Trans. Nonferrous Met. Soc. China 24(2014) 1785-1790
Effect of different fillers on oxidation behavior of low-temperature chromizing coating
Jun-sheng MENG1,2, Ze-sheng JI1
1. College of Materials Science and Engineering, Harbin University of Science and Technology, Harbin 150040, China;
2. College of Materials Science and Engineering, Heilongjiang University of Science and Technology, Harbin 150022, China
Received 27 June 2013; accepted 12 November 2013
Abstract: Three different chromizing coatings were produced on Ni substrate using a conventional pack-cementation method with Al2O3, Al2O3+CeO2 and CeO2 acting as filler, respectively, at a greatly decreased temperature (700 °C). Effects of different fillers on the isothermal and cyclic oxidation resistance of chromizing coating in air at 850 °C were comparably investigated. Microstructure results show that the addition of CeO2 into the filler significantly retards the grain growth of the chromizing coating. Oxidation results indicate that the chromizing coating using CeO2 as filler exhibits somewhat increased oxidation resistance than the normal chromizing coating, while the chromizing coating using Al2O3+CeO2 as filler exhibits much better oxidation resistance. The effects of different fillers on the oxidation behaviors were discussed in detail.
Key words: chromizing coating; filler; CeO2; oxidation; reactive element effect
1 Introduction
Chromium coatings, which were traditionally manufactured using a conventional pack cementation at temperatures above 1000 °C for duration of 6-10 h and limited by the diffusion and reaction kinetics involved, have been widespread to economically improve high temperature oxidation and corrosion resistance of metals [1-5]. Recently, a novel ultrafine-grained chromizing coating produced at low temperatures on the nanostructure materials was developed [6-8]. After some RE or RE oxides were added into the chromizing coatings through various techniques before chromizing, the oxidation resistance could be effectively improved [1-5,9,10]. The phenomenon was first reported in 1937 [11] and was referred to as “reactive element effect (REE)”. Various theories to elucidate the REE had been put forward but still were in dispute [12]. However, these methods not only added their technological step, but also improved the difficulty in preparation for the chromizing coatings. It was reported that the filler Al2O3 or Y2O3 particles could be entrapped into the outer layer of aluminide coating [13-15] due to the outer growth of the aluminide coating. ZHOU et al [10] found that the outer growth also contributed to the formation of chromizing coating at low temperature. By using CeO2 particles instead of part of Al2O3 acting as filler, the CeO2 was successfully entrapped into the low-temperature chromizing coatings [16]. Compared with other methods adding the REO into the chromizing coating, this method was relatively simple and only common chromizing equipment rather than any other special equipment was needed. Based on these works, the objective of the present work was to analyze the effect of different fillers on the isothermal and cyclic oxidation behavior of the low-temperature chromizing coatings.
2 Experimental
Samples with dimensions of 15 mm × 10 mm × 2 mm were cut from an electrolytic nickel plate. After being ultrasonically cleaned in acetone, the samples were aluminized using a conventional pack cementation in a homogeneous mixture of 75% Cr (mass fraction) powder with size of 75 mm as master alloy source, 20% inert fillers (namely 100% Al2O3; 50% CeO2 + 50% Al2O3; and 100% CeO2, respectively) and 5% NH4Cl as activator in a pure Ar atmosphere at 700 °C for 10 h, as listed in Table 1. Afterwards, the samples were brushed, cleaned in bubbling distilled water for 30 min and then ultrasonically cleaned in acetone to remove any loosely embedded pack particles.
Table 1 Sample of various chromizing coatings using different fillers

The isothermal oxidation experiments were carried out at 850 °C and cyclic oxidation at 850 °C was performed by automatically lifting samples from the hot zone of a vertical furnace after exposure period of 1 h followed with a 10 min cooling to room temperature. Mass changes of the oxidized specimens were measured after fixed time intervals using a balance with 0.01 mg sensitivity. The composition and phases of the various coatings before and after oxidation were investigated using Camscan MX2600FE type scanning electron microscope (SEM) equipped with energy dispersive spectroscopy (EDS) and D/Max-2500 pc type X-ray diffraction (XRD). The average chromium concentrations were analyzed using spot analysis at 1000 magnification. Ten replicate tests at different locations were carried out so as to minimize data scattering, and every value reported was an average of ten measurements. Electroless Ni-plating was plated on the surface of the oxidized specimens to prevent the spallation of the scales for observing cross-sections.
