利用铁酸钠脱除铝酸钠溶液中的S2-
来源期刊:中国有色金属学报(英文版)2016年第5期
论文作者:李小斌 牛飞 谭杰 刘桂华 齐天贵 彭志宏 周秋生
文章页码:1419 - 1424
关键词:铁酸钠;S2-;铝酸钠溶液;脱硫
Key words:sodium ferrite; S2-; sodium aluminate solution; desulfurization
摘 要:研究铁酸钠的合成及其脱除铝酸钠溶液中S2-的反应。热力学分析表明通过焙烧Fe2O3和Na2CO3的混合物合成铁酸钠的最低温度为810 K。结果表明:在1173 K焙烧60 min可以完全形成铁酸钠,且升高温度和减小Na2CO3粒径有利于加速铁酸钠的形成。铁酸钠可高效脱除铝酸钠溶液中的S2-,在铁硫摩尔比为1:1~1.5:1,温度为373 K,反应时间为60 min的条件下,脱硫率约为70%。脱硫过程是通过 与 S2- 在铝酸钠溶液中反应生成NaFeS2·2H2O沉淀实现的,脱硫效率取决于铁酸钠溶解生成的 。
Abstract: The synthesis of sodium ferrite and its desulfurization performance in S2--bearing sodium aluminate solutions were investigated. The thermodynamic analysis shows that the lowest temperature is about 810 K for synthesizing sodium ferrite by roasting the mixture of ferric oxide and sodium carbonate. The results indicate that the formation process of sodium ferrite can be completed at 1173 K for 60 min, meanwhile raising temperature and reducing Na2CO3 particle size are beneficial to accelerating the formation of sodium ferrite. Sodium ferrite is an efficient desulfurizer to remove the S2- in aluminate solution, and the desulfurization rate can reach approximately 70% at 373 K for 60 min with the molar ratio of iron to sulfur of 1:1-1.5:1. Furthermore, the desulfurization is achieved by NaFeS2·2H2O precipitation through the reaction of and S2- in aluminate solution, and the desulfurization efficiency relies on the generated by dissolving sodium ferrite.
Trans. Nonferrous Met. Soc. China 26(2016) 1419-1424
Xiao-bin LI1, Fei NIU1, Jie TAN1,2, Gui-hua LIU1, Tian-gui QI1, Zhi-hong PENG1, Qiu-sheng ZHOU1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Wanbao Mining Ltd., Beijing 100053, China
Received 24 July 2015; accepted 17 November 2015
Abstract: The synthesis of sodium ferrite and its desulfurization performance in S2--bearing sodium aluminate solutions were investigated. The thermodynamic analysis shows that the lowest temperature is about 810 K for synthesizing sodium ferrite by roasting the mixture of ferric oxide and sodium carbonate. The results indicate that the formation process of sodium ferrite can be completed at 1173 K for 60 min, meanwhile raising temperature and reducing Na2CO3 particle size are beneficial to accelerating the formation of sodium ferrite. Sodium ferrite is an efficient desulfurizer to remove the S2- in aluminate solution, and the desulfurization rate can reach approximately 70% at 373 K for 60 min with the molar ratio of iron to sulfur of 1:1-1.5:1. Furthermore, the desulfurization is achieved by NaFeS2·2H2O precipitation through the reaction of 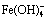 and S2- in aluminate solution, and the desulfurization efficiency relies on the
and S2- in aluminate solution, and the desulfurization efficiency relies on the 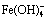 generated by dissolving sodium ferrite.
generated by dissolving sodium ferrite.
