DOI: 10.11817/j.ysxb.1004.0609.2020-36372
氟磷灰石还原的影响因素和等温动力学
栗艳锋,孙永升,韩跃新,王定政
(东北大学 资源与土木工程学院,沈阳 110819)
摘 要:为深入了解高磷铁矿煤基还原过程中氟磷灰石还原行为的影响因素和等温动力学参数,试验采用纯矿物配比的方式研究了二氧化硅含量、氧化铁含量、碳用量、还原时间和还原温度对磷灰石还原度的影响,并在此基础上,探明Ca10(PO4)6F2-SiO2-Fe2O3-C体系下氟磷灰石还原的等温动力学机理函数和动力学方程。结果表明:在一定的条件下,增加二氧化硅、氧化铁、碳用量、还原时间或还原温度均能够促进氟磷灰石的还原反应。在Ca10(PO4)6F2-SiO2-Fe2O3-C体系中,二氧化硅和氧化铁与氟磷灰石的最佳含量比分别为1.8和2.2,最佳C/O摩尔比为2.0。在最佳反应物用量条件下,氟磷灰石深度还原的最佳动力学机理函数为A1/3:1/3(1-α)[-ln(1-α)]-2;最佳动力学方程为:k(T)=3.89033×107exp(-282.748×103/RT),其中,指前因子为3.89033×107 min-1,活化能为282.748 kJ/mol。还原反应的限制环节为固态扩散。
关键词:氟磷灰石;Ca10(PO4)6F2-SiO2-Fe2O3-C体系;影响因素;动力学方程
文章编号:1004-0609(2020)-02-0431-07 中图分类号:TD91 文献标志码:A
随着我国钢铁工业的快速发展,铁矿石的需求量急剧增加,但是我国优质铁矿资源匮乏,仅占铁矿石总储量的2.5%,难选铁矿石储量巨大,但其开发利用率较低[1]。使得我国不得不每年高价从国外进口大量优质铁矿石,造成铁矿石对外依存度连年增高,如在2017年,我国进口铁矿石达到10.75亿t,对外依存度高达88.7%,创历史新高,因此,开发高效处理复杂难选铁矿石的新技术就显得尤为迫切。在我国复杂难选铁矿石中,高磷铁矿具有铁品位高(40%~55%)、储量大(约74.5亿t)、磷含量高(1%左右)的特点,因此解决高磷铁矿的高效利用问题具有重大意义。由于高磷铁矿石结构组成复杂,采用传统的选矿方法难以处理该类矿石[2-3]。深度还原-高效分选技术[4-7]作为一种能有效处理高磷铁矿的新工艺,然而在磷元素的调控方面仍存在一定的问题。因此对高磷铁矿石中磷矿物还原反应的深入研究就显得尤为重要。
氟磷灰石是高磷铁矿的主要含磷矿物,石英是高磷铁矿的主要脉石,因此有必要探索Ca10(PO4)6F2- SiO2-Fe2O3-C体系中各组分对氟磷灰石还原行为的影响,明确氟磷灰石还原过程的动力学方程、动力学参数及限制环节,为高磷铁矿的高效开发利用提供一定的理论参考。
1 实验
1.1 试验原料
本文所用的磷灰石采自湖北某磷灰石矿,经化学分析原矿中磷的品位为13.2%。对原矿进行XRD物相分析,分析结果见图1。由图1可知,磷灰石主要以氟磷灰石形式存在,还含有少量石英和方解石等。将原矿经过颚式破碎机粗碎、对辊破碎机细碎至粒径小于2 mm。将粉碎后的物料用球磨机磨至粒径小于74 μm占70%,磨细后的物料经浮选后得到磷灰石精矿。浮选采用油酸钠为捕收剂,水玻璃作为抑制剂、氢氧化钠为pH调整剂。浮选精矿中磷的品位达18.23%,而氟磷灰石中磷的理论品位为18.53%,计算可得浮选精矿中磷灰石的含量高达98.38%,可以作为试验用磷灰石纯矿物使用。试验用二氧化硅和氧化铁纯矿物均购自沈阳科密欧公司,为分析纯试剂。
试验用还原剂为焦炭,将焦炭经颚式破碎机和对辊破碎机破碎,并使其全部通过2 mm的筛孔。粉碎后的焦炭经混匀、缩分后,得到深度还原试验用样,并取少量焦炭样品做工业分析,结果见表1。由表1可知,该还原剂的固定碳含量高达85.44%,灰分和挥发分含量分别为12.46%和1.26%,硫的含量较低,为0.62%,超过二级焦炭的标准,可作为还原剂使用。
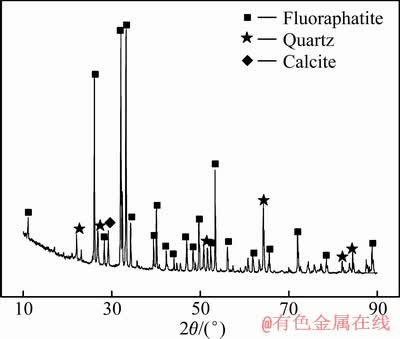
