废弃环氧树脂电路板的热解机理及动力学研究
湛志华,丘克强
(中南大学 化学化工学院,湖南 长沙,410083)
摘要:采用热分析技术(TGA)研究废弃环氧树脂电路板在氮气气氛和真空条件下热解过程的反应机理和动力学行为。将热解过程分为2个阶段进行机理和动力学研究。研究结果表明:环氧电路板的热解过程第1步是失去水分和小分子物质,第2步是有机材料的裂解。氮气氛围和真空2种条件下裂解反应第1阶段遵循共同的机理函数,是以成核及核成长为控制步骤的A3机理,反应级数为3级;第2阶段都是以幂函数不均匀生长为控制步骤的C1.5机理;真空热解有利于降低反应的活化能;氮气氛围裂解反应各阶段的表观活化能和频率因子分别为:E1=239.95 kJ/mol,A1=1.94×1022 s-1;E2=130.73 kJ/mol,A2=1.88×1013 s-1;在真空条件下,裂解反应各阶段的表观活化能和频率因子分别为:E1=74.24 kJ/mol,A1=1.52×108 s-1;E2=41.64 kJ/mol,A2=5.16×1010 s-1。
关键词:电路板;热裂解;反应机理;动力学
中图分类号:X705 文献标志码:A 文章编号:1672-7207(2011)03-0610-07
Mechanism and kinetics of pyrolysis of discarded epoxy printed circuit boards
ZHAN Zhi-hua, QIU Ke-qiang
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: Reaction mechanism and kinetic behavior of discarded epoxy printed circuit board pyrolysis were studied by means of the thermogravimetric analysis (TGA) technique in nitrogen and vacuum conditions. The results show that there are two steps in the thermal decomposition of PCB. In the first step, some moisture and smaller molecules are lost. And then macromolecule pyrolysis in PCB take place in the second step. The second step can disassemble into two phases. The pyrolysis reaction of the first phase in nitrogen and vacuum conditions belongs to the A3 mechanism with nucleation and nuclei growth reaction as the control step. The second phase in nitrogen and vacuum belongs to the C1.5 mechanism with power law reaction as control steps. The apparent active energy of two phases is: 239.95, 130.73 kJ/mol (in nitrogen), 74.24, 41.64 kJ/mol (in vacuum) respectively, and the frequency factor is 1.94×1022 s-1, 1.88×1013 s-1 (in nitrogen), 1.52×108 s-1, 5.16×1010 s-1 (in vacuum) respectively.
