文章编号:1004-0609(2013)S1-s0730-04
钛铝反应动力学及其控制
曲海涛1,2,任学平1 ,侯红亮2,赵 冰2
(1. 北京科技大学 材料科学与工程学院,北京 100083;
2. 北京航空制造工程研究所,北京 100024)
摘 要:通过差示扫描量热法(DSC)对钛铝反应进行动力学分析,研究钛铝反应过程和机理。结果表明:钛铝反应包括两个过程:前期是钛(固)与铝(液)直接反应生成TiAl3,反应激活能为(100±10) kJ/mol,后期是TiAl3中的Al通过TiAl、TiAl2和Ti3Al层向Ti中扩散,扩散激活能为(200±10) kJ/mol;若钛铝摩尔比为1:1,最终反应产物为TiAl,其反应速率控制步骤为Ti-TiAl3扩散偶中Al通过TiAl、Ti Al2和Ti3Al层的扩散。
关键词:钛铝反应;动力学;反应激活能;Kissinger法;Friedman法
中图分类号:TG146.2 文献标志码:A
Kinetic analysis and control action of titanium and aluminium
QU Hai-tao1,2, REN Xue-ping1, HOU Hong-liang2, ZHAO Bing2
(1. School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 10083, China;
2. Beijing Aeronautical Manufacturing Technology Research Institute, Beijing 100024, China)
Abstract: Kinetic analysis of Ti-Al reaction was conducted through differential scanning calorimetry (DSC), and mechanism of the preparation of titanium aluminides was elucidated. The results show that the reaction of titanium and aluminium involves two processes. First, TiAl3 is the priority generation phase of Ti-Al reaction, and the reaction activation energy is (100±10) kJ/mol. Second, Al element of TiAl3 diffused to Ti through the formed TiAl, TiAl2 and Ti3Al; and the reaction activation energy is (200±10) kJ/mol. However, after fully diffusing of Al, the final stable reaction product depends on Ti/Al atomic ratio. When the Ti/Al atomic ratio is 1:1, TiAl can be obtained.
Key words: Ti-Al reaction; kinetic analysis; reaction activation energy; Kissinger method; Friedman method
Ti-Al系金属间化合物具有高比强度和高熔点,以及优异的高温性能和抗氧化性能,成为未来最具发展前景的新型轻质耐高温材料[1-6]。因此,吸引国内外学者的广泛关注。但是,对于钛、铝反应生成钛铝金属间化合物的过程与机理却不确定。
目前,针对钛铝通过固相反应生成TiAl,ALMAN等[7]认为包括两个反应过程:
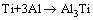 (1)
(1)
 (2)
(2)
但RAMOS等的研究成果[8-11]却表明:
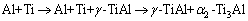
而LUO和ACOFF [12]利用钛箔、铝箔经冷轧和热
处理循环制备γ-TiAl,认为其反应过程如下:
