文章编号:1004-0609(2014)03-0814-06
金精矿碘化浸出过程动力学
李绍英1, 2,王海霞3,袁喜振1, 2,赵留成1, 2,孙春宝1, 2
(1. 北京科技大学 金属矿山高效开采与安全教育部重点实验室,北京 100083;
2. 北京科技大学 土木与环境工程学院,北京 100083;
3. 山西中条山工程设计有限公司,运城 043700)
摘 要:采用碘-碘化物体系对金精矿进行浸出,通过实际矿石的浸出试验考察搅拌速度、浸出温度、碘浓度及碘离子浓度对金精矿碘化浸出反应动力学的影响。结果表明:提高搅拌速度对金的浸出有不利影响;通过对影响该体系动力学参数的考察,发现其符合核收缩模型,反应过程总体由界面化学反应控制,其表观活化能为31.674 kJ/mol,碘浓度和碘离子浓度的反应级数分别为1和0.5,建立了金精矿碘化浸出过程的反应速率方程。
关键词:金精矿;碘化浸出;动力学;表观活化能;界面化学反应
中图分类号:TF111 文献标志码:A
Leaching dynamics of gold concentrates by iodine-iodide solution
LI Shao-ying1, 2, WANG Hai-xia3, YUAN Xi-zhen1, 2, ZHAO Liu-cheng1, 2, SUN Chun-bao1, 2
(1. Key Laboratory for High-Efficient Mining and Safety of Metal Mines, Ministry of Education,
University of Science and Technology Beijing, Beijing 100083, China;
2. School of Civil and Environmental Engineering, University of Science and Technology Beijing, Beijing 100083, China;
3. Shanxi Zhongtiaoshan Engineering Design & Research Co., Ltd., Yuncheng 043700, China)
Abstract: Gold concentrate was leached by iodine-iodide solution system. The effects of different parameters such as stirring speed, leaching temperature, iodine concentration and iodide concentration on the gold leaching dynamics were investigated. The results show that increasing stirring speed has a negative influence on the gold leaching. Through the study of dynamic parameters of this system, it is found that gold concentrates leaching by iodine-iodide solution is in accordance with the shrinking core model. The overall reaction is controlled by the interfacial chemical reaction with the apparent activation energy of 31.674 kJ/mol. The reaction orders of iodine and iodide concentrations are 1 and 0.5, respectively. The leaching reaction velocity equation of the process is established.