Key words: printed circuit boards; pyrolysis; reaction mechanism; kinetics
环氧树脂印刷线路板(PCB, Printed circuit boards)是一种热固性复合材料,其主要成分是环氧树脂、玻璃布和铜[1]。环氧树脂是电路板中主要的有机物质。国内每年环氧树脂用量仅在覆铜板行业就达3万t以上, 随着覆铜板工业的发展, 其需求量还在不断增 加[2],印刷线路板的使用量及其废弃物数量也在急剧增长。近几年,全世界PCB工业产量平均增长率为8.7%,中国大陆增长率为14.4%[3]。如何有效处理PCB废弃物,已成为一个热点问题[3-5]。用热解方法处理固体废物具有减量化、无害化和资源回收等优点[6-7]。真空热解有利于高分子发生裂解,促进热解反应得到的挥发物迅速从颗粒内部和表面离开,从而强化气相的挥发过程,有力限制二次裂解及再聚合反应发生[7],特别是降低了卤化氢(阻燃剂的裂解产物)发生二次反应生成卤代烃的几率,减少有毒气体的产生,有利于提高裂解油的产率和质量[8-9]。Chen等[10]在热重分析的基础上探讨了环氧树脂的热分解特性。孙路石等[11]对氧气气氛下PCB化学反应动力学进行了研究。彭绍洪等[6]研究了混合废旧电路板在真空下的热解特性。这些工作在研究PCB热解失重动力学时,对失重的动力学机制仅假设为最简单的幂函数形式而未进行广泛的检验,因此,所得每步分解机理和动力学参数准确性不高。为此,本文作者采用同步热分析仪(WRT-22P型) 及真空同步热分析仪(STA409PC型)对废旧环氧电路板进行真空条件和氮气保护下的热重实验,用Doyle 方程和Coats-Redfern方程[12-13],代入16个常见固相热分解机理的动力学机制函数,研究环氧树脂电路板热裂解反应机理及其动力学行为,以便为废弃环氧树脂电路板热裂解资源化的理论研究和工业化回收应用提供基础数据。
1 实验
1.1 试样选取
试样为长沙煤炭研究所电路板厂提供的废弃环氧树脂电路板。为研究电路板板材的热解特性,去除电子元件等对实验的干扰,选择光板作为实验样品。将电路板粉碎,颗粒直径小于1.0 mm。
1.2 实验方法
采用同步热分析仪(WRT-22P型,上海分析仪器厂制造)和真空同步热分析仪(STA409PC型,德国耐驰仪器制造有限公司制造)进行热解失重研究。分别于氮气气氛和真空环境中考察电路板的热解行为,升温速率均为5 ℃/min。氮气保护下的热重实验中,氮气流量为100 mL/min;真空热重实验反应系统稳定时压力为1.35 kPa。
2 结果与讨论
2.1 TG-DTG 热分解曲线分析
图1和图2所示为样品在氮气气氛和真空条件下的热重(TG)曲线和微分热重(DTG)曲线。从图1可知:在2种热解条件下,样品失重的起始温度基本一致;真空条件下的失重曲线相对氮气气氛下整体往高温区移动,在相同的升温速度下,达到相同转化率所需要的时间延长。在真空条件下,热滞后现象相对明显。真空条件下的热解最终固体剩余物质量与物料原始质量比比氮气气氛下的小,即真空热分解残余量少,说明真空条件下固体产物减少,挥发性产物增加。从图2可知:真空条件下的DTG图比氮气条件下的在失重的开始温度区多一个很小的失重峰,该峰由样品中所含的水分、小分子物质和气体等的挥发所导致,这些小分子物质逃逸固体反应物表面的温度为214~259 ℃。而从氮气气氛下得到的DTG曲线上只能观察到1个失重峰,其原因是在真空反应体系下,物质的表面蒸气压下降,沸点降低,与常压的氮气氛围相比,小分子物质逃逸固体反应物表面要容易很多,并且在真空
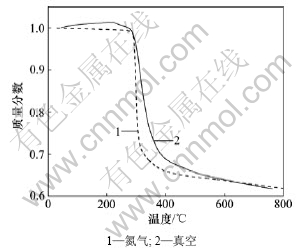
图1 不同裂解条件下的失重曲线