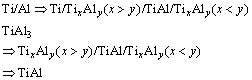 (3)
(3)
国内对钛铝反应的研究不多,其中李志强等[13]分析TiAl3是钛铝反应唯一初生相的热力学原因,同时指出TiAl2和Ti2Al5的生成须以先期生成TiAl为条件。
因此,针对钛铝反应研究存在的问题,为研究反应生成钛铝金属间化合物的过程与机理,本文通过差示扫描量热法(DSC)实验,采用Friedman法对钛铝反应进行动力学分析[14]。
1 实验
首先通过混粉机按照1:1的摩尔比将Ti6Al4V粉(纯度≥99.8%,粒径≤75 μm)与铝粉(纯度≥99%,粒径≤150 μm)进行均匀混合,并在400 MPa的压力下冷压成坯,然后在NETZSCH-STA409C热流型DSC仪上进行反应实验,升温速率分别为10、15、20 K/min,气氛为流动氩气,参比物为α-Al2O3。
物相结构采用X射线衍射仪进行分析,扫描范围为10°~100°步宽为0.02°,扫描速度10 (°)/min,Cu靶Kα,管电压为20 mA,并采用Jade5.0软件对XRD图谱进行分析。
2 分析与讨论
钛铝反应的典型DSC曲线如图1所示。分析表明,DSC曲线在670 ℃左右均存在一个吸热峰,可能是由于铝融化吸热引起。利用Origin8计算吸热峰的面积,得到铝融化吸热9.8 kJ/mol,接近真实值,误差在10%以内,从而证明本实验的DSC数据是可靠的。同时发现,DSC曲线在755 ℃左右存在一个放热峰,分析认为是由于钛铝发生放热反应而引起。且曲线上的放热峰紧邻吸热峰,说明铝粉融化,包围住钛粉,铝与钛接触面积增大,促使钛铝迅速发生放热反应,与杨兵[15]的研究结果吻合。
DSC试验后样品的XRD谱如图2所示,分析表明,反应产物为Ti3Al、TiAl、TiAl2、TiAl3,还有剩余的Ti。

图1 不同升温速率时钛铝反应的典型DSC试验曲线
Fig.1 Typical DSC curves of Ti-Al reaction at different heating rates
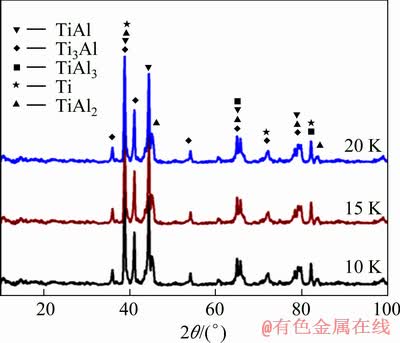
图2 DSC试验反应产物XRD谱
Fig.2 XRD patterns of reaction products
利用Kissinger微分法[16]对DSC曲线进行分析。假设DSC曲线的峰顶处为反应的最大速率发生处,与之相对应的温度为Tp,将反应动力学的基本方程(如式(4)所示):
 (4)
(4)
式中:α为转变率;T为温度;A为指前因子;β为升温速率;n为反应级数;Ea为反应激活能;R为理想气体常数。
在Tp处对温度取二阶导数并令(d2α)/(dT2)=0,可得式(5)。
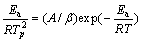 (5)
(5)
式中:Tp为峰顶处对应温度。
对式(5)两边取对数,并对1/Tp微分可得:
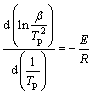 (6)
(6)
确定出与各β值相对应的峰顶温度Tp,并计算得1/Tp、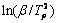 各数值,然后绘制出
各数值,然后绘制出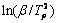 —1/Tp关系图,如图3所示,根据直线斜率,可计算求得反应活化能Ea=211 kJ/mol,接近于Ti-TiAl3扩散偶通过TiAl、TiAl2和Ti3Al的互扩散激活能206 kJ/mol[17]。
—1/Tp关系图,如图3所示,根据直线斜率,可计算求得反应活化能Ea=211 kJ/mol,接近于Ti-TiAl3扩散偶通过TiAl、TiAl2和Ti3Al的互扩散激活能206 kJ/mol[17]。
由图1可以看出,钛铝反应的放热峰呈光滑的钟型,但该反应不一定是简单的单步反应,因为固态反应多为复杂的多步反应过程,其反应峰仍可能具有光滑的钟型峰。因此,为研究钛铝反应过程与机理,采用可识别多步复杂固态反应的Friedman法,进一步研究钛铝反应动力学。
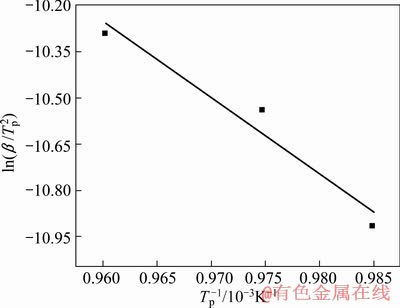
图3  —1/Tp关系图
—1/Tp关系图