图1 原磷灰石矿的XRD谱
Fig. 1 XRD pattern of fluorapatite raw ore
表1 焦炭化学成分
Table 1 Chemical compositions of coke (mass fraction, %)

1.2 研究方法
含氟磷灰石的深度还原试验在实验室马弗炉中进行。按试验条件取一定量的氟磷灰石、添加剂(SiO2和Fe2O3)和焦炭混合均匀,氟磷灰石与添加剂的用量采用质量比计量;碳用量采用理论配碳量倍数即C/O摩尔比(n(C)/n(O))表示;C/O摩尔比为1时,指氟磷灰石中的磷元素和氧化铁中铁元素被完全还原为单质,碳被氧化成一氧化碳时所需的碳用量。将混合均匀后的物料放入石墨坩埚中,在物料表面覆盖一层焦炭粉末,以保持坩埚内的还原气氛。待还原炉内温度达到指定值时,将装有混合物料的坩埚放入炉内,待炉内温度回升至预定值时,开始计时。还原进行到指定时间时取出坩埚进行水淬处理。水淬后的还原物料磨细至小于0.074 mm粒级含量占85%。将磨细后的物料放入烘箱烘干后取出15 g有代表性的产品,用XCSG-Φ50型磁选管在磁场强度为107 kA/m的条件下进行磁选。磁选精矿中的磷属于已被还原的磷,磁选尾矿中的磷属于尚未被还原的磷。将磁选尾矿称重后化验P含量,磷灰石的还原度可通过式(1)计算获得。
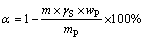 (1)
(1)
式中: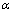 为P2O5还原率,%;m为还原产品的质量,g;
为P2O5还原率,%;m为还原产品的质量,g;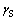 为磁选尾矿与磁选给矿的质量比;wP为磁选尾矿中的磷含量,%;mP为每次试验用氟磷灰石中P的质量,g。
为磁选尾矿与磁选给矿的质量比;wP为磁选尾矿中的磷含量,%;mP为每次试验用氟磷灰石中P的质量,g。
2 结果与讨论
2.1 不同因素条件对氟磷灰石还原度的影响
2.1.1 SiO2含量对氟磷灰石还原度影响
Ca10(PO4)6F2-SiO2-C体系在理论配碳量倍数为2.0、还原时间60 min、还原温度1573 K的条件下进行还原试验,随着二氧化硅含量增加,氟磷灰石的还原度变化规律如图2所示。由图2可知,当体系中二氧化硅与氟磷灰石的含量(质量)比低于1.8时,氟磷灰石的还原度随体系中二氧化硅的含量增加呈线性上升趋势。当体系中二氧化硅与氟磷灰石的含量比高于1.8时,氟磷灰石的还原度随体系中二氧化硅的含量增加而下降。研究资料表明[8-9],二氧化硅可以与氟磷灰石在高温下生成硅酸钙,降低氟磷灰石的起始还原温度,促进氟磷灰石的还原,使磷灰石的还原度提高;同时,二氧化硅作为一种酸性氧化物,过量的二氧化硅会增加物料粘度,阻碍氟磷灰石与焦炭反应,导致还原度达到峰值36.12%后,随着二氧化硅含量继续上升,氟磷灰石的还原度开始略微下降。Ca10(PO4)6F2-SiO2-C体系在高温下生成硅酸钙的反应如下:
Ca10(PO4)6F2+9SiO2+15C=CaF2+9CaSiO3+3P2+15CO (2)
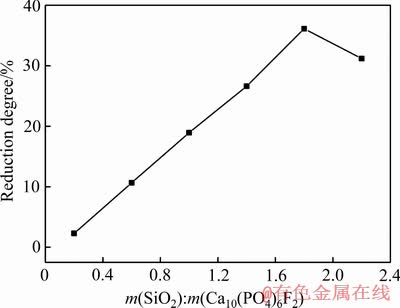
图2 SiO2含量对氟磷灰石还原度的影响
Fig. 2 Effect of SiO2 contents on reduction degree of fluorapatite
2.1.2 Fe2O3含量对氟磷灰石还原度影响