Key words: sodium ferrite; S2-; sodium aluminate solution; desulfurization
1 Introduction
With the boom of the alumina industry in recent decades, high quality bauxite resources is becoming depleted in China. However, there are over 560 million tons of high-sulfur bauxite [1,2] with high A/S (mass ratio of alumina to silica) which cannot be effectively utilized up till now, due to the negative impacts of sulfur [3-5] such as causing loss of caustic soda, degrading alumina product, corroding steel equipment and deteriorating red mud settling performance. Among the sulfur bearing minerals in bauxite, pyrite (FeS2) is the most harmful to the alumina production, and readily reacts with aluminate solution to produce S2- entering sodium aluminate solution in the Bayer digestion process [6]. Consequently, much attention has been paid to the desulfurization of high-sulfur bauxite, and the researches reported can be categorized into two aspects, i.e., the pretreatment of high-sulfur bauxite and removal of sulfur from solution. The former mainly includes mineral beneficiation [7] and roasting [8], characterized by poor desulfurization performance or cost pressure, while the latter involves the removal of  or S2- [1,2,9,10]. Either barium salt or lime is employed to remove
or S2- [1,2,9,10]. Either barium salt or lime is employed to remove  by precipitating BaSO4 or 3CaO·Al2O3·CaSO4·nH2O in solution, and zinc oxide was reported to remove S2- by forming insoluble ZnS. It was also reported that S2- was first oxidized by H2O2 or O2 into
by precipitating BaSO4 or 3CaO·Al2O3·CaSO4·nH2O in solution, and zinc oxide was reported to remove S2- by forming insoluble ZnS. It was also reported that S2- was first oxidized by H2O2 or O2 into  which was then removed by the methods mentioned above. However, these methods of desulfurization in sodium aluminate solution have insurmountable disadvantages, for instance, expensiveness of barium salt and zinc oxide, low desulfurization rate for lime and harmful
which was then removed by the methods mentioned above. However, these methods of desulfurization in sodium aluminate solution have insurmountable disadvantages, for instance, expensiveness of barium salt and zinc oxide, low desulfurization rate for lime and harmful 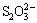 formation for H2O2 and O2.
formation for H2O2 and O2.
In view of low solubility of various iron sulfides in alkaline solution and existence of iron minerals in bauxite, it is fairly rewarding to develop a desulfurization method with the iron-based compounds to form an insoluble sulfur iron compounds. However, there are few publications about the removal of S2- by precipitation in concentrated caustic solution though iron sulfide complexes are readily formed in acid or neutral solutions [11-13]. We reported that S2- ion in aluminate solution can be removed by adding fresh Fe(OH)2 and Fe(OH)3 [14], and the removal efficiency can reach up to 92.70% and 86.10%, respectively. However, the preparations of Fe(OH)2 and Fe(OH)3 are relatively complicated and Fe(OH)2 is rather unstable. Therefore, it is worthwhile to investigate desulfurization by
precipitating S2- ion in sodium aluminate solution using other cheap and available iron-based desulfurizers. Considering facile formation of Fe(OH)3 by hydrolysis of sodium ferrite in aluminate solution, this work focused on the sodium ferrite synthesis, the desulfurization by adding sodium ferrite and its desulfurization mechanism.
2 Experimental
2.1 Materials
Sodium aluminate solutions with a designated molar ratio (αk) of caustic soda to alumina were prepared by dissolving industrial grade aluminum hydroxide (Chalco) into sodium hydroxide solution based on stoichiometry ratio. S2--bearing sodium aluminate solution was obtained by adding Na2S·9H2O (analytical grade) into the sodium aluminate solution. Both Fe2O3 and Na2CO3 used to synthesize sodium ferrite were analytical grade reagents.
2.2 Experimental procedure
2.2.1 Preparation of sodium ferrite
15 g mixture of Fe2O3 and Na2CO3 with the molar ratio of 1:1 was placed in a corundum crucible and then roasted with muffle furnace at preset temperature for a certain duration. After being cooled naturally in air to room temperature, the roasted product was then weighed in order to record the mass variation.
2.2.2 Desulfurization in solution with sodium ferrite
The sodium ferrite and 100mL S2--bearing sodium aluminate solution were added into a 150 mL stainless bomb, in which the amount of sodium ferrite was determined according to a given molar ratio of iron provided by sodium ferrite to sulfur existing in solution (n(Fe)/n(S)). The stainless bomb was then sealed and immersed in glycerol cell at preset temperature, after which the stainless bomb was rotated for a certain duration. In addition, two 18 mm in diameter and two 8 mm in diameter steel balls were also added into the bomb for strengthening agitation. The resultant slurry was filtered and washed by hot water, the residue was then obtained by drying the washed cake at (323±1) K.
2.2.3 Analytic methods
The concentrations of caustic soda (Na2O) and alumina (Al2O3) were determined by titration. The sulfur content in desulfurization residue was measured by an HDS3000 sulfur analyzer (Hunan Huade Electronics Corporation, China). While, the synthesized sodium ferrite and desulfurization residue were characterized using an X-ray diffractometer (XRD, D8-Advance, Bruker Corporation) with Cu Kα radiation and a scanning speed of 10.0 (°)/min.