Fig.3 Curve of  —1/Tp
—1/Tp
Friedman法是假设:1)放热曲线总面积正比于固化反应总放热量;2)固化反应速率与热流速率成正比。即:
 (7)
(7)
根据图1可得d(ΔH)/dt,即DSC曲线的纵坐标,对式(7)积分可得式(8):
 (8)
(8)
式中:F为DSC曲线上整个反应峰面积;S为与α相对应的点之前的那部分峰的积分面积;T0和T分别为反应开始温度和与α相对应的温度。
根据式(7)求出不同升温速率β下的dα/dt,再做出不同升温速率β下的lg(dα/dt)—1/T关系图,即Friedman图,如图4所示。在图4中,对转变率α相同的点进行线性拟合,由直线的斜率可得到该转变率α下的反应表观激活能Ea。根据Friedman法求得的反应表观激活能Ea,做出E—α关系图,如图5所示。

图4 不同升温速率下Friedman图
Fig.4 Curve of Friedman at different heating rates
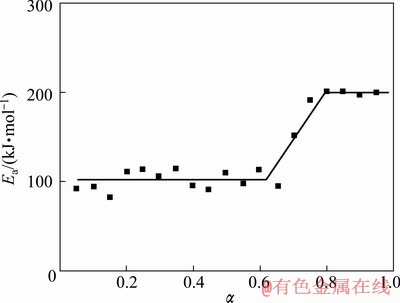
图5 Ea—α关系图
Fig.5 Curve of Ea—α
分析表明Friedman曲线由两个平台组成,钛铝反应并不是简单的单步反应,包括两个过程:平台1的反应表观激活能为(100±10) kJ/mol;平台2的反应表观激活能为(200±10) kJ/mol。与文献中钛铝反应的激活能对比[15],可知平台1的激活能接近于钛(固)与铝(液)直接反应生成TiAl3的反应激活能(97 kJ/mol),而平台2的激活能接近于Ti-TiAl3扩散偶中通过TiAl、TiAl2和Ti3Al层的扩散激活能(206 kJ/mol)。
因此,在反应初期钛(固)与铝(液)迅速反应生成Al3Ti层。随着反应的进行,TiAl3与Ti进一步反应生成TiAl、TiAl2和Ti3Al;若钛铝原子比为1:1,最终产物为TiAl。钛与铝反应生成TiAl的反应速率控制步骤为Ti-TiAl3扩散偶中Al通过TiAl、TiAl2和Ti3Al层的扩散。
3 结论
1) 钛铝反应包括两个过程:反应前期,钛(固)与铝(液)直接反应生成TiAl3,反应激活能为(100±10) kJ/mol;反应后期,TiAl3中的Al通过TiAl、TiAl2和Ti3Al层向Ti中扩散,最终生成TiAl,反应激活能为(200±10) kJ/mol。
2) 钛与铝反应生成TiAl的反应速率控制步骤为Ti-TiAl3扩散偶中Al通过TiAl、TiAl2和Ti3Al层的扩散。
REFERENCES
[1] 孔凡涛, 陈玉勇, 田 竞, 陈予勇. TiAl基金属间化合物研究进展[J]. 材料科学与工艺, 2003, 11(4): 441-444.
KONG Fan-tao, CHEN Yu-yong, TIAN Jing, CHEN Zi-yong. Developments in TiAl based intermetallics research[J]. Materials Science & Technology, 2003, 11(4): 441-444.
[2] VASSEL A. Continuous fibre reinforced titanium and aluminium composites: A comparison[J]. Materials Science and Engineering A, 1999, 263 (2): 305-313.
[3] BRINDLEY P K, BARTOLOTTA P A. Failure mechanisms during isothermal fatigue of SiC/Ti-24A1-11Nb composites[J]. Materials Science and Engineering A, 1995, 200(1/2): 55-67.
[4] VEERARAGHAVAN D, WANG Ping, VAAUDEVAN V K. Kinetics and thermodynamics of the α→γm massive transformation in a Ti-47.5at% Al alloy[J]. Acta Mater, 1999, 47(11): 3313-3330.
