Cu(Ⅱ)-Fe(Ⅱ)-H2O2协同催化氧化降解甲基橙
徐夫元,宋天顺,陈英文,沈树宝
(南京工业大学 制药与生命科学学院,江苏 南京,210009)
摘 要:考察pH值、温度、H2O2、Cu(Ⅱ)和Fe(Ⅱ)添加量对Cu(Ⅱ)-Fe(Ⅱ)-H2O2催化氧化降解甲基橙(MO)的影响。提出了羟基自由基降解甲基橙的机理,并通过数据处理得到了甲基橙的降解动力学模型。研究结果表明:Cu(Ⅱ)和Fe(II)对甲基橙的降解存在协同催化效应,处理200 mL质量浓度为1.5 g/L的甲基橙模拟废水的最佳催化氧化条件为:pH 3.0,温度60 ℃,过氧化氢(体积分数30%)10 g/L,硫酸铜4.0 g/L,硫酸亚铁0.1 g/L,反应速率常数0.943 min-1;Cu(Ⅱ)-Fe(Ⅱ)-H2O2催化体系对甲基橙的降解速率高,5 min即可实现对甲基橙的完全降解。
关键词:催化氧化; 甲基橙; 铜; 亚铁; 过氧化氢; 反应速率常数
中图分类号:O643.6 文献标识码:A 文章编号:1672-7207(2007)03-0480-06
Synergetic degradation of Cu(Ⅱ)-Fe(Ⅱ)-H2O2 for methyl orange
XU Fu-yuan, SONG Tian-shun, CHEN Ying-wen, SHEN Shu-bao
(College of Life-science and Pharmacy, Nanjing University of Technology, Nanjing 210009, China)
Abstract: The effects of pH value, temperature, and the quantities of H2O2, Cu(Ⅱ) and Fe(Ⅱ) on the degradation efficiency of Cu(Ⅱ)-Fe(Ⅱ)-H2O2 for methyl orange(MO) were investigated, and the synergetic catalytic effects of Cu(Ⅱ) and Fe(Ⅱ) on the catalytic oxidation for methyl orange were found. The mechanism of producing hydroxyl radicals by Cu(Ⅱ) or Fe(Ⅱ) and its catalytic oxidation mechanism for methyl orange were proposed, and the overall oxidation dynamic equation was obtained. The results show that the optimum conditions of degrading 200 mL methyl orange simulated wastewater are obtained as follows: the concentration of 1.5 g/L, pH 3.0, temperature 60 ℃, H2O2 10.0 mg/L, CuSO4 4.0 g/L and FeSO4 0.1 g/L. The constant of reaction rate is 0.943 min-1. The degradation rate of Cu(Ⅱ)-Fe(Ⅱ)-H2O2 for methyl orange is high, i.e. methyl orange can be completely degraded within 5 min.
Key words: catalytic oxidation; methyl orange; copper; ferrous; hydrogen peroxide; constant of reaction rate
人们关于催化氧化处理染料废水的研究较多集中在以过渡金属为催化剂、以过氧化氢为氧化剂的催化氧化体系。该体系对污染物氧化彻底,处理效率高,不会带来二次污染,但对采用2种催化剂均相复合催化氧化处理废水的研究鲜有报道。
