DOI: 10.11817/j.issn.1672-7207.2015.11.005
2种阴离子捕收剂在石英表面的吸附机理
寇珏,郭玉,孙体昌,徐世红,徐承焱
(北京科技大学 土木与环境工程学院,北京,100083)
摘要:用石英晶体微天平(QCM-D)实时原位测定油酸钠和混合脂肪酸(KS-I)在经Ca2+活化的SiO2表面的吸附量,并结合单矿物浮选、原子力显微镜(AFM)和Zeta电位,研究捕收剂的吸附机理。研究结果表明:当矿浆pH为12.0时,油酸钠的浮选效果比KS-I的好,且活化剂和捕收剂用量都比KS-I的小。矿浆中Ca(OH)2浓度为6.48×10-5 mol/L且油酸钠用量为30 mol/L时,石英的回收率可达到97.9%;而KS-I在Ca(OH)2浓度为2.16×10-4 mol/L且捕收剂用量为90 mol/L的条件下得到最佳的回收率仅为78.6%。Ca2+在SiO2表面的吸附分为2个阶段,油酸钠在活化后的SiO2表面形成吸附量为5.4×10-6 g/cm2的黏弹性吸附层,且只有1个吸附阶段。而KS-I在SiO2表面的吸附量只有2.5×10-8 g/cm2。油酸钠在SiO2表面形成15.2~97.3 nm的吸附层,而KS-I在SiO2表面的吸附层最厚仅为10 nm,且2种药剂在整个表面的吸附并不均匀。油酸钠与KS-I在活化的石英表面均发生静电吸附作用,但油酸钠的吸附量比KS-I的吸附量大。
关键词:油酸钠;混合脂肪酸;石英;石英晶体微天平(QCM-D);原子力显微镜(AFM);Zeta电位
中图分类号:TD923 文献标志码:A 文章编号:1672-7207(2015)11-4005-10
Adsorption mechanism of two different anionic collectors on quartz surface
KOU Jue, GUO Yu, SUN Tichang, XU Shihong, XU Chengyan
(School of Civil and Environment Engineering, University of Science and Technology Beijing, Beijing 100083, China)
Abstract: A real-time and in-situ measurement of adsorption mass of sodium oleate and KS-I on SiO2 surface activated by Ca2+ was carried out by using quartz crystal microbalance with dissipation (QCM-D). The adsorption mechanism was investigated in combination with single mineral flotation, atomic force microscope (AFM) and Zeta potential as well, and the adsorption mechanism was discussed. The results show that sodium oleate has better flotation performance than KS-I at pH 12.0, and the dosages of both activator and collectors are smaller than those of KS-I. The highest quartz recovery of 97.9% is obtained with 6.48×10-5 mol/L Ca(OH)2 and 30 mg/L sodium oleate. However, by using 90 mg/L KS-I and 2.16×10-4 mol/L Ca(OH)2, the optimum quartz recovery is only 78.6%. Two distinct adsorption behaviors of Ca2+ on SiO2 surface are identified by QCM-D results. Sodium oleate forms a high viscosity and elasticity adsorption layer with adsorption quantity of 5.4×10-6 g/cm2 on the activated SiO2 surface, and there is only one stage of adsorption. However, the adsorption quantity of KS-I on SiO2 is only 2.5×10-8 g/cm2. Sodium oleate forms a adsorption layer with thickness ranging from 15.2 to 97.3 nm on SiO2 surface, but the highest adsorption layer of KS-I is only 10 nm. The electrostatic adsorption occurs on activated quartz surface with both sodium oleate and KS-I,but the adsorption amount of sodium oleate is larger than that of KS-I.