2.3 Data processing
2.3.1 Relative yield of sodium ferrite
The yield of sodium ferrite was determined by the mass variation of reactants and resultants owing to the release of CO2 in the reaction. Therefore, the relative yield (Q) of sodium ferrite, representing its content in roasted product, can be calculated according to the following equation:
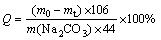 (1)
(1)
where Q is the yield of sodium ferrite, m0 and mt are the mass of mixture and roasted product, respectively (g), m(Na2CO3) is the mass of Na2CO3 in mixture (g).
2.3.2 Desulfurization rate
The desulfurization rate with sodium ferrite was calculated based on Eq. (2).
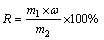 (2)
(2)
where R is the desulfurization rate, m1 is the mass of desulfurization residue (g), ω is the sulfur content in desulfurization residue, m2 is the mass of sulfur added in the sodium aluminate solution (g).
3 Results and discussion
3.1 Synthesis of sodium ferrite
3.1.1 Thermodynamic analysis
Ferric oxide in bauxite can react with sodium carbonate to generate sodium ferrite [15] in the soda-lime sintering process at about 1573 K, as shown by the following equation:
Fe2O3+Na2CO3=2NaFeO2+CO2 (3)
To understand the formation rule of sodium ferrite at low temperature, the thermodynamic analysis of the reaction between ferric oxide and sodium carbonate was carried out with the aid of the alumina thermodynamic database [16]. The calculation results are shown in Fig. 1.
Figure 1 shows that the reaction Gibbs free energy change (ΔGΘ) decreases with increasing the reaction temperature. Meanwhile, the reaction of sodium ferrite formation may proceed spontaneously even at low temperature of 810 K, because the ΔGΘ for Reaction (3) changes from positive to negative at this temperature. The abrupt changes in the temperature range of 800-1000 K are attributed to the phase transitions of α-NaFeO2 to β-NaFeO2 (at 870 K) and α-Fe2O3 to β-Fe2O3 (at 960 K) [16]. Thus, the sodium ferrite can be thermodynamically synthesized at temperature much lower than 1573 K.
3.1.2 Effect of temperature on formation of sodium ferrite
In order to save the energy consumption for the synthesis of sodium ferrite, the low temperature roasting is desired. According to the above thermodynamic calculation, the mixture should be roasted at the temperature higher than 810 K. Therefore, the sodium ferrite synthesis was investigated at temperature ranging from 873 to 1273 K, and the XRD patterns of roasted products are shown in Fig. 2.
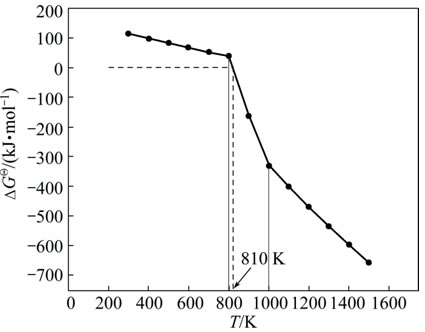
Fig. 1 Relationship between reaction Gibbs free energy change (ΔGΘ) and temperature (T) for Reaction (3)
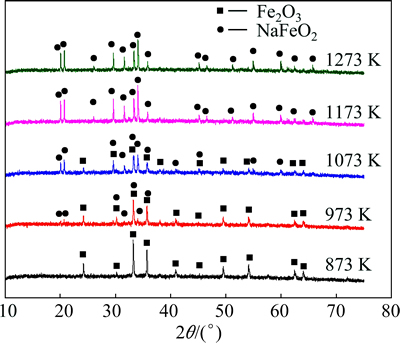
Fig. 2 XRD patterns of products by roasting mixture of Na2CO3 and Fe2O3 at various temperatures for 60 min
As shown in Fig. 2, there are both sodium ferrite and ferric oxide in the products obtained at 973 and 1073 K, while only sodium ferrite exists in roasted products obtained at 1173 and 1273 K. Whereas, no visible characteristic peaks can be observed for sodium ferrite at 873 K, which may be due to dynamic factors. Therefore, although elevating temperature favors the reaction between Fe2O3 and Na2CO3, the sodium ferrite can be manufactured by roasting at relative low temperature.