本文作者选取染料甲基橙为降解对象,考察Cu(Ⅱ)-Fe(Ⅱ)-H2O2对甲基橙的催化氧化降解效果。由于该染料毒性较高、结构复杂并难于生物降解,研究其降解性能对于其他染料和指示剂体系的催化氧化降解具有普遍的参考价值[1]。
1 实验部分
1.1 实验试剂及仪器
试剂:甲基橙;CuSO4.5H2O;FeSO4.7H2O;30%H2O2;KH2PO4;K2HPO4。以上试剂均为分析纯,溶液用蒸馏水配制。
仪器:温度计;恒温水浴锅;搅拌器;自制。
反应器(见图1);LAMBDA25 UV/VIS分光光度计(美国PE公司);pH计(上海雷磁,pHs-3c)
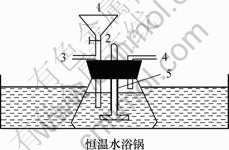
1—加料口;2—搅拌轴;3—取样口;
4—排气管;5—液封套管
图1 催化氧化甲基橙的反应装置图
Fig.1 Schematic diagram of device for MO catalytic oxidation
1.2 实验分析方法
取200 mL(1.5 g/L,参考文献[1,2],并作适当改变)甲基橙模拟废水,加入硫酸铜、硫酸亚铁,调节pH值,混匀,加入恒温的反应器,用搅拌器搅拌,待升温至设定值后,加入H2O2(30%),计时。隔时取样稀释,于波长464 nm下测定其吸光度,计算甲基橙的质量浓度,并计算降解率:

对于m级反应动力学方程,有:-dρ/dt=kρm。
在反应初始阶段,ρm近似等于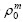 (常数),即
(常数),即
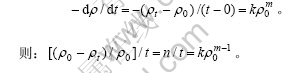
式中:n/t(即为甲基橙的降解率曲线零点处切线的斜率)正比于初始反应速率常数k,以此判断初始反应速率常数的大小。数据用STATISTICA6.0软件处理。
2 实验结果与分析
2.1 pH的影响
条件:硫酸铜0.4 g;硫酸亚铁0.02 g;H2O2 2.0 mL;温度60 ℃;pH分别为2.0,3.0,5.0,7.0和11.0。结果见图2。
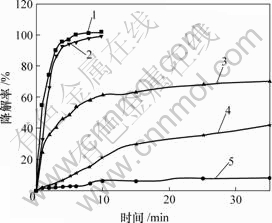
pH: 1—3.0; 2—2.0; 3—5.0; 4—7.0; 5—11.0
图2 pH对甲基橙降解率的影响
Fig.2 Influence of pH on degradation of MO
由图2可知,pH过高或过低,本体系对甲基橙的催化氧化降解效果均较差。pH为3.0时,降解效果最好,故pH选定为3.0。
2.2 反应温度的影响
条件:pH=3.0;温度分别为40,60和80℃;其他条件同上。结果见图3。
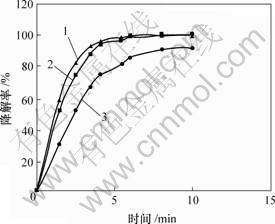
1—80 ℃; 2—60 ℃; 3—40 ℃
图3 温度对甲基橙降解率的影响
Fig.3 Influence of temperature on degradation of MO
由图3可知,80 和60 ℃时的降解率高于优于 40 ℃时的降解率,故反应温度选60 ℃为宜。
2.3 H2O2添加量的影响
条件:温度60 ℃,H2O2分别为0,0.5,1.0,2.0,3.0 mL,其他条件同上。结果见图4。

V(H2O2)/mL: 1—2.0; 2—1.0; 3—3.0; 4—0.5; 5—0
图4 H2O2对甲基橙降解率的影响
Fig.4 Influence of H2O2 on degradation of MO
由图4可知,不加H2O2、只加催化剂时,甲基橙不能被降解;随H2O2添加量的增加,甲基橙降解的初始反应速率常数先增加后减小,故H2O2最佳添加量为2.0 mL。
2.4 硫酸铜添加量的影响
条件:H2O2 2.0 mL;硫酸铜分别为0,0.2,0.4,0.8,1.6 g;其他条件同上。结果见图5。
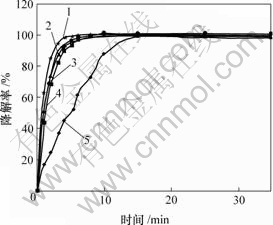
m(CuSO4)/g: 1—0.8; 2—0.4; 3—0.2 g; 4—1.6; 5—0