3 Results
3.1 Microstructure
Figure 1 shows the surface morphologies of different chromizing coatings. It is clear that the addition of CeO2 into the filler retards the grain growth of the chromizing coating. The grain refinement enhances the diffusion of chromium during pack cementation, leading to higher Cr content at a given distance from the coating surface, as shown in Fig. 2.
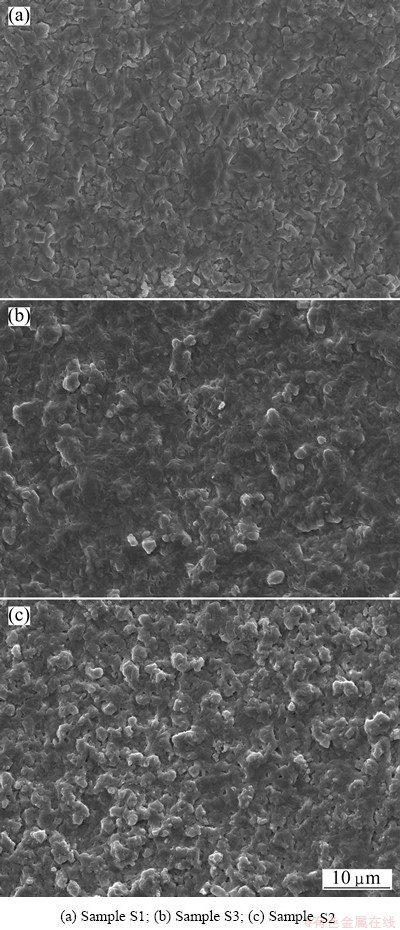
Fig. 1 SEM images of surface morphologies of various chromizing coatings
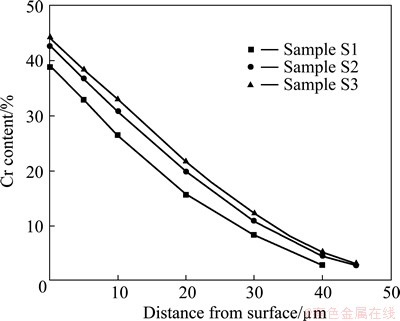
Fig. 2 Chromium content profiles for various chromizing coatings
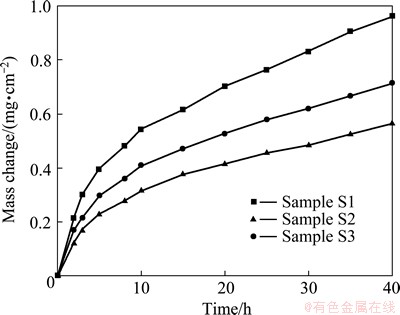
Fig. 3 Isothermal-oxidation kinetics of various chromizing coatings at 850 °C for 40 h
3.2 Isothermal oxidation
Figure 3 shows the isothermal-oxidation curves of various chromizing coatings in air at 850 °C for 40 h. During oxidation and cooling, no spallation occurs for the three chromizing coatings. All chromizing coatings obey the parabolic rate law to a good approximation. The parabolic rate constant is 6.4×10-12 g2·cm-4·s-1 for sample S1, 3.5×10-12 g2·cm-4·s-1 for sample S3, and 2.2×10-12 g2·cm-4·s-1 for sample S2, respectively. The results suggest that the addition of CeO2 into the fillers (samples S2 and S3) decreases the oxidation rate of the chromizing coatings, and sample S2 exhibits the best oxidation resistance.
XRD characterization shows that a mixture of NiO, NiCr2O4 and Cr2O3 is formed on sample S1 but only a mixture of Cr2O3 and Cr2O4 is formed on sample S3 and sample S2, as shown in Fig. 4. For oxidations of sample S3 and sample S2, a higher intensity peak from the coating substrate appears (Figs. 4(b) and (c)), suggesting that the chromia scale formed is thinner in this case. The above results suggest that the CeO2 addition suppresses the growth of NiO, and a purer and denser chromia scale is formed on samples S3 and S2, especially on sample S2. This is consistent with the SEM investigation, as shown in Figs. 5 and 6.