[5] DJANARTHANY S, VIALA J C, BOUIX J, Development of SiC/TiAl composites processing and interfacial phenomena[J]. Materials Science and Engineering A, 2001, 300(1/2): 211-218.
[6] 钱余海, 李美栓, 张亚明. Ti3Al基合金在700-850 ℃空气中的循环氧化行为[J]. 中国有色金属学报, 2004, 14(9): 1609-1614.
QIAN Yu-hai, LI Mei-shuan, ZHANG Ya-ming. Cyclic oxidation behaviors of Ti3Al-based alloys at 700-850℃ in air[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(9): 1609-1614.
[7] ALMAN D E, RAWERS J C, HAWK J A.Microstructural and failure characteristics of metal-intermetallic layered sheet composites[J]. Metallurgical and Materials Transactions A, 1995, 26: 589-599.
[8] RAMOS A S, VIEIRA M T. Kinetics of the thin films transformation Ti/Al multilayer→γ-TiAl[J]. Surface & Coatings Technology, 2005(200): 326-329.
[9] RAMOS A S, VIEIRA M T, DUARTE L I, VIEIRA M F, CALINAS R. Nanometric multilayers: A new approach for joining TiAl[J]. Intermetallics, 2006(14): 1157-1162.
[10] RAMOS A S, CALINAS R, VIEIRA M T. The formation of γ-TiAl from Ti/Al multilayers with different periods[J]. Surface & Coatings Technology, 2006(200): 6196-6200.
[11] RAMOS A S, VIEIRA M T, MORGIEL J, GRZONKA J, SIMOSE S, VIEIRA M F. Production of intermetallic compounds from Ti/Al and Ni/Al multilayer thin films-A comparative study[J]. Journal of Alloys and Compounds, 2009(484): 335-340.
[12] LUO J G, ACOFF V L. Processing gamma-based TiAl sheet materials by cyclic cold roll bonding and annealing of elemental titanium and aluminum foils[J]. Materials Science and Engineering A, 2006(433): 334-342.
[13] 李志强, 韩杰才, 赫晓东, 张幸红. 燃烧合成TiAl金属间化合物的反应机制[J]. 稀有金属材料与工程, 2002, 31(1): 4-7.
LI Zhi-qiang, HAN Jie-cai, HE Xiao-dong, ZHANG Xing-hong. Reaction mechanism of combustion synthesis of gamma titanium aluminide: an overview[J]. Rare Metal Materials and Engineering, 2002, 31(1): 4-7.
[14] 朱丽萍, 张廷安, 张含博, 豆志河. 铝热还原制备高钛铁的热力学和动力学[J]. 中国有色金属学报, 2010, 20: s425-s428.
NIU Li-ping, ZHANG Ting-an, ZHANG Han-bo, DOU Zhi-he. Thermodynamics and kinetics of preparation of high titanium ferroalloy by thermite reation[J]. The Chinese Journal of Nonferrous Metals, 2010, 20: s425-s428.
[15] 杨 兵. 元素粉末法制备TiAl基合金[J]. 粉末冶金技术, 1999, 17(4): 286-290.
YANG Bing. Manufacturing of TiAl based alloy through elemental powder process[J]. Powder Metallurgy Technique, 1999, 17(4): 286-290.
[16] KISSINGER H E. Reaction kinetics on differential thermal analysis[J]. Anal Chem, 1957, 29(11): 1702-1706.
[17] 张俊善, 汪 涛, 祝美丽, 刘瑞岩. 燃烧合成TiAl3化学反应动力学研究[J]. 金属学报, 2002, 38(10): 1027-1030.
ZHANG Jun-shan, WANG Tao, ZHU Mei-li, LIU Rui-yan. Chemical kinetics research on the combustion synthesis of TiAl3[J]. Acta Metallurgica Sinica, 2002, 38(10): 1027-1030.
(编辑 王 超)
基金项目:国家自然科学基金资助(51205376)
收稿日期:2013-07-28;修订日期:2013-10-10
通信作者:任学平,教授,博士;电话:010-82376475;E-mail:rxp33@ustb.edu.cn