] CHEN C C, EVANS L B. A local composition model for the excess Gibbs energy of aqueous electrolyte systems [J]. AiChE Journal, 1986, 32: 444-454.
[9] HAGHTALAB A, PAPANGELAKIS V G, ZHU X. The electrolyte NRTL model and speciation approach as applied to multicomponent aqueous solutions of H2SO4, Fe2(SO4)3, MgSO4 and Al2(SO4)3 at 230-270 °C [J]. Fluid Phase Equilibria, 2004, 220: 199-209.
[10] ABRAMS D S, PRAUSNITZ J M. Statistical thermodynamics of liquid mixtures: A new expression for the excess Gibbs energy of partly or completely miscible systems [J]. AiChE Journal, 1975, 21: 116-128.
[11] THOMSEN K, RASMUSSEN P, GANI R. Correlation and prediction of thermal properties and phase behavior for a class of aqueous electrolyte systems [J]. Chemical Engineering Science, 1996, 51: 3675-3683.
[12] GBOR PHILIP K, HOQUE S J, CHARLES Q. Dissolution behavior of Fe, Co, and Ni from non-ferrous smelter slag in aqueous sulphur dioxide [J]. Hydrometallurgy, 2006, 81: 130-141.
[13] LIU H, PAPANGELAKIS V G. Thermodynamic equilibrium of the O2-ZnSO4-H2SO4-H2O system from 25 to 250 °C [J]. Fluid Phase Equilibria, 2005, 234: 122-130.
[14] LIU H, PAPANGELAKIS, VLADIMIROS G. Chemical modeling of high temperature aqueous processes [J]. Hydrometallurgy, 2005, 79: 48-61.
[15] JU S, TANG M, YANG S, TANG C. Thermodynamics of Cu(II)-NH3-NH4C1-H2O System [J]. Transactions of Nonferrous Metals Society of China, 2005, 15(6): 1414-1419.
NH4Cl体系浸出焙烧后湿法炼锌铁矾渣的热力学
巨少华1,2,3,张利波1,2,3,彭金辉1,2,3,施 哲3,郭胜惠1,2,3,刘秉国1,2,3,王亚健1
1. 云南省特种冶金重点实验室,昆明 650093;
2. 昆明理工大学 非常规冶金教育部重点实验室,昆明 650093;
3. 昆明理工大学 冶金与能源工程学院,昆明 650093
摘 要:研究湿法炼锌铁矾渣的热分解过程,及其分解后各种氧化物在NH4Cl-H2O体系中的溶解度。铁矾渣的热分解实验结果表明,渣中不可溶物相ZnFe2O4在500~650 °C下焙烧1 h后发生分解。采用OLI System软件研究热分解得到的渣中各金属氧化物在NH4Cl-H2O溶液中的溶解度。结果表明,在pH为4.0~9.0范围内,ZnO、PbO、CdO、CuO和Ag2O的溶解度较大,而Fe2O3的溶解度仅为10-4 mol/L。计算结果与实验结果相吻合。
关键词:铁矾渣;湿法炼锌;热力学;NH4Cl体系
(Edited by Sai-qian YUAN)
Foundation item: Project (51090385) supported by the National Natural Science Foundation of China
Corresponding author: Jin-hui PENG; Tel: +86-871-5147924; Fax: +86-871-5192076; E-mail: jhpeng72@hotmail.com
DOI: 10.1016/S1003-6326(13)62581-9