3.1.3 Effect of Na2CO3 particle size and duration on formation of sodium ferrite
Furthermore, the effect of Na2CO3 particle size and duration on the relative yield of sodium ferrite was also investigated, and the results are listed in Table 1.
Table 1 Effect of Na2CO3 particle size on relative yield of sodium ferrite
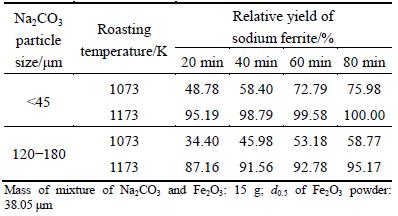
As shown in Table 1, the relative yields (Q) of sodium ferrite formed with fine sodium carbonate powder are higher than those with coarse one especially at low roasting temperature. The influence of Na2CO3 particle size abates with the increase of temperature. For fine Na2CO3 powder, Q can reach about 100% at 1173 K for 60 min. In addition, Q increases with the temperature elevating from 1073 to 1173 K for the same residence time, further confirming the positive effect of temperature on the sodium ferrite formation.
3.2 Desulfurization by adding sodium ferrite
3.2.1 Effect of synthesis condition of sodium ferrite on desulfurization rate
The desulfurization reactions were conducted in S2--bearing sodium aluminate solution using sodium ferrites synthesized under different conditions, and the results are listed in Table 2.
Table 2 Effect of sodium ferrite on desulfurization rate
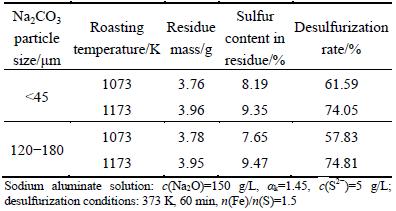
Table 2 indicates that the desulfurization rate (R) of about 75% can be achieved with synthesized sodium ferrite. The sodium ferrite prepared by the fine Na2CO3 powder at 1073 K is conducive to the desulfurization, while R almost remains unchanged for the sodium ferrite synthesized at 1173 K with either coarse or fine Na2CO3 powder. In addition, R with sodium ferrite synthesized at 1073 K is much less than that at 1173 K, which is attributed to the incomplete formation of sodium ferrite at low temperature (Table 1). Namely, the desulfurization rate positively correlates to the relative yields of sodium ferrite. Consequently, the sodium ferrite employed for following desulfurization experiments was synthesized by the fine Na2CO3 powder at 1173 K for 60 min.
3.2.2 Effect of dosage of sodium ferrite on desulfurization rate
In view of the desulfurization rate directly related to the dosage of sodium ferrite, the experiments for effect of sodium ferrite dosage on desulfurization rate were carried out, and the results are listed in Table 3.
Table 3 Effect of dosage of sodium ferrite on desulfurization rate
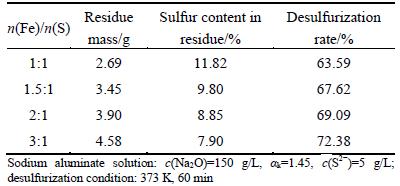
Table 3 exhibits that a certain extent increase of desulfurization rate can be made with increasing n(Fe)/n(S) from 1:1 to 3:1, implying that excessive sodium ferrite enhances slightly the desulfurization rate. Thus, n(Fe)/n(S) was determined in the range of 1:1 to 1.5:1 in the following experiments.
3.2.3 Effect of duration and S2- ion concentration on desulfurization rate
The dependence of desulfurization rate by sodium ferrite on duration is shown in Fig. 3. As shown in Fig. 3, the desulfurization rate reaches 68.47% for 10 min, and then remains almost unchanged with prolonging duration, implying that the reaction of sodium ferrite and S2- in sodium aluminate solution can take place very quickly.