Key words: gold concentrates; iodine leaching; dynamics; apparent activity energy; interfacial chemical reaction
黄金的使用可以追溯到公元前3400年,由于其迷人的金属光泽及独特的物理和化学性质,已在多个行业广泛应用。目前,应用最广泛的金浸出方法仍然是氰化法,这是由其成本低及工艺简单所决定的[1]。氰化法最主要的缺点是浸金速度慢、氰化物有剧毒及浸出过程易于受到铜、铁、铅、锌和硫等杂质的影响[2-3]。长期以来,国内外学者对非氰、选择性浸金技术进行了深入研究并且取得了较多的研究成果。目前国内外非氰浸金工艺主要有硫脲法、硫代硫酸盐法、石硫合剂法、卤素提金法、丙二腈法(有机腈法)、硫氰化物法和多硫化物法等[4-6]。
碘化浸金作为一种卤素提金法,与其他卤素提金相比更加具有较大优越性。根据俄罗斯莫斯科国立贵金属勘探研究院对金的络合物AX2(X为阴离子)稳定性研究,AX2的稳定性由强到弱的顺序为CN-、I-、Br-、Cl-、NCS-、NCO-。由此可知,金氰络合物的稳定性是最高的,其次是金碘络合物[7-8]。MARUN等[9]指出:在水稳定区域内,无论是AuCl4-还是AuBr4-都只是在很小的pH值范围内稳定;而Au在Au-I--H2O体系中形成的两种配位体(AuI4-和AuI2-)却在pH值0~14范围内均能保持稳定。BAGHALHA[10] 针对含碳矿石(有机碳含量为1.6%,质量分数)及氧化矿石分别进行了碘化浸出试验,并对碘化浸出动力学模型进行了探讨,认为碘化浸出反应与I3-浓度呈一级反应。李桂春等[11]对戈塘金矿含碳原生矿进行了碘化浸出试验,结果表明碘化浸金浸出速度快,金浸出率高。WANG等[12]针对某金精矿进行了碘化浸出试验研究,同样得到了较为理想的浸出效果。QI等[13-14]研究了金在碘-碘化物溶液中的动力学及电化学行为,结果表明,金的溶解遵循一级反应动力学,一级反应速度与圆盘旋转速度的二次方成正比。ANGELIDIS等[15]利用旋转圆盘技术对金在碘-碘化物体系中的溶解进行了研究,发现适当调节溶液中碘和碘化物的浓度,金的溶解即可自发进行,且金的溶解速率远比在氰化物或硫脲中的快。
虽然部分研究人员已经对碘化浸出的动力学做了一些研究,但尚无利用实际矿石的浸出对其进行较为全面的动力学研究。为此,本文作者以浮选金精矿为研究对象,以碘-碘化物作为浸出剂进行浸出,在分析碘化浸金基本原理的基础上,通过对影响金精矿浸出动力学参数的考察,分析金精矿碘化浸出速度的变化规律,求出该金精矿碘化浸出的表观活化能并推导出碘化浸出速率方程。通过该研究可为更加深入地了 解碘化浸金动力学提供一定的理论依据及技术指导。
1 碘化浸金基本原理
金在碘-碘化物溶液中溶解时发生的化学反应如下:
I2(s)=I2(l) (1)
I2(l)+I-=I3- (2)
阳极反应为
Au+2I-=AuI2-+e (3)
Au+4I-=AuI4-+3e (4)
阴极反应为
I3-+2e=3I- (5)
总反应为
2Au+I-+I3-=2AuI2- (6)
2Au+3I3-=2AuI4-+I- (7)
根据热力学计算可知,式(6)和(7)的标准电动势分别为-0.038及-0.036 V。可见碘化浸金反应在标准状态下不能自发进行。
反应(6)和(7)的能斯特方程分别为
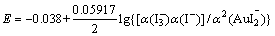 (8)
(8)
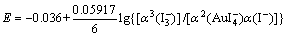 (9)
(9)
式中:α为不同离子的活度。
由于体系中存在过量的碘离子,反应生成的AuI4-会迅速发生歧化反应,生成碘化金,进而又溶解在过量的碘化物中[16]:
2AuI4- =2AuI+3I2(10)
AuI+I-=AuI2-(11)
因此,在碘化浸出时,反应主要以式(6)进行。