Fig.1 TG curves of the samples at different pyrolysis conditions
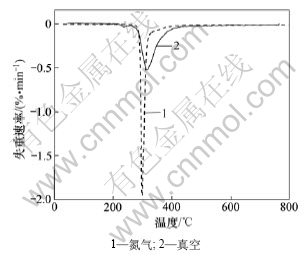
图2 不同裂解条件下的DTG曲线
Fig.2 DTG curves of the samples at different pyrolysis conditions
泵的机械力作用下,使小分子物质逃逸变得更容易。发生热裂解的物质主要是环氧树脂,而高分子物质主链发生裂解之前,侧链先断裂反应生成小分子物质。真空条件下热裂解活化能要比常压氮气保护条件下的热裂解活化能降低约50%。可见,真空条件有利于热解主反应发生前小分子物质的生成与逃逸。从图2可以看出:热解发生的起始温度与前1个失重峰尾对应温度比较接近。综合上述原因可见:在氮气条件下得到的DTG曲线只能观察到1个失重峰。
2种条件下电路板热失重温度区间基本一致,为270~594 ℃,起始分解温度均为270 ℃。594 ℃时失重反应基本完成,对应失重率均为36%。从图2可以看出:2种条件下的热分解速率相差较大,在氮气条件下DTG曲线较窄且尖锐,在氮气和真空条件下最大分解速率分别为1.95%/min和0.5%/min,它们的对应温度分别为298 ℃和317 ℃。
2.2 热分解反应机理及动力学分析
根据非等温反应动力学理论,线性升温条件下固相物质的分解反应动力学方程[14-15]为,
 (1)
(1)
式中:β为线性升温速率(℃/min);a为在温度T时的
反应物转化率;f(a)为动力学机制函数;k(T)为反应速率常数。
根据Arrhenius公式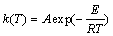 ,代入式(1)得:
,代入式(1)得:
 (2)
(2)
式中:A 为频率因子(min-1);E为活化能(kJ/mol)。为了据试样的单条TG 曲线对固相分解反应非等温动力学进行研究,本研究采用Doyle机理方程[12]:
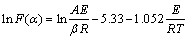 (3)
(3)
式中: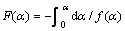 。由式(3)可知:
。由式(3)可知: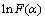 对1/T作图应是1条直线。取16个常见固体热分解反应机理[15-17]的
对1/T作图应是1条直线。取16个常见固体热分解反应机理[15-17]的 值(见表2)分别代入式(3),用最小二乘法求其线性相关系数r。同时,从直线的斜率求得反应活化能E,从截距求得反应频率因子A。
值(见表2)分别代入式(3),用最小二乘法求其线性相关系数r。同时,从直线的斜率求得反应活化能E,从截距求得反应频率因子A。
为了进行对比,本研究也采用了Coats-Redfern方程[13]求取动力学参数:
 (4)
(4)
表1 常见固体热分解反应机理
Table 1 Most frequently used mechanisms of solid state processes
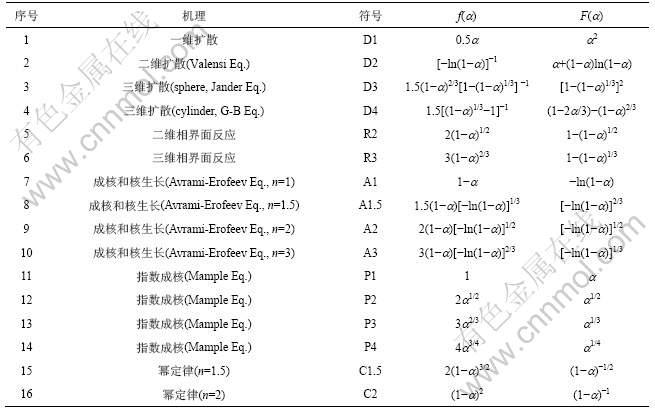
2RT/Ea随温度变化很小,在程序升温过程中可视为常数,故ln[F(a)/T2]对1/T作图应是1条直线,据斜率可求得反应活化能E,据截距求得反应频率因 子A。
以上2种积分方法是直接由实验数据a和T求算动力学参数,避开了由da/dT求算可能引入的计算误算,是较合理的。
采用Doyle方程和Coats-Redfern方程代入a和T的实验数据,分别对环氧树脂板在氮气气氛和真空条件下热分解反应动力学参数进行计算,结果见表2(氮气条件)和表3(真空条件)(其中s 为平均标准偏差)。
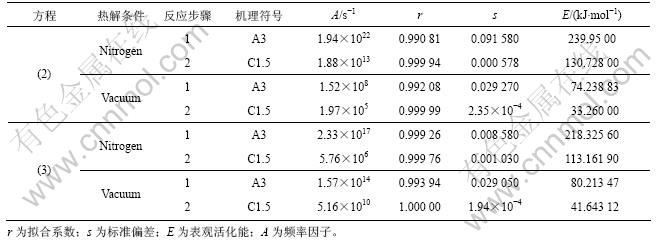