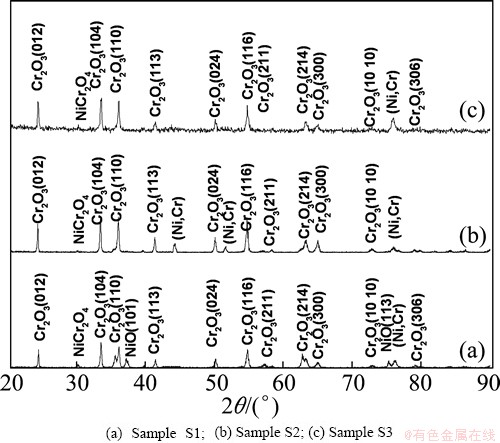
Fig. 4 XRD patterns of various chromizing coatings after isothermal oxidation at 850 °C for 40 h
Figure 5 shows the SEM top-views of the scales formed on various chromizing coatings after 40 h exposure at 850 °C. As compared to the chromia feature on sample S1 (Fig. 5(a)), the chromia grains on samples S3 and S2 have a noticeably finer-grain structure (Figs. 5(b) and (c)), especially on sample S2.
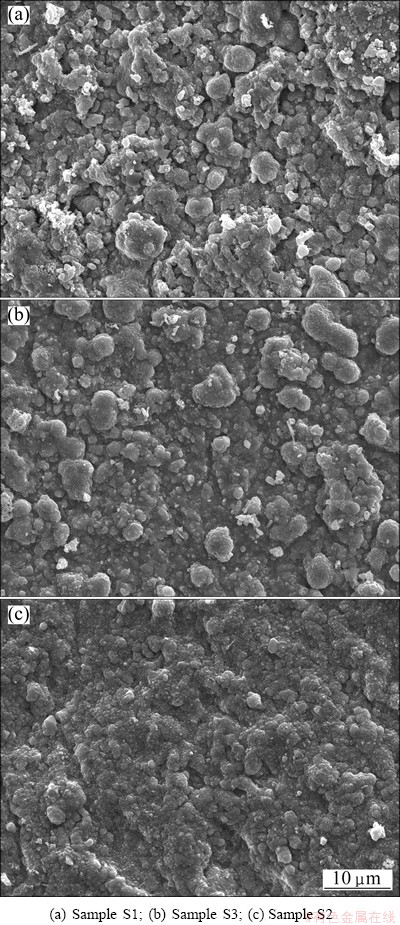
Fig. 5 Surface images of different specimens after isothermal oxidation at 850 °C for 40 h
By comparing the cross-sectional images of the chromia scale on the different coatings after isothermal oxidation in Fig. 6, it can be seen that thicker scales with significant voids are formed on sample S1 (Fig. 6(a)). However, thinner scales with less voids can be observed on sample S3 (Fig. 6(b)) and sample S2 (Fig. 6(c)), especially on sample S2.
3.3 Cyclic oxidation
Figure 7 shows the mass change versus time curves of the three chromizing coatings during 40 h cyclic oxidation in air at 850 °C. For sample S1, significant mass loss occurs at 25 cycles. However, minor mass loss occurs at 35 cycles for sample S3. In contrast, the oxidation rate of sample S2 steadily decreases, and the scale spallation dose not occur. Above results can also be seen from its top view of the oxidized samples shown in Fig. 8. Clearly, heavy spallation and the formation of nodules on the spallation area appear on sample S1. However, minor spallation with few cracks occurs on sample S3. In contrast, no spallation and cracks occurs on sample S2. At the same time, they are all somewhat convoluted. As compared to the chromia feature on the other two chromizing coatings (Figs. 8(a) and (b)), the chromia grains on sample S2 have a noticeably finer- grain structure, which is similar as isothermal oxidation results, as shown in Fig. 5.