由式(8)和(9)可知,改变体系中I-、I3-、AuI2-和AuI4-的浓度,反应电动势可能变为正值,即反应可自发进行。当体系中I-浓度和金碘络合物离子浓度一定时,浸金反应的电动势随着I3-浓度的增加而增大,反应的吉布斯自由能随之减小,反应进行的程度随之增大。而在碘-碘化物溶液中,当I-浓度一定时,I3-浓度随着I2用量的增加而增大(I2+I- I3-)。因此,在碘化浸金体系中,金的浸出率随着I2加入量的增加而增大。
I3-)。因此,在碘化浸金体系中,金的浸出率随着I2加入量的增加而增大。
从金精矿中回收金是固-液多相反应。根据湿法冶金动力学原理及核收缩模型,当反应中有固相产生或起始物中残留有不被浸出的物料层时,多相反应浸出历程一般经历吸附、扩散和化学反应等几个阶段。浸出速度一般由这几个阶段中速度最小者决定,由于吸附很快达到平衡,所以多相反应的速度主要由化学反应或反应物扩散决定。当以化学反应为控制步骤时称反应处于化学反应控制;当以扩散为控制步骤时称多相反应为扩散控制,扩散控制又分为液膜扩散控制(也称为外扩散)和固膜扩散控制(也称为内扩散)[17]。
通常情况下,反应速度公式为
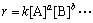 (12)
(12)
式中:k为速率常数;a为组分A的反应级数;b为组分B的反应级数。
当反应物浓度一定时,温度与速率常数k之间的关系可用阿累尼乌斯经验公式表示:
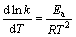 (13)
(13)
式中:Ea为反应活化能,kJ/mol;R为摩尔气体常数;T为热力学温度。
对式(13)进行不定积分可得
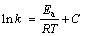 (14)
(14)
式中:C为积分常数。
式(14)还可以写成如下形式:
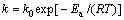 (15)
(15)
式中: k0为频率因子。
将式(12)与式(15)合并,可得化学反应速率方程为
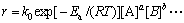 (16)
(16)
对于碘化浸金:
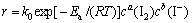 (17)
(17)
以下将对搅拌速度、a、b和T 4个参数进行试验研究。
2 实验
2.1 实验原料
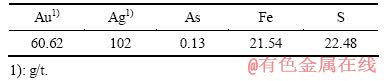
所用矿样为福建双旗山矿业责任有限公司生产的浮选金精矿。双旗山金矿矿物组成简单,金属矿物主要是黄铁矿,脉石矿物主要为石英和长石。金大多以自然金形式存在。在原生矿石中,自然金呈他形粒状充填于黄铁矿孔洞中,或呈短脉状、粒状充填于黄铁矿碎裂缝和裂隙中,少量呈他形粒状、脉状分布在石英中。在风化矿石中,自然金多呈齿状、浸染状、星点状与褐铁矿相嵌连生,或包裹于褐铁矿中[18]。对浮选金精矿进行多元素分析,分析结果如表1所列。
表1 化学多元素分析结果
Table 1 Results of chemical multielement analysis for gold concentrate (mass fraction, %)
2.2 实验方法
采用XMB-d200 mm×240 mm棒磨机进行磨矿。磨矿结束后,过滤,低温烘干,混匀,缩分,每份矿样称取50 g以备后续实验使用。用500 mL的烧杯作为反应器,用MY3000-6智能型混凝试验搅拌仪搅拌,并且计时进行浸出实验。浸出完成后,用循环水式多用真空泵抽滤。尾渣洗涤干净后送至北京矿冶研究总院进行金含量分析,并由此计算金的浸出率或溶液中金的浓度。
3 结果与讨论
3.1 搅拌速度对金浸出率的影响
碘化物选用碘化钾,固定磨矿细度为粒径小于45 μm的占80%,I2浓度为0.0394 mol/L,I-浓度为0.3152 mol/L,浸出液pH值为7,液固比为4:1,浸出时间为4 h,浸出液温度为25 ℃。搅拌速度分别为200、400、600和800 r/min,实验结果如图1所示。