判断固相分解反应机理一般以相关性(r)为主要判据,如相关性均较接近,可选取平均标准偏差及相关经验为辅助判据。表4所示为2 种计算方法的最优裂解反应机理及活化能与频率因子。从表4可以看出:2种计算方法结果较接近,但由于应用Doyle 方程的条件比Coats-Redfern方程的条件更严格,故其拟合的相关性较Coats-Redfern 法普遍有所提高。
图3和图4所示为a从0.05开始以增幅0.025增加到0.90,共35个数据16个机理函数的lnF(a)-1/T
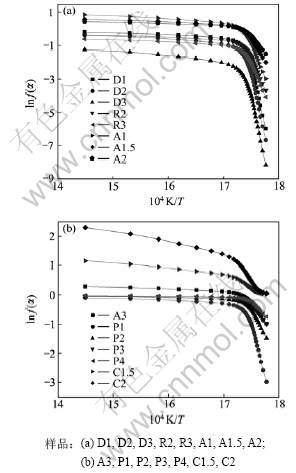
图3 氮气气氛下的lnF(a)-1/T曲线
Fig.3 lnF(a)-1/T curves of sample in nitrogen gas condition
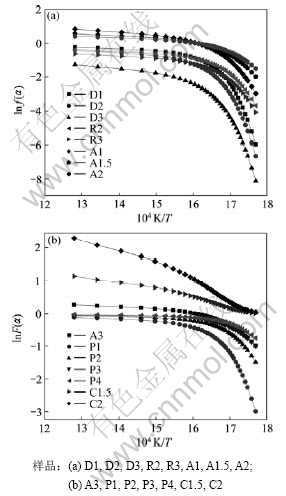
图4 真空条件下的lnF(a)-1/T曲线
Fig.4 lnF(a)-1/T curves of sample in vacuum condition
图,每种裂解条件(氮气气氛和真空)得到16条曲线。从图3和图4可知:各曲线均存在明显的拐点,说明至少有2个活化能,可能需要多个机理函数表示。因此,将lnF(a)-1/T曲线按对应温度由低到高分为2段分别拟合求动力学参数。真空条件下的lnF(a)-1/T曲线比氮气气氛下的曲线光滑,拐点不明显,体现在拟合系数均比氮气气氛下的拟合系数略低。
求得的热分解活化能在热分解温度范围内满足Doyle 方程的假设20≤E/(RT)≤60,说明采用Doyle方程对热解反应是合理的,E2=33.26 kJ/mol 不满足要求,故E2和A2的取值应采用方程(4)的计算结果(见表4),即E2=41.64 kJ/mol,A2=5.16×1010 s-1;利用方程(3)的拟合数据与利用方程(4)的拟合计算结果比较接近(见表4),由方程(2)求解的动力学参数是可信的(E2和A2除外,因不满足方程(3)假设条件),故相应的动力学方程式分别为:
在氮气气氛条件下,
表2 据式(3)拟合的环氧树脂电路板氮气气氛下热分解反应固相反应机理
Table 2 Calculated results using Eq.(3) for different solid state reaction mechanisms of pyrolysis of PCBs
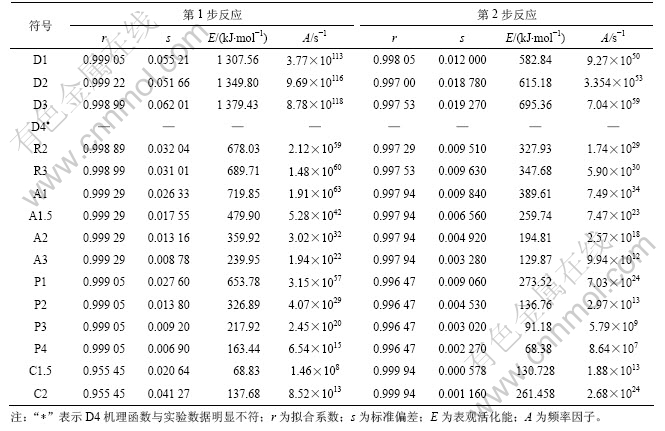
表3 据式(3)拟合的环氧树脂电路板真空热分解反应固相反应机理
Table 3 Calculated results using Eq.(3) for different solid state reaction mechanisms of pyrolysis of PCBs
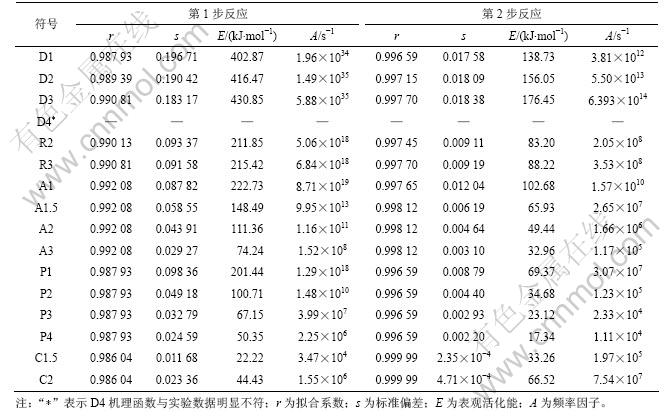
表4 据式(3)和(4)拟合的PCBs最优热分解机理及活化能
Table 4 Calculated results using Eqs.(3) and (4) for the best fit mechanisms of PCBs pyrolysis and apparent activation energy

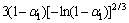

在真空条件下,

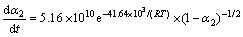
从表2和表3可知:氮气氛围和真空2种条件下裂解的主反应阶段(第1阶段)遵循共同的机理函数,是以成核及核成长为控制步骤的A3 机理,反应级数为3级;第2阶段都是为幂函数不均匀生长为控制步骤的C1.5机理。文献报道的数据大多是在假设裂解机理函数为A1的前提条件下进行拟合计算得到的动力学参数。16个机理函数与实验数据的拟合曲线相关系数均很高,氮气条件下的r均在0.99以上,真空条件下大多数的r也均在0.99以上,说明“在环氧树脂电路板热解动力学求算过程中先假设某一机理函数,然后进行拟合,根据拟合系数很高而判断假设的机理函数正确”是不合理的。