Ca10(PO4)6F2-Fe2O3-C体系在还原条件为C/O摩尔比2.0、还原时间60 min、还原温度1573 K时,氟磷灰石的还原度与体系中Fe2O3的含量关系如图3所示。由图3可以看出,在该条件下,氟磷灰石的还原度随Fe2O3含量的上升逐渐增大,而还原度的增长速率逐渐减小。当体系中Fe2O3与氟磷灰石的含量(质量)比大于2.2时,氟磷灰石的还原度高达73%以上。研究资料表明[9],Fe2O3对氟磷灰石还原度的影响远大于SiO2对氟磷灰石还原度的影响,这是由于氟磷灰石还原生成的磷单质极易进入Fe2O3的还原产物单质铁中,形成FexP和P-Fe固溶体,进而促进了氟磷灰石还原反应的进行。Ca10(PO4)6F2-Fe2O3-C体系在高温下主要发生的化学反应如下:
Ca10(PO4)6F2+15C+18Fe=CaF2+9CaO+6Fe3P+15CO (3)
Ca10(PO4)6F2+15C+12Fe=CaF2+9CaO+6Fe2P+15CO (4)
Ca10(PO4)6F2+15C+3Fe=CaF2+9CaO+3FeP2+15CO (5)
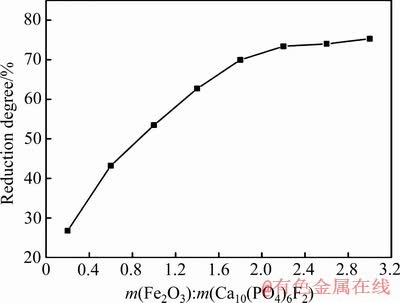
图3 Fe2O3含量对氟磷灰石还原度的影响
Fig. 3 Effect of Fe2O3 contents on reduction degree of fluorapatite
2.1.3 配碳量对氟磷灰石还原度影响

图4 C/O摩尔比对氟磷灰石还原度的影响
Fig. 4 Effect of C/O molar ratio on reduction degree of fluorapatite
Ca10(PO4)6F2-SiO2-Fe2O3-C体系在还原条件为还原时间60 min、还原温度1573 K、体系中SiO2和Fe2O3与氟磷灰石含量比分别为1.8和2.2时,调整C/O摩尔比并考察C/O摩尔比对氟磷灰石还原度的影响,结果如图4所示。由图4可知,随着体系中C/O摩尔比增大,氟磷灰石的还原度逐渐升高,并逐渐达到平衡。这是因为C/O摩尔比的增加会增大氟磷灰石与还原剂的接触面积,有利于氟磷灰石还原;再加上二氧化硅和氧化铁对氟磷灰石还原的促进作用都十分显著,在二者的共同作用下,当C/O摩尔比增加至2.0时,还原度达到峰值87.43%;但是过量添加碳也会阻碍氟磷灰石与二氧化硅、金属铁(氧化铁还原生成)的接触,导致氟磷灰石的还原度基本不再增加。
2.1.4 还原温度对氟磷灰石还原度影响
Ca10(PO4)6F2-SiO2-Fe2O3-C体系在SiO2和Fe2O3与氟磷灰石含量比依次为1.8、2.2,C/O摩尔比为2.0,还原时间为60 min的条件下,氟磷灰石的还原度随还原温度的变化如图5所示。由图5可知,还原温度对氟磷灰石的还原影响显著。随着还原温度的由1473 K升高到1573 K,氟磷灰石的还原度不断增加,且还原速率逐渐增大。这是因为升高还原温度有利于提高还原剂的反应活性,使还原反应速率增大,氟磷灰石的还原度快速升高。
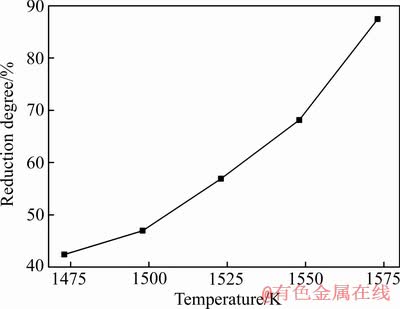
图5 还原温度对氟磷灰石还原度影响
Fig. 5 Effect of reduction temperature on reduction degree of fluorapatite
2.1.5 还原时间对氟磷灰石还原度影响
Ca10(PO4)6F2-SiO2-Fe2O3-C体系在SiO2和Fe2O3与氟磷灰石含量比依次为1.8、2.2,C/O摩尔比为2.0,还原温度为1573 K的条件下,氟磷灰石的还原度随还原时间的变化如图6所示。由图6可知,当还原时间小于15 min时,氟磷灰石的还原度快速上升至74.11%;当还原时间由15 min延长到75 min时,氟磷灰石的还原度缓慢升高至88.12%。表明在一定温度下,随着还原时间的延长,氟磷灰石的还原度逐渐升高,但还原速率逐渐降低;这是因为在初始阶段,由于反应物比较充足,还原反应剧烈发生,氟磷灰石还原度快速升高;随着反应时间的延长,反应物逐渐减少,反应环境逐渐恶化,导致还原反应速率降低,氟磷灰石还原度升高缓慢。
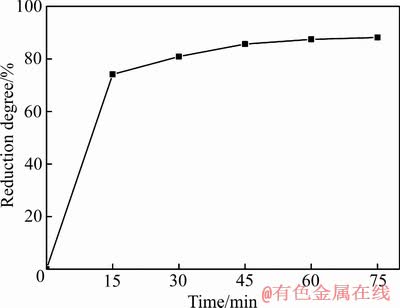
图6 还原时间对氟磷灰石还原度的影响