图5 硫酸铜对甲基橙降解率的影响
Fig.5 Influence of CuSO4 on degradation of MO
由图5可知,随着硫酸铜添加量的增加,对甲基橙的反应速率常数先增加后减小。综合考虑效率与经济,硫酸铜的最佳添加量为0.4 g。
2.5 硫酸亚铁添加量的影响
条件:硫酸铜0.4 g;硫酸亚铁添加量分别为0,0.005,0.010,0.020,0.040 g;其他条件同上。结果见图6。
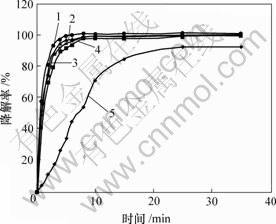
m(FeSO4)/g: 1—0.04; 2—0.02; 3—0.01; 4—0.005; 5—0
图6 硫酸亚铁对甲基橙降解率的影响
Fig.6 Influence of FeSO4 on degradation of MO
由图6可知,随着硫酸亚铁添加量的增加,处理效果变好,直至最后效果变化不太明显。
2.6 Cu(II)和Fe(II)协同催化氧化效应
为考察铁、铜离子之间是否存在协同催化效应,进行4组对照实验,试剂添加量H2O2 (mL)?硫酸亚铁(g)?硫酸铜(g)分别为:2?0?0,2?0.02?0,2?0?0.4,2?0.02?0.4,其他条件同上,结果见图7。

V(H2O2)?m(FeSO4)?m(CuSO4): 1—2/0.02/0.4; 2—2/0.02/0;3—2/0/0.4; 4—2/0.0
图7 铁铜离子协同效应
Fig.7 Synergetic effects of Fe(Ⅱ) and Cu(Ⅱ)
由图7可知,单加H2O2时甲基橙不能被降解,再加Cu(Ⅱ)或者Fe(Ⅱ)后,甲基橙迅速被降解脱色;在初始段加入2种催化剂的处理效果优于加入单一离子的效果,这表明铁、铜离子之间对甲基橙的降解存在协同催化效应。
2.7 甲基橙降解机理
对于羟基自由基氧化降解底物的报道很多[2-13],针对本文研究体系,归纳提出如下羟基自由基降解甲基橙的催化氧化机理,见表1。
表1 甲基橙降解机理
Table 1 Mechanism of MO degradation
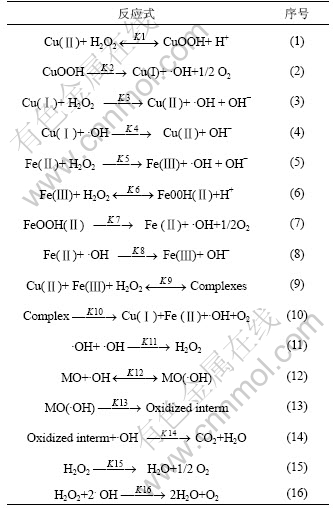
从实验中观察到,甲基橙降解过程中颜色转变过程为:深红→褐色→浅褐色→无色。当氧化剂添加量较少时,溶液呈褐色;当氧化剂添加量充足时,溶液呈无色。溶液中的褐色物质是甲基橙被羟基自由基氧化形成的中间产物,可被进一步氧化为CO2和H2O[3, 5-8]。反应(1),(3)~(6)和(8)中涉及H+和OH-浓度,其大小将影响到相关反应的进行,pH值较高时很容易将催化剂沉淀,降低催化能力,不利于对底物的降解。因此,pH值要适当。H2O2作为促生羟基自由基的反应物,其添加量将影响到羟基自由基的产生量,涉及的反应有式(1),(3),(5)和(6);H2O2浓度过大,将导致自身无效分解以及对羟基自由基的淬灭,如式(15)和(16)所示,并不会提高降解效果。Cu(Ⅱ)和Fe(Ⅱ)作为促生羟基自由基的激发剂和催化剂,涉及的反应有式(1)~(10),当在低浓度范围内增大Cu(Ⅱ)和Fe(Ⅱ)的浓度时,产生的羟基自由基增加,并有效氧化甲基橙;当Cu(Ⅱ)和Fe(Ⅱ)浓度过大时,对甲基橙的处理效果提高很小,甚至变差。这因为大量催化剂的加入导致瞬间产生大量的羟基自由基,但受底物浓度及降解反应速率等因素的限制,这些自由基不能快速有效地用于氧化甲基橙,部分羟基自由基发生复合或者与H2O2淬灭,如式(11)和(16) 所示,导致羟基自由基的利用率低。
实验发现,Cu(Ⅱ)和Fe(Ⅱ)之间存在协同催化作用。单加H2O2或者催化剂,甲基橙均不能降解;当同时加入两者时,底物被快速降解。这表明甲基橙是被催化剂激发氧化剂产生的强氧化性自由基所氧化降解,由此推测单加Cu(Ⅱ)或Fe(Ⅱ)与同时加入2种离子催化氧化甲基橙的机理不同。Cu(Ⅱ)和Fe(Ⅱ)均含有空的d轨道,易接受孤对电子,形成配合物。单加Cu(Ⅱ)或者Fe(Ⅱ)时发生的反应分别为式(1)~(4),(5)~(8);当同时加入2种金属离子时,发生的主体反应为式(9)~(10),即2种离子与H2O2形成了较为复杂的多核金属配合物[5-6],改变了羟基自由基的产生途径,更加利于羟基自由基的产生。