Key words: sodium oleate; mixed fatty acid; quartz; quartz crystal microbalance with dissipation (QCM-D); atomic force microscope (AFM); Zeta potential
反浮选工艺是处理细粒嵌布型难选铁矿石、提铁降硅的有效方法之一。铁矿石反浮选石英所用捕收剂包括阳离子捕收剂和阴离子捕收剂。阳离子捕收剂对矿泥敏感、泡沫黏度大,而阴离子捕收剂选择性好、对矿石适应性强[1]。因此,为获得高品位铁精矿,实际生产中常采用阴离子捕收剂反浮选石英来降低铁精矿中SiO2含量。油酸钠是反浮选石英最常见的阴离子捕收剂,其在未活化的石英表面的吸附主要是靠静电力和氢键作用,这种吸附是可逆的,吸附强度低[2]。混合脂肪酸(KS-I)是某选矿厂反浮选石英用的阴离子捕收剂,它的主要成分是工业纯的C12~C18的脂肪酸钠的混合物。使用阴离子捕收剂反浮选石英时通常需要先用金属阳离子对石英表面进行活化[3-6]。关于金属离子对石英的活化,Fuerstenau等[7-9]认为一价羟基金属离子在石英表面的吸附是活化石英的原因;而王淀佐等[10-11]根据金属离子在固液界面及溶液中的性质差异,计算得出金属氢氧化物在石英表面开始沉积的pH对应了石英开始浮选时的pH,因而认为石英的活化是金属氢氧化物在石英表面沉淀的结果。目前,尽管对于阴离子捕收剂在石英表面的作用机理已有很多研究,但大部分研究都是采用物理或化学分析测试手段,如红外光谱、XPS及SEM等,对矿物与药剂作用前后表面结构和成分进行研究,这些测试方法大都是在真空干燥的环境下得到的非实时或非原位的测定结果,而浮选过程是在液态环境中进行的,捕收剂与矿物表面的作用是一个动态的过程。因此,为了进一步揭示矿浆环境下药剂在矿物表面的吸附过程,有必要进行原位实时的测定和研究。石英晶体微天平(quartz crystal microbalance with dissipation,QCM-D)是一种用来研究表面作用过程和表面质量变化的精密设备,其最大的特点就是可以在液态环境中实时原位测定物质在某一特定表面的吸附量和吸附过程,并且可同时得到吸附层黏弹性模量,由此可推测出吸附层的结构变化。QCM-D的核心原理是利用石英的压电效应来实时监控待测表面的质量变化。具体方法是在具有压电效应的石英晶体上施加一个交变电场,晶片就会产生机械振动,当外加交变电压的频率为某一特定值f时,振幅明显加大并发生压电谐振。当石英晶体谐振器表面质量发生变化,就会对石英晶体共振频率f引发1个变化量△f,通过测定QCM-D输出的频率变化△f,就可以计算并模拟得到电极表面的质量变化△m。此外,QCM-D在交变电流反复断开的过程中,石英晶体的振动频率呈指数衰减,这个衰减被记录下来,就得到了石英晶体谐振器的能量耗散因子D[12-14]。如果有物质吸附在石英晶体谐振器表面,吸附层就会在频率衰减过程中发生机械耦合,使得系统的D产生一个改变量△D。吸附层的黏弹性不同,△D也会产生很大的差异,因此吸附层的力学性质就反映在了谐振器的电学性质上[15]。QCM-D通过周期性闭合和断开石英晶体谐振器的震荡电路来获得△f和△D,从而得到谐振器表面的△m及吸附层的黏弹性和松散程度[14, 16]。QCM-D不仅测定精度可以达到纳克级,而且可以在液态环境下进行实时原位测定,很好地弥补了传统研究手段的不足。Teng等[17-18]应用QCM-D研究了Ag+等金属离子对闪锌矿表面的活化过程,Kou等[19-20]采用QCM-D研究了胺类阳离子捕收剂在石英表面的吸附以及脂肪酸类捕收剂在磷酸盐矿物表面的吸附。本文作者采用QCM-D这一测试手段,在液态环境下分别对油酸钠和KS-I在Ca2+活化后的石英表面的动态吸附全过程进行实时原位研究。考察一定Ca2+浓度和捕收剂用量下捕收剂在石英表面吸附量随时间的变化情况,分析活化和吸附这2个阶段的吸附层结构性质,并结合单矿物浮选、AFM和Zeta电位测定的方法探讨并比较这2种阴离子捕收剂在石英表面的吸附效果与作用机理。
1 试验材料与方法
1.1 试样与预处理
单矿物浮选及Zeta电位测定试验所用纯石英矿样产自浙江诸暨。试验前先对块状石英进行破碎,然后用陶瓷球磨机进行研磨,将研磨后的石英矿样用稀释后的盐酸(质量分数为36.5%浓盐酸与去离子水体积比为2:1)浸泡2次,每次24 h,然后用去离子水多次清洗,直至样品呈中性,烘干后的石英经化验,纯度为99.37%。再用陶瓷球磨机对处理后的石英矿样进行研磨,并筛分成2个粒级,38~74 μm粒级用于单矿物浮选;小于38 μm粒级用于Zeta电位测定。