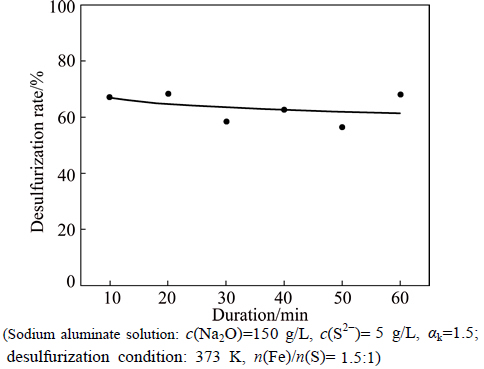
Fig. 3 Effect of duration on desulfurization rate by sodium ferrite (Sodium aluminate solution
In order to determine the influence of S2- ion concentration on desulfurization rate, sodium aluminate solutions with different S2- concentrations were adopted in the desulfurization experiments. The results are shown in Table 4.
Table 4 Effect of concentration of S2- on desulfurization rate
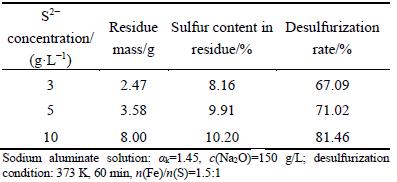
The results in Table 4 indicate that increasing the S2- concentration can enhance the desulfurization rate with sodium ferrite. When the S2- ion concentration increases from 3 to 10 g/L, the sulfur content in desulfurization residues arises from 8.16% to 10.20%, and the desulfurization rate arises correspondingly from 67.09% to 81.46%.
3.2.4 Effect of caustic soda concentration on desulfurization rate
The caustic soda concentration in sodium aluminate solution varies significantly in alumina production by Bayer process. In order to determine an appropriate caustic soda concentration for desulfurization, the effect of the caustic soda concentration on desulfurization with sodium ferrite was studied and the results are listed in Table 5.
Table 5 Effect of caustic soda concentration on desulfurization rate
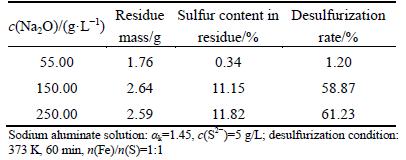
As seen from Table 5, the increase of Na2O concentration benefits the desulfurization, i.e., the desulfurization rate strongly depends on the caustic soda concentration. The desulfurization rate is only 1.2% in aluminate solution with caustic soda concentration of 55 g/L, while they are promoted to 58.87% and 61.23% with correspondingly increasing the Na2O concentration to 150 and 250 g/L.
Aiming to further understand the desulfurization mechanism by adding sodium ferrite in sodium aluminate solution, the phase analyses of the hydrolysis product and desulfurization residue were conducted. The XRD analyses show that the hydrolysis product of sodium ferrite in sodium aluminate solution is amorphous iron hydroxide oxide (Fig. 4), while the predominant phase is NaFeS2·2H2O (Fig. 5) in the desulfurization residue.
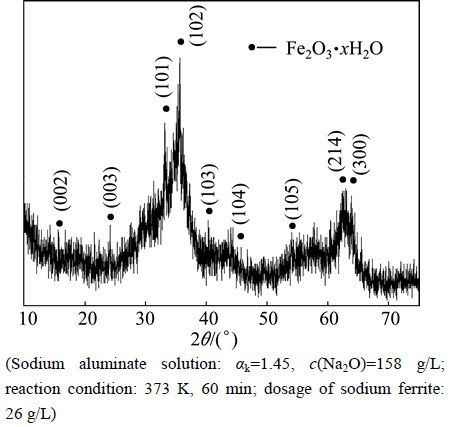
Fig. 4 XRD pattern of hydrolysis product of sodium ferrite
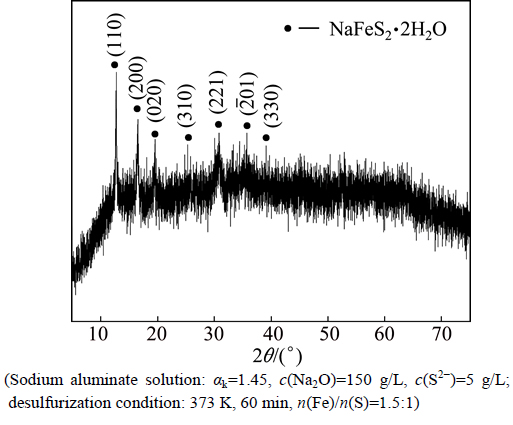
Fig. 5 XRD pattern of desulfurization residue (Sodium aluminate solution
The results discussed above confirm that the sodium ferrite as an efficient desulfurizer can remove S2- from S2--bearing sodium aluminate solution. Apparently, sodium ferrite hydrolyzes in the solution, forming insoluble precipitate of Fe2O3·H2O [17], Fe(OH)3 and 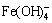 [18] as described by Eqs. (4)-(6).