2.8 甲基橙降解动力学
在60 ℃,pH=3.0时,对不同的初始H2O2浓度ρA、硫酸铜浓度ρB、硫酸亚铁浓度ρC的实验数据,用一级动力学模型(ln(ρ0/ρt)=kt)拟合,得到反应速率常数k。结果见图8。

ρA?ρB?ρC: 1—10?4?0.1; 2—10?2?0.2; 3—10?2?/0.1; 4—10?2?0.05; 5—10?2?0.02; 6—10?1?0.1; 7—5?2?0.1;8—2.5?2?0.1
图8 数据一级动力拟合
Fig.8 Fit of experimental data with first-order kinetic models
由图8可知,当ρA?ρB?ρC=10?4?0.1时,甲基橙降解的反应速率常数最大,为0.943 min-1。又
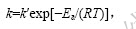
当ρA,ρB和ρC不变时,k′为常数。式中: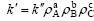 [ 8-13]。对不同温度数据拟合得k,并用lnk对1/T作图,见图9。
[ 8-13]。对不同温度数据拟合得k,并用lnk对1/T作图,见图9。
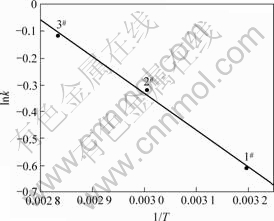
图9 lnk—1/T关系图
Fig.9 Diagram of lnk—1/T
由纵截距求得Ea/R=1 370.2,则
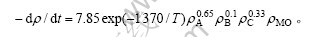
将T=333 K代入得:
lnk=-4.1+lnk″+alnρA+blnρB+clnρC。
以lnρA,lnρB和lnρC为自变量,以lnk为因变量线性拟合得:lnk=-2.04+0.65lnρA + 0.10lnρB+0.33lnρC,拟合度R=0.99。
为了进一步考察拟合结果的可靠性[14],考察了拟合的误差分布情况,见图10。由图10可知,拟合误差是随机分布于0的两侧,表明拟合得到的参数可靠。
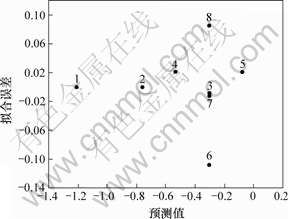
图10 拟合误差分布
Fig.10 Distribution of fit error
求得动力学方程为
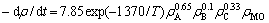 。
。
2.9 紫外-可见光谱图
取不同催化氧化时间的水样,进行全波长扫描。结果见图11。由图11可知,原水样在464 nm左右有一明显的吸收峰,这是偶氮键与苯环组成的共轭体系的特征吸收峰。随着反应的进行,该吸收峰的峰高逐渐降低,表明分子结构中的共轭体系被破坏,从而导致特征吸收的减弱[3]。

1—0 min时试样; 2—2 min时试样; 3—4 min时试样; 4—6 min时试样; 5—8 min时试样; 6—10 min时试样
图11 紫外-可见光谱图
Fig.11 UV-Vis absorption spectrum
3 结 论
a. pH值越高,Cu(Ⅱ)-Fe(Ⅱ)-H2O2对甲基橙的降解效果越差,故pH选3.0为宜;高温较低温有利于羟基自由基的产生,但温度过高,H2O2蒸发损失加快,故温度选60 ℃为宜。
b. 对200 mL的甲基橙模拟废水,H2O2(30%)、硫酸铜、硫酸亚铁的适宜加量分别为10,4.0和0.1 g/L,反应速率常数为0.943 min-1,并发现Cu(Ⅱ)和Fe(Ⅱ)之间存在协同催化效应,降解速率高。
c. 提出了Cu(Ⅱ)-Fe(Ⅱ)-H2O2催化氧化降解甲基橙的机理,并由此解释了实验结果和现象;通过对实验数据拟合发现,甲基橙的降解满足一级动力学模型,并得到动力学模型:
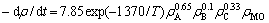 。
。
d. 从紫外-可见光谱图可以看出甲基橙的脱色机理在于其共轭键的断裂。
参考文献:
[1] 杨润昌, 周书天. 高浓度甲基橙低压湿式催化氧化及动力学研究[J]. 湘潭大学自然科学学报, 2000, 22(3): 59-62.