从图1可知,随着搅拌速度的增加,金浸出率不增反降。在固-液多相浸出反应中,当液膜扩散为控制步骤时,搅拌强度对浸出率影响极大,通常可提高浸出率 40%~70%[17, 19],而该金精矿碘化浸出时随着搅拌速度的增加,金浸出率反而下降,由此可以判断,该金精矿在此条件下浸出反应控制步骤不是由液膜扩散控制。当搅拌速度继续降低时,实验中可以看出有矿物沉积于反应器底部,故后续实验采用搅拌速度为200 r/min。
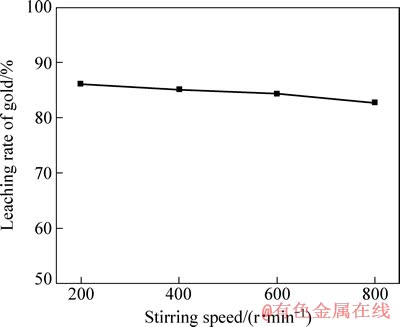
图1 搅拌速度对金浸出率的影响
Fig. 1 Effect of stirring speed on leaching rate of gold
3.2 温度对金浸出速度的影响
其他实验条件同3.1节所示,考察不同温度(15、25和35 ℃)对金浸出速度的影响,结果如图2所示。
由图2可以看出,相同时间内金的溶解量随着温度的升高而增大,图2中3条趋势线斜率(线性相关系数的绝对值均大于0.99)即为不同温度时金的浸出速率常数k。以ln k对1/T作图,并进行线性拟合,可得浸出反应过程的阿累尼乌斯曲线(见图3),由图3可知,该直线方程y=-3809.715x+5.417。结合图3与式(14)可求得碘化浸出时其表观活化能Ea=31.674 kJ/mol,该活化能数据远大于4~12 kJ/mol,故该金精矿在此条件下浸出反应控制步骤不是由固膜扩散控制。对于大多数浸出反应来说,由界面化学反应控制时其表观活化能在30~85 kJ/mol的范围内变化[20-21],这说明该金精矿浸出过程总体由界面化学反应控制。
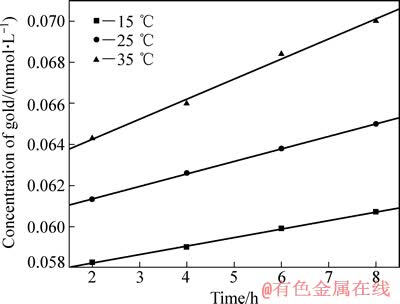
图2 温度对金浸出速度的影响
Fig. 2 Effect of temperature on leaching velocity of gold
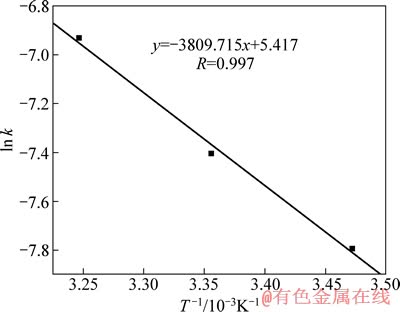
图3 金浸出过程的ln k-1/T曲线
Fig. 3 Curve of ln k-1/T for leaching process of gold
3.3 I2浓度对浸金速度的影响及反应级数的计算
其他条件同3.1节所示,考察I2浓度对金精矿碘化浸出反应速度的影响,其中,I2浓度分别为0.03152、0.0394和0.0473 mol/L,结果见图4。
由图4可知,在相同时间内,金的溶解量随着单质碘浓度的增加而增加,图4中趋势线的斜率即为不同I2浓度下金的浸出速率常数k,金的浸出速率常数也随着I2浓度的增加而增大。以lg k对lg[c(I2)]作图并进行线性拟合,得图5。由图5可以看出,该趋势线斜率为1.00222,非常接近1,因此,该金精矿碘化浸出时I2浓度的反应级数为1。
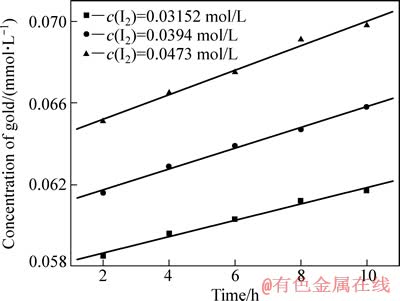
图4 I2浓度对金浸出速度的影响
Fig. 4 Effect of I2 concentration on leaching velocity of gold
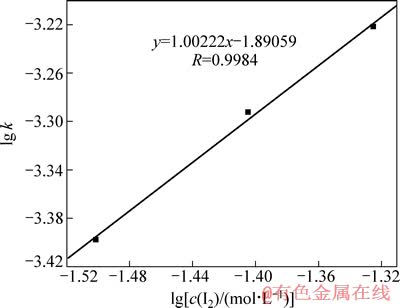
图5 金精矿碘化浸出时的lg k-lg[c(I2)]曲线
Fig. 5 Curve of lg k-lg[c(I2)] for gold concentrate by iodine leaching
3.4 I-浓度对浸金速度的影响及反应级数的计算
其他条件同3.1节所示,考察I-浓度对金精矿碘化浸出反应速度的影响,其中,I-浓度分别为0.2364、0.3152和0.394 mol/L,结果见图6。
由图6可以看出,在相同时间内,随着浸出液中碘离子浓度的增加,金的溶解量也随之增加,图6中趋势线(线性相关系数的绝对值均大于0.99)的斜率即为不同碘离子浓度下金的浸出速率常数k,该浸出速率常数也随着I-浓度的增加而增大。以lg k对lgc(I-)作图,并进行线性拟合,得图7。由图7可得,该趋势线斜率为0.50381,接近于0.5,因此,该金精矿碘化浸出时I-浓度的反应级数为0.5。