1.2 研究方法
1.2.1 单矿物浮选试验
石英单矿物浮选试验在XFGII5型挂槽浮选机中进行。每次称取2.0 g矿样于浮选槽内,加入30 mL去离子,配成质量分数为6.25%的矿浆,控制浮选机转速为1 700 r/min。充分搅拌后加入饱和Ca(OH)2溶液搅拌2 min,之后加入适量pH调整剂充分搅拌2 min,随后加入捕收剂再搅拌2min并测定矿浆pH(pH测试误差为±0.2),最后加2号油(工业纯)作为起泡剂,充分搅拌2 min之后进行浮选,采取手工刮泡,浮选至所有泡沫产品完全被刮出为止。然后分别将精矿和尾矿过滤、烘干、称量,并按各产品的质量计算回收率。
1.2.2 石英晶体微天平(QCM-D)测定
研究所用QCM-D的型号为Q-sense E4,产自瑞典Biolin Scientific公司,其主要工作单元包括石英晶体谐振器、流动池、样品平台、驱动电路、蠕动泵和控制软件(Q soft 401)。
石英晶体谐振器是QCM-D的核心部件。该谐振器是由1块厚0.3 mm、直径14 mm的AT切割型石英晶体夹在2片金电极之间构成,其共振基频f为5 MHz。在一侧的金电极表面修饰有厚300 nm、面积为0.2 cm2的SiO2作为捕收剂吸附试验的待测表面。试验时,将表面干净的石英晶体谐振器安装在QCM-D的流动池中,首先在空气介质中进行测定,待△f和△D信号稳定后,向样品池中通入去离子水作为本底值直至信号稳定,然后以一定速率向系统中通入饱和Ca(OH)2溶液活化SiO2,一段时间后通入待测的捕收剂溶液,捕收剂即在SiO2表面发生吸附作用。在此过程中每隔0.5 s测定1次△f和△D,直至整个吸附过程结束。整个试验过程溶液温度稳定在(25±0.05) ℃。
QCM-D测试可以连续先后通入不同的待测溶液,以考察药剂之间的相互影响。整个测定过程可以得到液态环境中捕收剂吸附过程的实时原位数据。数据分析主要采用分析软件Q tools。根据Sauerbrey方程[13] (式(1)),△f正比于△m,因此由测定的△f可以计算得到△m。
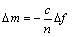 (1)
(1)
然而Sauerbrey方程仅适用于吸附层为刚性且没有内部摩擦或滑动的情况,此时△D≤5×10-6。而当△D>5×10-6时,形成的吸附层较松散且为黏弹性膜,则需采用Voight模型[21-22](式(2)和(3))来模拟计算吸附质量。根据Voight模型,△f和△D与密度ρ、厚度h、剪切弹性模量μ、剪切黏度η、剪切波的穿透深度△以及角频率ω有关。

 (2)
(2)
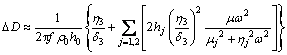 (3)
(3)
1.2.3 原子力显微镜(AFM)成像
试验所用AFM为Bruker公司的Dimension FastScan,试验采用氮化硅探针(FASTSCAN-B,Bruker),共振频率为400kHz。测试样品为干净的SiO2谐振器和经QCM-D吸附测试后自然干燥的SiO2谐振器。测试采用快速扫描模式在室温((25±0.5) ℃)空气条件下对待测表面进行扫描。得到的原始表面形貌用NanoScope Analysis软件进行自动样品校正,并对其进行二维和三维形貌分析。
1.2.4 Zeta电位测试
Zeta电位测试使用的是美国Brookhaven公司ZetaPlus型的Zeta电位及粒度分析仪。每次试验取50 mg纯石英矿样置于50 mL的离心管中,加入50 mL已配好的不同浓度的溶液,搅拌均匀配成质量浓度为1 g/L的悬浊液,再加一定量pH调整剂,所用pH调整试剂为0.5 mol/L HCl和0.5 mol/L NaOH溶液,充分搅拌5 min,然后超声(40 kHz)分散20 min,使矿物颗粒充分分散,静置10 min后取上层含有微细颗粒的悬浊液,测其Zeta电位并测定pH。测定温度为25 ℃,每个样品测定5次取平均值。
2 结果与讨论
2.1 单矿物浮选结果分析
首先在不同pH下对2种阴离子捕收剂进行单矿物浮选,考察并对比2种药剂的浮选效果。研究中Ca2+浓度为2.16×10-4 mol/L,油酸钠和KS-I用量均为30 mg/L,结果如图1所示。由图1可以看出:矿浆pH对油酸钠和KS-I浮选石英的效果影响很大。当pH为8.5~10.2时,油酸钠和KS-I对石英的浮选回收率都很低;当pH=10.2时,油酸钠的浮选回收率为10.4%,KS-I的回收率为6.5%;当pH>10.2时,油酸钠浮选石英的回收率急剧增大,在pH=11.0时回收率达到96.0%,继续增大pH回收率缓慢增大,且在pH=12.0时达最大值98.6%。而KS-I浮选石英的回收率在整个pH范围内均增大较缓慢,最高回收率仅为15.3%。