YANG Run-chang, ZHOU Shu-tian. Low pressure wet catalytic oxidation of high concentrated methyl orange and its kinetic studies[J]. Natural Science Journal of Xiangtan University, 2000, 22(3): 59-62.
[2] 谢 磊, 杨润昌. 高浓度甲基橙湿式过氧化氢氧化及机理初探[J]. 环境工程, 2002, 20(2): 22-24.
XIE Lei, YANG Run-chang. Wet hydrogen peroxide oxidation of high concentration methyl orange and its mechanism[J]. Environment Engineering, 2002, 20(2): 22-24.
[3] 陈传好, 谢 波. Fenton试剂处理废水中各因子的作用机制[J]. 环境科学, 2000, 21(3): 93-96.
CHEN Chuan-hao, XIE Bo. The mechanisms of affecting factors in treating wastewater by Fenton reagent[J]. Environmental Science, 2000, 21(3): 93-96.
[4] 刘春英, 弓晓峰, 曾 珍. Fenton试剂在染料废水处理中的应用[J]. 环境保护科学, 2004, 30(2): 9-12.
LIU Chun-ying, GONG Xiao-feng, ZENG Zhen. Application of Fenton reagent in dyeing wastewater treatment[J]. Science of Environment Protection, 2004, 30(2): 9-12.
[5] Perez-Benito J F. Reaction pathways in the decomposition of hydrogen peroxide catalyzed by copper(Ⅱ)[J]. Journal of Inorganic Biochemistry, 2004, 98(3): 430-438.
[6] Salem I A, El-Maazawi M S. Kinetics and mechanism of color removal of methylene blue with hydrogen peroxide catalyzed by some supported alumina surfaces[J]. Chemosphere, 2000, 8(41): 1173-1180.
[7] Salem I A. Activation of H2O2 by Amberlyst-15 resin supported with copper(Ⅱ)-complexes towards oxidation of crystal violet[J]. Chemosphere, 2001, 5(44): 1109-1119.
[8] El-Daly H A, Habib A F M, Borhan Ei-Din M A. Kinetics and mechanism of the oxidative color removal from Durazol Blue 8G with hydrogen peroxide[J]. Dyes and Pigments, 2003, 57(3): 197-210.
[9] Salem I A. Kinetics of the oxidative color removal and degradation of bromophenol blue with hydrogen peroxide catalyzed by copper(Ⅱ)-supported alumina and zirconia[J]. Applied Ca talysis B: Environmental, 2000, 28(3/4): 153-162.
[10] Lin S H, Lo C C. Fenton process for treatment of desizing waste-water [J]. Water Research, 1997, 31(8): 2050-2056.
[11] Sharma V K, Millero F J, Homonnay Z. The kinetics of the complex formation between iron (Ⅲ)-ethylene diamine tetraacetate and hydrogen peroxide in aqueous solution[J]. Inorganica Chimica Acta, 2004, 357(12): 3583-3587.
[12] LU Ming-chun, CHEN Jong-nan, CHANG Cheu-ping. Oxidation of dichlorvos with hydrogen peroxide using ferrous ion as catalyst[J]. Journal of Hazardous Materials, 1999, 65(3): 277-288.
[13] Lin S H, Lin C M, Leu H G. Operating characteristics and kinetic studies of surfactant waste-water treatment by Fenton oxidation[J]. Water Research, 1999, 33(7): 1735-1741.
[14] 马正飞, 殷 翔. 数学计算方法与软件的工程应用[M]. 北京:化学工业出版社, 2002.
MA Zheng-fei, YIN Xiang. Mathematics calculating method and engineering application of the software[M]. Beijing: Chemical Industry Press, 2002.
收稿日期:2006- 06-28
基金项目:国家高技术研究发展计划资助项目(20060102Z2009); 国家自然科学基金资助项目(20376034); 江苏省自然科学基金资助项目(BK2006181); 江苏省省级污染防治专项资金资助项目(2006-1-W-19)
作者简介:徐夫元(1982-),男,江苏新沂人,博士研究生,从事废水处理的研究
通讯作者:沈树宝,男,教授;电话:025-83587349;E-mail: zsbshen@njut.edu.cn