由此,根据式(17)及上述实验结果可求得该金精矿碘化浸金的反应速率为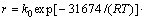
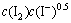 。
。
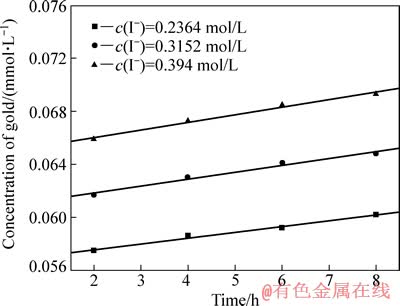
图6 I-浓度对金浸出速度的影响
Fig. 6 Effect of I- concentration on leaching velocity of gold
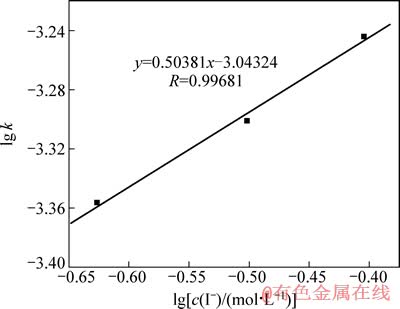
图7 金精矿碘化浸出时的lg k-lg[c(I-)]曲线
Fig. 7 Curve of lg k-lg[c(I-)] for gold concentrate by iodine leaching
4 结论
1) 在一定搅拌速度(200~800 r/min)下,随着搅拌速度的增加,金浸出率不增反降,说明液膜扩散(外扩散)对浸出没有明显的限制作用。金精矿碘化浸出速率常数随着单质碘浓度、碘离子浓度及温度的增加而增大。
2) 通过对金精矿碘化体系浸出过程动力学参数的考察,发现其符合核收缩模型,浸出过程受界面化学反应控制,其表观活化能为31.674 kJ/mol,浸出反应速率方程为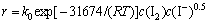 。
。
REFERENCES
[1] SYED S. Recovery of gold from secondary sources—A review[J]. Hydrometallurgy, 2012, 115/116: 30-51.
[2] WILMOT J C, MILOSAVLJEVIC E B, SOLUJIC L, HENDRIX J L. Fate of cyanide in gold ores containing reduced sulphur minerals[C]//Proceedings of the 24th International Mineral Processing Congress. Beijing: Science Press, 2008: 2958-2964.
[3] DAI X, JEFFREY M. The effect of sulfide minerals on the leaching of gold in aerated cyanide solutions[J]. Hydrometallurgy, 2006, 82: 118-125.
[4] HILSON G, MONHEMIUS A J. Alternatives to cyanide in the gold mining industry: What prospects for the future[J]. Journal of Cleaner Production, 2006, 14: 1158-1167.
[5] 玉 涵, 胡显智. 氰化及非氰化提金方法综述[J]. 云南冶金, 2010, 39(3): 9-12.
YU Han, HU Xian-zhi. Review of gold-leaching technologies by cyanidation and non-cyanidation[J]. Yunnan Metallurgy, 2010, 39(3): 9-12.
[6] 李桂春, 卢寿慈. 非氰化提金技术的发展[J]. 中国矿业, 2003, 12(3): 1-5.
LI Gui-chun, LU Shou-ci. The development of non-cyanidation gold lixiviating technology[J]. China Mining Magazine, 2003, 12(3): 1-5.
[7] SEDELNIKOVA G V, KRYLOV G S. Iodinated and brominated solvent of gold[J]. Schnigri, 2001, 3: 43-52.