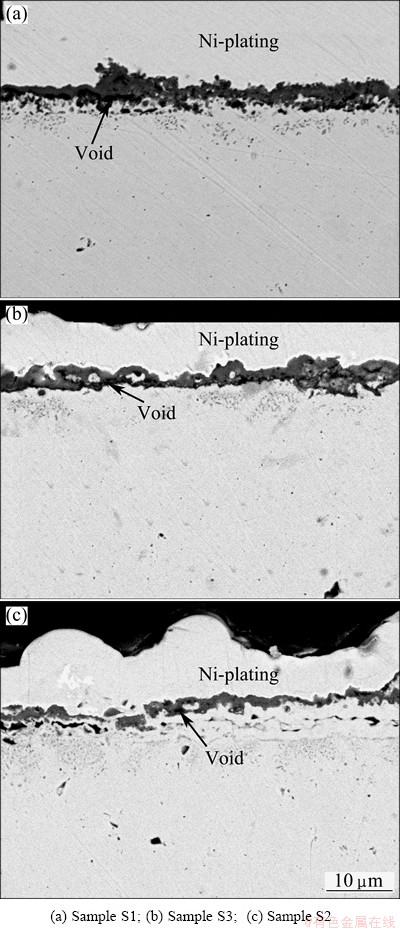
Fig. 6 Cross-sectional images of different specimens after 40 h isothermal-oxidation at 850 °C
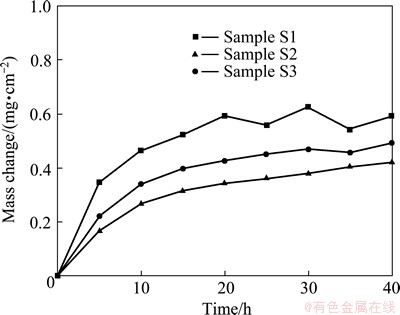
Fig. 7 Cyclic-oxidation kinetics of various chromizing coatings at 850 °C for 40 h

Fig. 8 Surface images of different specimens after cyclic-oxidation at 850 °C for 40 h
Figure 9 shows the cross sections of various samples after cyclic oxidation. Clearly, nodule oxides are observed on sample S1, which agree well with the top-surface SEM results in Fig. 8(a). From Figs. 9(b) and (c), it can be also found that a chromia scale formed on sample S2 is thinner than that on the other two chromizing coatings.
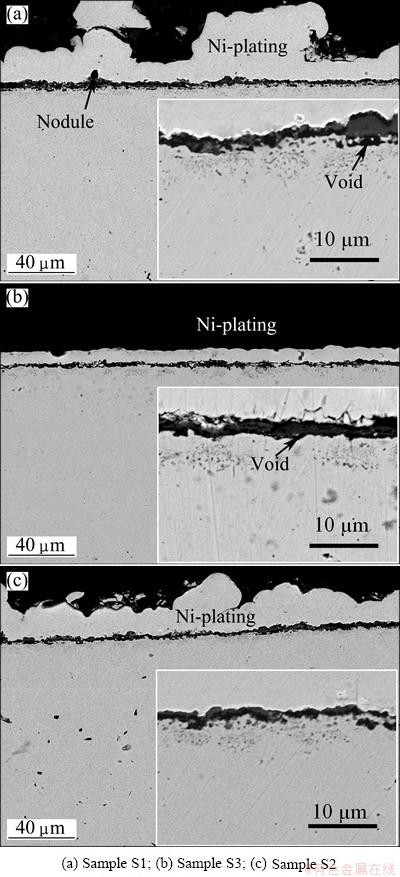
Fig. 9 Cross-sectional images of different specimens after 40 h cyclic-oxidation at 850 °C
4 Discussion
Previous results [10] indicated that even with 50% Cr, a NiO-rich outer layer can still form at 900 °C on the chromizing coatings. In this work, the chromizing coatings prepared on sample S1 have a coarse structure and low Cr concentration (low than 40%). In this case, they cannot exclusively develop a continuous chromia scale. For samples S2 and S3, with a finer grain structure and high Cr concentration, they can quickly and exclusively develop a continuous chromia scale in a short transient oxidation stage due to increased sites for chromia nucleating on the finer-grain boundaries and enhanced grain boundary diffusion of Cr to the oxidation front. Thus, a finer and compact chromia scale is formed on the samples S2 and S3, especially on sample S2. After the establishment of the continuous chromia scale, the scale growth rate should be faster on samples S2 and S3 than that on sample S1, because the chromia scale on samples S2 and S3 is finer grained, especially on sample S3. However, the case is not seen from the oxidation kinetics, suggesting that grain-boundary diffusion of Cr is hindered to a great extent. The reason may be that the chromia scale is incorporated by Ce ions released from the dispersed CeO2 when they are incorporated into the growing scale. Then, they will segregate to the scale grain-boundaries and then transport outwards, which are driven by the oxygen potential gradient between the interfaces of the metal/scale and the scale/gas [17]. Once incorporated into the growing scale, they would potentially improve the oxidation resistance by blocking the dominant grain boundary outward diffusion of chromium [17], leading to a decrease of oxidation and more rapid formation of a purer, denser chromia scale in a short transient oxidation stage. The comparison of the oxidations of samples S3 and S2 also exhibits that the higher CeO2 content in the filler causes worse isothermal oxidation resistance. The reason may be due to higher CeO2 particles content entrapped in the chromizing coating, which needs further investigations. However, the addition of CeO2 into the filler still improves the isothermal oxidation resistance of the chromizing coatings.