3 结论
(1) 真空条件下的失重曲线相对氮气气氛下的失重曲线整体往高温区移动,在相同的升温速率下,达到相同转化率所需要的时间延长。真空条件下热分解残余量少,说明在真空条件下,有利于减少固体产物的产生,增加挥发性产物(热解油或气的产率增加)。
(2) 氮气氛围和真空2种条件下裂解的主反应阶段(第1阶段)遵循共同的机理函数,是以成核及核成长为控制步骤的A3 机理,反应级数为3级;第2阶段都是为幂函数不均匀生长为控制步骤的C1.5机理。
(3) 真空热解有利于降低反应的活化能。氮气氛围裂解反应各阶段的表观活化能和频率因子分别为:E1=239.95 kJ/mol,A1=1.94×1022 s-1;E2=130.73 kJ/mol,A2=1.88×1013 s-1。在真空条件下,裂解反应各阶段的表观活化能和频率因子分别为:E1= 74.24 kJ/mol,A1=1.52×108 s-1;E2=41.64 kJ/mol,A2= 5.16×1010 s-1。
参考文献:
[1] 熊祖鸿, 李海滨, 吴创之, 等. 印刷线路板废弃物的热解与动力学实验研究[J]. 环境污染治理技术与设备, 2006, 7(10): 47-50.
XIONG Zu-hong, LI Hai-bin, WU Chuang-zhi, et al. A study on pyrolysis and kinetics of printed circuit boards wastes[J]. Techniques and Equipment for Environmental Pollution Control, 2006, 7(10): 47-50.
[2] 汤冬英, 邓海波. FR-4覆铜板专用溴化环氧树脂合成新工艺[J].化工进展, 2005, 24(4): 441-444.
TANG Dong-ying, DENG Hai-bo. New synthesis process of brominized epoxy resin for FR-4 copper clad panel[J]. Chemical Industry and Engineering Progress, 2005, 24(4): 441-444.
[3] HUANG Kui, GUO Jie, XU Zhen-ming. Recycling of waste printed circuit boards: A review of current technologies and treatment status in China[J]. J Anal Appl Pyrolysis, 2009, 164(2/3): 399-408.
[4] 李芝华, 谢科予, 郑子樵. 磨碎玻璃纤维/聚氨酯/环氧灌封材料的形貌结构与力学性能[J]. 中南大学学报: 自然科学版, 2007, 38(1): 51-55.
LI Zhi-hua, XIE Ke-yu, ZHENG Zi-qiao. Morphology and mechanical properties of MG/PU/EP encapsulating composite[J]. Journal of Central South University: Science and Technology, 2007, 38(1): 51-55.
[5] 李芝华, 任冬燕, 郑子樵, 等. 聚氨酯改性TDE-85/MeTHPA环氧树脂体系的结构表征[J]. 中南大学学报: 自然科学版, 2007, 38(3): 399-403.