[18] as described by Eqs. (4)-(6).
2NaFeO2+2H2O=2NaOH+Fe2O3·H2O (4)
NaFeO2+2H2O=NaOH+Fe(OH)3 (5)
NaFeO2+2H2O+OH-=NaOH+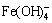 (6)
(6)
Therefore, it is interesting to determine that either Fe(OH)3 or 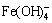 reacts with S2- in solution for desulfurization. The respective reaction and its Gibbs free energy change at 373 K are demonstrated in Eqs. (7) and (8) [14].
reacts with S2- in solution for desulfurization. The respective reaction and its Gibbs free energy change at 373 K are demonstrated in Eqs. (7) and (8) [14].
Fe(OH)3+2S2-+Na++2H2O=NaFeS2·2H2O+3OH-,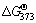 =16.20 kJ/mol (7)
=16.20 kJ/mol (7)
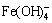 +2S2-+Na++2H2O=NaFeS2·2H2O+4OH-,
+2S2-+Na++2H2O=NaFeS2·2H2O+4OH-,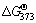 =-19.58 kJ/mol (8)
=-19.58 kJ/mol (8)
The Gibbs free energy change of Reaction (7) is positive while that of Reaction (8) is negative, indicating that the desulfurization is predominantly through Reaction (8) to form NaFeS2·2H2O. Based on this mechanism, the dependences of various factors, especially caustic concentration, on desulfurization rate can be rationally explained. Moreover, the desulfurization with adding fresh Fe(OH)3, reported in our previous work [14], is also achieved via the formation of 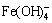 .
.
Furthermore, the caustic soda consumption for equivalent S can be saved about 75% with NaFeS2·2H2O formed in the desulfurization with sodium ferrite, compared with Na2S formed in the direct digestion of high-sulfur bauxite. Additionally, the preparation of sodium ferrite can be accomplished by sintering process for bauxite, resulting in the causticization of sodium carbonate and utilization of iron minerals in bauxite.
4 Conclusions
1) Elevated temperature and fine Na2CO3 powder favor the formation of sodium ferrite by roasting the mixture of Na2CO3 and Fe2O3, while the formation reaction can be completed at 1173 K for 60 min.
2) Sodium ferrite is an efficient reagent for the removal of S2- in sodium aluminate solution, and the desulfurization rate reaches about 70% at 373 K for 60 min with n(Fe)/n(S) ranging from 1:1 to 1.5:1.
3) The mechanism for desulfurization of S2--bearing sodium aluminate solution with sodium ferrite can be described as the consequence of 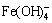 reacting with S2- to precipitate NaFeS2·2H2O.
reacting with S2- to precipitate NaFeS2·2H2O.
References
[1] PENG Xin, JIN Li-ye. Development and application of bauxite containing high sulfur [J]. Light Metals, 2010(11): 14-17. (in Chinese)
[2] YIN Jian-guo, XIA Wen-tang, HAN Ming-rong. Resource utilization of high-sulfur bauxite of low-median grade in chongqing china [C]//LINDSAY S J. Light Metals. San Diego, California: Wiley, 2011: 19-22.
[3] KUZNETSOV S I, GRACHEV V V, TYURIN N G. Interaction of iron and sulfur in alkaline aluminate solutions [J]. Zhurnal Prikladnoi Khimii, 1975, 48(4): 748-750. (in Russian)
[4] XIE Q L, CHEN W M. Corrosion behavior of 16Mn low alloy steel in sulfide-containing Bayer solutions [J]. Corrosion Science, 2014, 86: 252-260.
[5] XIE Q L, CHEN W M, YANG Q. Influence of sulfur anions on corrosion of 16Mn low-alloy steel in sulfide-containing Bayer solutions [J]. Corrosion, 2014, 70(8): 842-849.