The higher oxidation rate will cause fast degradation of the chromizing coatings (Fig. 4) and formation of large interface cavities due to condensation of cation vacancies injected from the growing chromia scale for counterbalancing the outward Cr diffusion. The interface cavities greatly decrease the critical stress for scale decohesion. The spallation mainly occurs during cooling, due to the large thermal stress generated during cooling as a consequence of mismatching coefficients of thermal expansion between the alumina scale and the coating. The scale cracking and spallation expose the underlying coating directly to the air during the subsequent oxidation, which causes a high mass loss and the formation of oxides nodule on the spallation area, as shown in Fig. 8(a). However, with the addition of CeO2 particles into the filler, the scale formed is adherent due to the addressed reasons: 1) a low consumption rate of chromium by oxidation reduces the flux of the cation vacancies toward the interface, leading to a reduction of the interface voiding kinetics [1-5,9,10]; 2) CeO2 particles act as sinks for vacancies condensation to prevent the large interface cavity formation [18,19]; 3) CeO2 particles eliminate the unbeneficial sulfur effect [20]; and 4) fine-grained scale on samples S3 and S2 will be more adherent, because of easier operation of plastic deformation in the thermal cycling [21].
5 Conclusions
1) The addition of CeO2 into filler retards the grain growth of the chromizing coating.
2) The chromizing coating using CeO2 as filler exhibits somewhat increased oxidation resistance than the normal chromizing coating, while the chromizing coating using Al2O3+CeO2 as filler exhibits much better oxidation resistance.
References
[1] ZHU L, PENG X, YAN J, WANG F. Oxidation of a novel chromium coating with CeO2 dispersions [J]. Oxidation of Metals, 2004, 62: 411-426.
[2] YAN J, PENG X, WANG F. Oxidation of a novel CeO2-dispersion- strengthened chromium coating in simulated coal-combustion gases [J]. Materials Science and Engineering A, 2006, 426 : 266-273.
[3] PENG X, YAN J, XU C, WANG F. Oxidation at 900 °C of the chromized coatings on A3 carbon steel with the electrodeposition pretreatment of Ni or Ni-CeO2 film [J]. Metallurgical and Materials Transactions A, 2008, 39: 119-129.
[4] PENG X, YAN J, DONG Z, XU C, WANG F. Discontinuous oxidation and erosion-oxidation of a CeO2-dispersion-strengthened chromium coating [J]. Corrosion Science, 2010, 52: 1863-1873.
[5] PENG X, YAN J, ZHENG L, WANG F. Oxidation of a novel CeO2-dispersed chromium coating in wet air [J]. Materials and Corrosion, 2011, 62(6): 514-520.
[6] WANG Z B, TAO N R, TONG W P, LU J, LU K. Diffusion of chromium in nanocrystalline iron produced by means of surface mechanical attrition treatment [J]. Acta Materialia, 2003, 51: 4319-4329.
[7] WANG Z B, LU J, LU K. Chromizing behaviors of a low carbon steel processed by means of surface mechanical attrition treatment [J]. Acta Materialia, 2005, 53: 2081-2089.
[8] WANG Z B, LU J, LU K. Wear and corrosion properties of a low carbon steel processed by means of SMAT followed by lower temperature chromizing treatment [J]. Surface and Coatings Technology, 2006, 201: 2796-2801.