Fig. 6 Effect of reduction time on reduction degree of fluorapatite
2.2 等温动力学分析
2.2.1 等温动力学试验
为了解氟磷灰石在不同还原温度下的反应速率,确定反应过程的动力学参数和限制环节。采用等温法对Ca10(PO4)6F2-SiO2-Fe2O3-C体系进行等温动力学试验研究,调整SiO2和Fe2O3与氟磷灰石含量比依次为1.8、2.2,C/O摩尔比为2.0,还原温度分别为1473、1498、1523、1548和1573 K。一定温度下,氟磷灰石的还原度与还原时间的关系如图7所示。
2.2.2 机理函数确定
在等温条件下,均相或非均相化学反应的动力学方程[10-11]如式(6)所示:
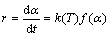 (6)
(6)
式中:r为反应速率,%/min;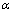 为磷灰石的还原度,%;T为还原时间,min;k(T)为反应速率常数,min-1;
为磷灰石的还原度,%;T为还原时间,min;k(T)为反应速率常数,min-1;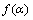 反应机理函数。
反应机理函数。
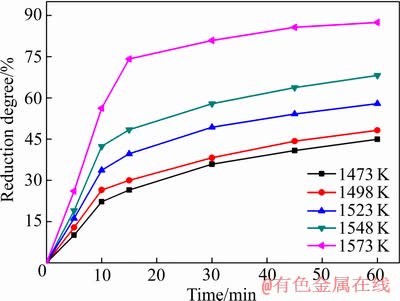
图7 氟磷灰石还原度与还原时间的关系
Fig. 7 Relationship between reduction degree of fluorapatite and reduction time
随着反应的进行,氟磷灰石的还原度不断改变,反应速率也随之变化,所以采用反应速率常数k(T)来表示反应的速率。而对上式进行积分得:
 (7)
(7)
式中: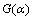 为机理函数的积分形式。
为机理函数的积分形式。
本文采用试错法对30种常用的动力学机理函数模型进行分析[12-14],选择线性相关度最高的动力学机理函数。将图7中的数据代入式7,对30种动力学机理函数进行线性拟合,得到各机理函数线性相关系数。经过对比分析,初步选定Avrami-Erofeev中A1/3模型: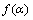 =
=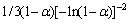 和扩散D5模型:
和扩散D5模型: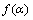 =
=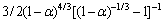 为该体系对应的机理函数,两者在不同温度下的线性相关系数均大于0.97。机理函数对应的
为该体系对应的机理函数,两者在不同温度下的线性相关系数均大于0.97。机理函数对应的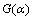 随时间变化的拟合曲线如图8所示。
随时间变化的拟合曲线如图8所示。
2.2.3 动力学参数确定
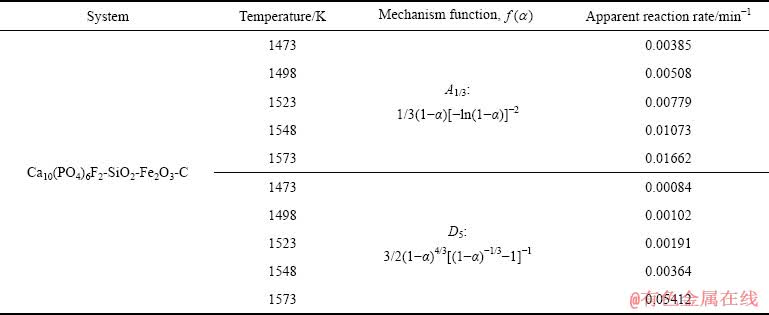
由图8所示各回归直线的斜率可得两种机理函数不同温度对应的反应速率常数k(T),如表2所示。
对于一定的化学反应,反应速率常数k(T)和温度有关,可由Arrhenius方程表示出,如式(8)和式(9)所示。
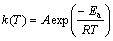 (8)
(8)
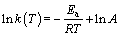 (9)
(9)
式中:A为指前因子,min-1;T为还原温度,K;Ea为反应的表现活化能,J/mol;R为理想气体常数,R=8.314 J/(mol·K)。