[8] LI Gui-chun, LU Shou-ci. Iodide leaching gold from carbonaceous refractory gold ores[C]//Proceedings of the 24th International Mineral Processing Congress. Beijing: Science Press, 2008: 1819-1822.
[9] MARUN J N, MEISSL R J, LARA R F. Gold bearing ore processing with iodine-iodide solutions[C]//Proceedings of the 10th International Mineral Processing Congress. Beijing: Science Press, 1997: 381-391.
[10] BAGHALHA M. The leaching kinetics of an oxide gold ore with iodide/iodine solutions[J]. Hydrometallurgy, 2012, 113: 42-50.
[11] 李桂春, 卢寿慈. 碘化浸金试验研究[J]. 中国矿业, 2004, 13(7): 66-68.
LI Gui-chun, LU Shou-ci. Experimental investigation for iodine leaching of gold[J]. China Mining Magazine, 2004, 13(7): 66-68.
[12] WANG Hai-xia, SUN Chun-bao, LI Shao-ying. Study on gold concentrate leaching by iodine-iodide[J]. International Journal of Minerals, Metallurgy and Materials, 2013, 20(4): 323-328.
[13] QI P H, HISKEY J B. Dissolution kinetics of gold in iodide solutions[J]. Hydrometallurgy, 1991, 27: 47-62.
[14] QI P H, HISKEY J B. Electrochemical behavior of gold in iodide solutions[J]. Hydrometallurgy, 1993, 32: 161-179.
[15] ANGELIDIS T N, KYDROS KA, MATIS K A. A fundamental rotating disk study of gold dissolution in iodine-iodide solutions[J]. Hydrometallurgy, 1993, 34: 49-64.
[16] 曹永吉. 关于应用碘法回收金的探讨[J]. 贵金属, 1989, 10(3): 8-12.
CAO Yong-ji. A method for recovery of gold with Iodine[J]. Perious Metals, 1989, 10(3): 8-12.
[17] 胡天觉, 曾光明, 袁兴中. 湿法炼锌废渣中硫脲浸出银的动力学[J]. 中国有色金属学报, 2001, 11(5): 933-937.
HU Tian-jue, ZENG Guang-ming, YUAN Xing-zhong. Leaching kinetics of silver extracted by thiourea from residue in hydrometallurgy of zinc[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(5): 933-937.
[18] 李绍英, 王海霞, 孙春宝. 碘化物对金精矿碘化浸出的影响[J]. 中国有色金属学报,2013, 23(5): 1434-1439.
LI Shao-ying, WANG Hai-xia, SUN Chun-bao. Effect of different iodide on gold concentrates leaching process in iodine-iodide solution[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(5): 1434-1439.
[19] 李浩然, 冯雅丽, 罗小兵. 湿法浸出粘土矿中钒的动力学[J]. 中南大学学报: 自然科学版, 2008, 39(6): 1181-1184.
LI Hao-ran, FENG Ya-li, LUO Xiao-bing. Leaching kinetics of extraction of vanadium pentoxide from clay mineral[J]. Journal of Central South University: Science and Technology, 2008, 39(6): 1181-1184.
[20] 李洪桂. 冶金原理[M]. 北京: 科学出版社, 2005: 291-316.
LI Hong-gui. Principles of metallurgy[M]. Beijing: Science Press, 2005: 291-316.
[21] 薛娟琴, 毛维博, 卢 曦. 超声波辅助硫化镍矿氧化浸出动力学[J]. 中国有色金属学报, 2010,20(5): 1013-1020.
XUE Juan-qin, MAO Wei-bo, LU Xi. Dynamics of ultrasound-assisted oxidation leaching of nickel sulfide concentrate[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(5): 1013-1020.
(编辑 陈卫萍)
基金项目:高等学校博士学科点专项科研基金项目(20100006110003)
收稿日期:2013-06-27;修订日期:2013-09-17
通信作者:孙春宝,教授,博士;电话:010-62334953;E-mail:suncb@ustb.edu.cn