[9] ZHANG H, PENG X, ZHAO J, WANG F. Prior electrodeposition of nanocrystalline Ni-CeO2 film fabricating an oxidation-resistant chromized coating on carbon steels [J]. Electrochemical and Solid-State Letters C, 2007, 10(3): 12-5.
[10] ZHOU Y B, CHEN H, ZHANG H, WANG Y. Preparation and oxidation of an Y2O3-dispersed chromizing coating by pack-cementation at 800 °C [J]. Vacuum, 2008, 82: 748-753.
[11] PFEIL B. Improvement in heat-resisting alloys: UK, 459848 [P]. 1937.
[12] MOON D P. Role of reactive elements in alloy protection [J]. Material Science and Technology, 1989, 5: 754-764.
[13] GOWARD G W, BOONE D H. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys [J]. Oxidation of Metals, 1971, 3: 475-495.
[14] XIANG Z D, DATTA P K. Pack cementation process for the formation of refractory metal modified aluminide coatings on nickel-base superalloys [J]. Journal of Materials Science, 2003, 38: 3721-3728.
[15] ZHOU W, ZHAO Y G, LI W, TIAN B, HU S W, QIN Q D. Oxidation behavior of the Y2O3-modified aluminide coating on Ti-6Al-4V alloy [J]. Materials Science and Engineering A, 2007, 458: 34-38.
[16] SUN Jian-feng, ZHOU Yue-bo, ZHANG Hai-jun. Preparation and oxidation behavior of a novel CeO2-modified chromizing coating [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 1375-1381.
[17] PINT B A, HOBBS L W. Experimental observations in support of the dynamic segregation theory to explain the reactive-element effect [J]. Oxidation of Metals, 1996, 45: 1-37.
[18] KUMAR A, NASRALLAH M M, DOUGLASS D L. The effect of yttrium and thorium on the oxidation behavior of Ni-Cr-Al alloys [J]. Oxidation of Metals, 1974, 8: 227-263.
[19] BRUMM M W, GRABKE H J. Oxidation behaviour of NiAl—II cavity formation beneath the oxide scale on NiAl of different stoichiometries [J]. Corrosion Science, 1993, 34: 547-561.
[20] LEES D G. On the reasons for the effects of dispersions of stable oxides and additions of reactive elements on the adhesion and growth-mechanisms of chromia and alumina scales-the “sulfur effect” [J]. Oxidation of Metals, 1987, 27: 75-81.
[21] WANG F, TIAN X, LI Q, LI L, PENG X. Oxidation and hot corrosion behavior of sputtered nanocrystalline coating of superalloy K52 [J]. Thin Solid Films, 2008, 516: 5740-5747.
填充剂对低温渗铬涂层氧化性能的影响
孟君晟1,2,吉泽升1
1. 哈尔滨理工大学 材料科学与工程学院,哈尔滨 150040;2. 黑龙江科技大学 材料科学与工程学院,哈尔滨 150022
摘 要:分别采用Al2O3、Al2O3+CeO2和CeO2作为填充剂,于700 °C在Ni基体上进行低温渗铬,制备3种渗铬涂层,并在850 °C对其进行恒温和循环氧化性能对比研究,研究填充剂对渗铬涂层氧化性能的影响。结果表明:填充剂中CeO2颗粒的加入有利于得到晶粒细小的渗铬涂层。以纯CeO2作为填充剂制备的渗铬涂层,其抗氧化性能明显优于普通的以Al2O3作为填充剂制备的渗铬涂层,而以Al2O3+CeO2作为填充剂制备的渗铬涂层表现出最佳的抗氧化性能。详细分析不同填充剂对渗铬涂层氧化性能的影响。
关键词:渗铬涂层;填充剂;CeO2;氧化;活性元素效应
(Edited by Chao WANG)
Foundation item: Project (11551419) supported by Scientific Research Fund of Heilongjiang Provincial Education Department; Project (12511469) supported by Heilongjiang Provincial Science and Technology Department
Corresponding author: Jun-sheng MENG; Tel: +86-451-88036695; E-mail: mengjs2008@live.cn
DOI: 10.1016/S1003-6326(14)63254-4