LI Zhi-hua, REN Dong-yan, ZHENG Zi-qiao, et al. Structural identification of PU-modified TDE-85/MeTHPA epoxy resin[J]. Journal of Central South University: Science and Technology, 2007, 38(3): 399-403.
[6] 彭绍洪, 陈烈强, 甘舸, 等. 废旧电路板真空热解[J]. 化工学报, 2006, 57(11): 2720-2725.
PENG Shao-hong, CHEN Lie-qiang, GAN Ge, et al. Vacuum pyrolysis of waste printed circuit boards[J]. Journal of Chemical Industry and Engineering, 2006, 57(11): 2720- 2726.
[7] Chien Y C, Wang H P, Lin K S, et al. Fate of bromine in pyrolysis of printed circuit board wastes[J]. Chemosphere, 2000, 40(4): 383-387.
[8] Hall W J, Williams P T. Separation and recovery of materials from scrap printed boards[J]. Resources, Conservation and Recycling, 2007, 51(3): 691-709.
[9] Lee J C, Song H T, Yoo J M. Present status of the recycling of waste electrical and electronic equipment in Korea[J]. Resources, Conservation and Recycling, 2007, 50(4): 380-397.
[10] Chen K S, Yeh R Z. Pyrolysis kinetics of epoxy resin in a nitrogen atmosphere[J]. Journal of Hazardous Materials, 1996, 49(2/3): 105-113.
[11] 孙路石, 陆继东, 曾 丽, 等. 印刷线路板热分解动力学特性[J]. 华中科技大学学报, 2001, 29(12): 40-42.
SUN Lu-shi, LU Ji-dong, ZENG Li, et al. Kinetic study on thermal degradation of printed circuit boards[J]. Journal of Huazhong University of Science and Technology, 2001, 29(12): 40-42.
[12] Rosu D, Cascaval C N, Mustata F, et al. Cure kinetics of epoxy resins studied by non-isothermal DSC data[J]. Thermochim Acta, 2002, 383(1/2): 119-127.
[13] Miranda R, Yang J, Roy C, et al. Vacuum pyrolysis of commingled plastics containing PVC. Ⅰ: Kinetic study[J]. Polymer Degradation and Stability, 2001, 72(3): 469-491.
[14] 李峰, 何静, 杜以波, 等. a-磷酸锆的制备及热分解非等温动力学研究[J]. 无机化学学报, 1999, 15(1): 55-60.
LI Feng, He Jing, DU Yi-bo, et al. Thermal decomposition of a-Zirconium phosphate[J]. Journal of Inorganic Chemistry, 1999, 15(1): 55-60.
[15] 黄小芳, 吴玉龙, 杨明德, 等. 水氯镁石的热解机理及动力学[J]. 过程工程学报, 2006, 6(5): 729-733.
HUANG Xiao-fang, WU Yu-long, YANG Ming-de, et al. Mechanism and kinetics of thermal decomposition of bischofite[J]. The Chinese Journal of Process Engineering, 2006, 6(5): 729-733.
[16] 马伟, 王苏, 崔季平, 等. 酚醛树酯的热解动力学模型[J]. 物理化学学报, 2008, 24(6): 1090-1094.
MA Wei, WANG Su, CUI Ji-ping, et al. Thermal decomposition kinetic model of phenolic resin[J]. Acta Phys-Chim Sin, 2008, 24(6): 1090-1094.
[17] Gao X, Dollimore D. The thermal decomposition of oxalates 26: A kinetic study of the thermal decomposition of manganese(Ⅱ) oxalate dehydrant[J]. Thermochimica Acta, 1993, 215: 47-63.
(编辑 张曾荣)
收稿日期:2010-02-30;修回日期:2010-04-10
基金项目:国家“863”计划项目(2006AA06Z375);广东省社会发展计划项目(2006A36703002)
通信作者:丘克强(1956-),男,广东梅县人,博士,教授,博士生导师,从事真空分离理论与工程,高纯材料加工和功能纳米材料制备理论与技术及二次资源绿色循环化学与技术等研究;电话:0731-88877364;E-mail: qiuwhs@sohu.com