将表2中各温度下的反应速率k(T)代入式(9)中,对lnk(T)与1/T进行线性拟合,所得的拟合曲线如图9所示。根据图9回归直线的斜率、截距计算得出氟磷灰石还原反应动力学方程的活化能与指前因子如表3所示。
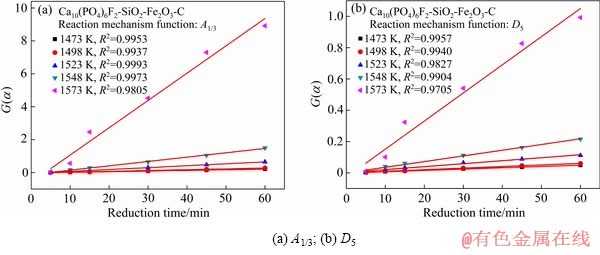
图8  随还原时间变化拟合曲线
随还原时间变化拟合曲线
Fig. 8 Linear fitting of  versus time of reduction of fluorapatite reduction: (a) A1/3; (b) D5
versus time of reduction of fluorapatite reduction: (a) A1/3; (b) D5
表2 氟磷灰石还原反应机理函数及反应速率常数
Table 2 Mechanism function and apparent reaction rates of fluorapatite reduction
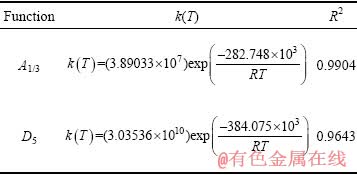
对比表3中两种机理函数的相关系数可知,氟磷灰石在Ca10(PO4)6F2-SiO2-Fe2O3-C体系下还原反应的最佳动力学机理函数为A1/3:1/3(1-α)[-ln(1-α)]-2,符合Avrami-Erofeev方程的随机成核和随后生长模型;最佳动力学方程为
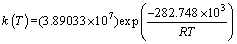 (10)
(10)
式中:指前因子为3.89033×107 min-1;活化能为282.748 kJ/mol。有资料显示[15-16],活化能大于90 kJ/mol时,化学反应的限制环节为固态扩散。因此,氟磷灰石在Ca10(PO4)6F2-SiO2-Fe2O3-C体系下还原反应的限制环节为固态扩散。
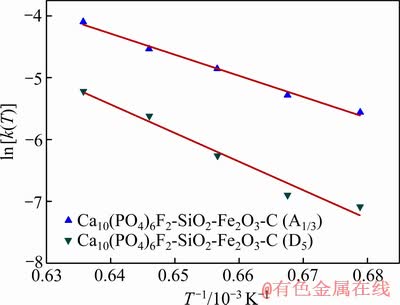
图9 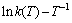 拟合曲线
拟合曲线
Fig. 9 Linear fitting of 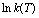 versus
versus 
表3 氟磷灰石还原动力学参数
Table 3 Kinetics parameters of fluorapatite reduction
3 结论
1) 在Ca10(PO4)6F2-SiO2-Fe2O3-C体系中,SiO2和Fe2O3与氟磷灰石含量的最佳比分别为1.8和2.2,最佳C/O摩尔比为2.0;且增加还原温度和还原时间均有利于氟磷灰石的还原反应进行。
2) 在本试验条件下,氟磷灰石深度还原的最佳动力学机理函数为A1/3:1/3(1-α)[-ln(1-α)]-2,符合Avrami-Erofeev方程的随机成核和随后生长模型;最佳动力学方程为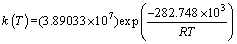 ,其中,指前因子为3.89033×107 min-1,活化能为282.748 kJ/mol,还原反应的限制环节为固态扩散。
,其中,指前因子为3.89033×107 min-1,活化能为282.748 kJ/mol,还原反应的限制环节为固态扩散。
REFERENCES
[1] 张宗旺, 李 健, 李 燕, 潘聪超. 国内难选铁矿的开发利用现状及发展[J]. 有色金属科学与工程, 2012, 3(1): 72-77.
ZHANG Zong-wang, LI Jian, LI Yan, PAN Cong-chao. The development and utilization status of China’s refractory ore[J]. Nonferrous Metals Science and Engineering, 2012, 3(1): 72-77.