[6] LI Xiao bin, LI Chong yang, QI Tian gui, ZHOU Qiu sheng, LIU Gui hua, PENG Zhi hong. Reaction behavior of pyrite during Bayer digestion at high temperature [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(3): 829-835. (in Chinese)
[7] WANG Xiao-min, ZHANG Ting-an,  Guo-zhi, BAO Li. Selection of flotation desulfurization collector for high-sulfur bauxite [J]. Journal of Northeastern University: Natural Science, 2010(4): 555-558. (in Chinese)
Guo-zhi, BAO Li. Selection of flotation desulfurization collector for high-sulfur bauxite [J]. Journal of Northeastern University: Natural Science, 2010(4): 555-558. (in Chinese)
[8] HU Xiao-lian, CHEN Wen-mi, XIE Qiao-ling. Sulfur phase and sulfur removal in high sulfur-containing bauxite [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(7): 1641-1647.
[9] HE Run de, TIAN Zhong liang. Discussion on reasonable cost of sulphur removal with barium aluminate from industrial sodium aluminate solution [J]. Journal of Guizhou University of Technology, 2000, 29(6): 54-58. (in Chinese)
[10] HU Xiao-lian, CHEN Wen-mi. Desulfurization from sodium aluminate solution by wet oxidation [J]. Journal of Central South University: Science and Technology, 2011, 42(10): 2911-2916. (in Chinese)
[11]  H. Sulfide removal in petroleum refinery wastewater by chemical precipitation [J]. Journal of Hazardous Materials, 2008, 153(1-2): 462-469.
H. Sulfide removal in petroleum refinery wastewater by chemical precipitation [J]. Journal of Hazardous Materials, 2008, 153(1-2): 462-469.
[12] RICKARD D, LUTHER G W. Chemistry of iron sulfides [J]. Chemical Reviews, 2007, 107(2): 514-562.
[13] RICHARD D. Kinetics of pyrite formation by the H2S oxidation of iron(II) monosulfide in aqueous solutions between 25 and 125 °C: The rate equation [J]. Geochimica et Cosmochimica Acta, 1997, 61(1): 115-134.
[14] LI Xiao-bin, LI Chong-yang, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng, QI Tian-gui. Interaction of sulfur with iron compounds in sodium aluminate solutions [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(2): 608-614.
[15] ZHOU Qiu-sheng, QI Tian-gui, PENG Zhi-hong, LIU Gui-hua, LI Xiao-bin. Thermodynamics of reaction behavior of ferric oxide during sinter-preparing process [J]. The Chinese Journal of Nonferrous Metals, 2007, 17(6): 973-978. (in Chinese)
[16] REN Wan-neng. The optimization and application of thermodynamic database for alumina production [D]. Changsha: Central South University, 2005. (in Chinese)
[17] YANG Zhong yu. Process technology of alumina [M]. Beijing: Metallurgical Industry Press, 1993: 228.
[18] TREMAINE P R, LEBLANC J C. The solubility of magnetite and the hydrolysis and oxidation of Fe2+ in water to 300 °C [J]. Journal of Solution Chemistry, 1980, 9(6): 415-442.
李小斌1,牛 飞1,谭 杰1, 2,刘桂华1,齐天贵1,彭志宏1,周秋生1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 万宝矿产有限公司,北京 100053
摘 要:研究铁酸钠的合成及其脱除铝酸钠溶液中S2-的反应。热力学分析表明通过焙烧Fe2O3和Na2CO3的混合物合成铁酸钠的最低温度为810 K。结果表明:在1173 K焙烧60 min可以完全形成铁酸钠,且升高温度和减小Na2CO3粒径有利于加速铁酸钠的形成。铁酸钠可高效脱除铝酸钠溶液中的S2-,在铁硫摩尔比为1:1~1.5:1,温度为373 K,反应时间为60 min的条件下,脱硫率约为70%。脱硫过程是通过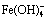 与 S2- 在铝酸钠溶液中反应生成NaFeS2·2H2O沉淀实现的,脱硫效率取决于铁酸钠溶解生成的
与 S2- 在铝酸钠溶液中反应生成NaFeS2·2H2O沉淀实现的,脱硫效率取决于铁酸钠溶解生成的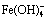 。
。
关键词:铁酸钠;S2-;铝酸钠溶液;脱硫
(Edited by Mu-lan QIN)
Foundation item: Project (51374239) supported by the National Natural Science Foundation of China
Corresponding author: Tian-gui QI; Tel/Fax: +86-731-88830453; E-mail: qitiangui@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64246-2