[2] 郝先耀, 戴惠新, 赵志强. 高磷铁矿石降磷的现状与存在问题探讨[J]. 金属矿山, 2007(1): 7-10.
HAO Xian-yao, DAI Hui-xin, ZHAO Zhi-qiang. State quo of phosphorous reduction of high phosphorus iron ore and discussion on its problems[J]. Metal Mine, 2007(1): 7-10.
[3] 孙永升, 韩跃新, 高 鹏, 周满庚. 高磷鲕状赤铁矿石工艺矿物学研究[J]. 东北大学学报(自然科学版), 2013, 34(12): 1773-1777.
SUN Yong-sheng, HAN Yue-xin, GAO Peng, ZHOU Man-geng. Study on process mineralogy of a high phosphorus oolitic hematite ore[J]. Journal of Northeastern University (Natural Science), 2013, 34(12): 1773-1777.
[4] LI Shu-fei, SUN Yong-sheng, HAN Yue-xin, SHI Guang-quan, GAO Peng. Fundamental research in utilization of an oolitic hematite by deep reduction[J]. Advanced Materials Research, 2011, 158: 106-112.
[5] 栗艳锋, 韩跃新, 孙永升, 高 鹏, 宫贵臣. 鲕状赤铁矿深度还原过程中铁颗粒粒度和形貌特征分析[J]. 中南大学学报(自然科学版), 2018, 49(4): 15-21.
LI Yan-feng, HAN Yue-xin, SUN Yong-sheng, GAO Peng, GONG Gui-chen. Characteristic analysis of iron particle size and microtopography of oolitic hematite in coal-based reduction process[J]. Journal of Central South University (Science and Technology), 2018, 49(4): 15-21.
[6] HAN Yue-xin, LI Guo-feng, GAO Peng, SUN Yong-sheng. Reduction behaviour of apatite in oolitic hematite ore using coal as a reductant[J]. Ironmaking & Steelmaking, 2016, 44(4): 287-293.
[7] 高 鹏, 孙永升, 邹春林, 韩跃新. 深度还原工艺对铁颗粒粒度影响规律研究[J]. 中国矿业大学学报, 2012, 41( 5): 817-820.
GAO Peng, SUN Yong-sheng, ZOU Chun-lin, HAN Yue-xin. Effect of reduction process on size of iron grain[J]. Journal of China University of Mining & Technology, 2012, 41( 5): 817-820
[8] 邱礼有, 梁 斌. 氟磷灰石固态还原过程的实验研究[J]. 化工学报, 1996, 47(1): 65-71.
QIU Li-you, LIANG Bin. Investigation on the solid state reduction of fluorapatite[J]. CIESC Journal, 1996, 47(1): 65-71.
[9] 孙永升, 栗艳锋, 王定政, 韩跃新. 氟磷灰石还原过程热力学分析[J]. 东北大学学报(自然科学版), 2019, 40(6): 875-880.
SUN Yong-sheng, LI Yan-feng, WAN Ding-zheng, HAN Yue-xin. Thermodynamic analysis of the reduction process of fluorapatite[J]. Journal of Northeastern University (Natural Science), 2019, 40(6): 875-880.
[10] WANG Ze-hong, LI Guo-feng, SUN Yong-sheng, HE Ming-zhao. Reduction behavior of hematite in the presence of coke[J]. International Journal of Minerals, Metallurgy, and Materials, 2016, 23(11): 1244-1251.
[11] CHEN Chao-yi, CHEN Hui-lin, MA Ya-qin, LIU Jing. Hydrogen desorption kinetics mechanism of Mg-Ni hydride under isothermal and non-isothermal conditions[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(1): 160-166.
[12] LI Peng, YU Qing-bo, QIN Qin, LEI Wei. Kinetics of CO2/coal gasification in molten blast furnace slag[J]. Industrial & Engineering Chemistry Research, 2012, 51(49): 15872-15883.
[13] LI Peng, YU Qing-bo, XIE Hua-qing, QIN Qin. CO2 gasification rate analysis of Datong coal using slag granules as heat carrier for heat recovery from blast furnace slag by using a chemical reaction[J]. Energy Fuels, 2013, 27(8): 4810-4817.
[14] SUNT T, ZHAI Y C, JIANG H, GONG H. Studies on the thermal decomposition kinetics and mechanism of ammonium niobium oxalate[J]. Journal of Thermal Analysis & Calorimetry, 2009, 98(2): 449-455.
[15] TIERNAN M J, BARNES P A, PARKES G M B. Reduction of iron oxide catalysts: The investigation of kinetic parameters using rate perturbation and linear heating thermoanalytical techniques[J]. Journal of Physical Chemistry B, 2001, 105(1): 220-228.
[16] HAMDY K M. Isothermal reduction kenetics of Fe2O3 mixed with 1-10% Cr2O3 at 1173 K-1473 K[J]. Transactions of the Iron & Steel Institute of Japan, 2007, 40(4): 309-314.
Influencing factors and isothermal kinetics of fluorapatite reduction
LI Yan-feng, SUN Yong-sheng, HAN Yue-xin, WANG Ding-zheng
(College of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China)
Abstract: To study the influencing factors and isothermal kinetic parameters of fluoropatite reduction in the process of coal-based reduction of high-phosphorus iron ore, the effects of reduction time, reduction temperature, and content of silica, iron oxide or carbon on reduction degree of fluorapatite were studied by mixing pure minerals in the experiment. On this basis, the reduction kinetic mechanism function and isothermal kinetic equation of fluoroapatite in Ca10(PO4)6F2-SiO2-Fe2O3-C system were investigated. The results show that increasing the amounts of silica, iron oxide and carbon, and decreasing time and temperature, can accelerate the reduction reactions of fluoroapatite under experiment conditions. In Ca10(PO4)6F2-SiO2-Fe2O3-C system, the optimum ratios of silica, iron oxide comparing to fluorapatite are 1.8 and 2.2, respectively, and the optimal C/O molar ratio is 2.0. Based on the optimum amount of reactants, the best mechanism function for the reduction of fluoroapatite is the A1/3: 1/3(1-α)[-ln(1-α)]-2; the best kinetic equation is K(T)=3.89033×107exp(-282.748×103/RT), where the pre-exponential factor is 3.89033×107 min-1, and the activation energy is 282.748 kJ/mol. The limiting factor of the reduction reaction is solid-state diffusion.
Key words: fluoropatite; Ca10(PO4)6F2-SiO2-Fe2O3-C system; influencing factors; kinetic equation
Foundation item: Project(51604063) supported by the National Natural Science Foundation of China
Received date: 2019-02-26; Accepted date: 2019-10-12
Corresponding author: SUN Yong-sheng; Tel: +86-24-83689272; E-mail: neusunyongsheng@163.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51604063)
收稿日期:2019-02-26;修订日期:2019-10-12
通信作者:孙永升,副教授,博士;电话:024-83687120;E-mail:neusunyongsheng@163.com