图1 pH对油酸钠和KS-I浮选石英回收率的影响
Fig. 1 Effect of pH on quartz recovery using sodium oleate and KS-I as collectors
由此可见,2种阴离子捕收剂都是在pH>10.0的强碱性条件下对石英具有捕收作用,且在pH为11.0~12.0范围内浮选回收率最高。但在相同Ca2+浓度和药剂用量下,KS-I的浮选效果比油酸钠的差。为了考察不同Ca2+浓度对2种捕收剂浮选效果的影响,调节矿浆pH为12.0,当油酸钠和KS-I的用量均为60 mg/L时向浮选矿浆内添加不同用量Ca(OH)2饱和溶液,得到了Ca2+浓度对浮选回收率的影响规律,结果如图2所示。由图2可知:用油酸钠作为捕收剂,当Ca2+浓度<4.32×10-5 mol/L时,石英的回收率低于10%,当Ca2+浓度增大到1.01×10-4 mol/L时,石英回收率快速提高至90.2%。此后,随着Ca2+浓度增大,石英回收率继续增大,在1.51×10-4 mol/L时达最大值99.1%;相比之下,KS-I浮选石英的回收率始终都比较低,当Ca2+浓度为2.16×10-4 mol/L时,石英的回收率为31.0%,但随着Ca(OH)2用量增加,回收率缓慢增大,在Ca2+浓度为2.16×10-3 mol/L时才达到75.7%。比较这2种药剂对石英的浮选效果可以看出:油酸钠浮选石英时,在Ca2+浓度较低条件下就可以得到很好的石英回收率;而用KS-I浮选石英时,Ca2+浓度达到了油酸钠的10倍以上时,石英的最大回收率比油酸钠的最大回收率低20%多。

图2 Ca2+浓度对油酸钠和KS-I浮选石英回收率的影响
Fig. 2 Effect of Ca2+ concentrations on quartz recovery using sodium oleate and KS-I as collectors
为考察捕收剂用量对石英回收率的影响规律,固定Ca2+浓度,当矿浆pH为12.0时,进行不同捕收剂用量的条件试验,结果如图3所示。由图3可知:当Ca2+浓度为6.48×10-5 mol/L时,当油酸钠用量从3 mg/L增加到30 mg/L时,石英回收率从59.2%增大到97.9%;继续增大油酸钠的用量,石英回收率反而降低,油酸钠用量增加到50 mg/L时,石英回收率为95.3%,而当油酸钠用量为80 mg/L时,石英回收率为89.7%。邱继存等[4]认为钙的氢氧化物的溶度积远大于其金属油酸盐的溶度积,其在石英表面的吸附,可以被过量的油酸钠解脱,因此导致石英回收率降低;而石云良等[6, 10]认为捕收剂的半胶束反向吸附是捕收剂过量抑制石英浮选的主要原因。
由图3还可知:当Ca2+浓度为2.16×10-4 mol/L时,石英回收率开始时随KS-I用量的增加而增大,当KS-I用量为90 mg/L时,回收率达最大值78.6%,此后继续增大KS-I的用量,石英回收率出现下降。由此可见,阴离子捕收剂在浮选石英过程中存在最佳用量范围。相比之下,油酸钠浮选石英时可以在更低Ca2+浓度和更少的药剂用量下取得最大回收率,因此,纯油酸钠的浮选效果要优于混合脂肪酸的浮选效果。

图3 不同油酸钠和KS-I用量下石英的浮选回收率
Fig. 3 Quartz recoveries at different dosages of sodium oleate and KS-I
2.2 QCM-D测试结果分析
为考察油酸钠和KS-I在SiO2表面的吸附作用过程,用QCM-D分别对这2种捕收剂在SiO2镀层的石英晶体谐振器表面的吸附全过程进行了实时原位测定。结果如图4所示,图4(a)中曲线1和4分别为油酸钠吸附的△D和△f,曲线2和3分别为KS-I的△D和△f。开始试验后0~10 min,先向系统中通入去离子水,在这个过程中△f和△D始终都是0,说明石英晶体谐振器表面并没有发生任何质量变化。箭头A表示开始向系统中注入Ca(OH)2饱和溶液,可以看到油酸钠和KS-I的△f都立即出现明显的下降,并迅速在-5 Hz附近达到吸附平衡,同时△D也有较小幅度的上升,这与Deng等[23]用QCM-D研究石膏过饱和溶液对SiO2表面性质影响的结果相一致。△f的下降,说明Ca2+在石英晶体谐振器的SiO2镀层表面发生活化作用,使得谐振器的质量发生了变化,而且这个活化速度很快;此外在10~35 min时,由△D<5×10-6,可知Ca2+形成的活化层对系统的能量耗散较小,因此可以判断为紧密的刚性吸附。Cao等[8]认为,由于SiO2表面存在部分Si—O键断裂,并且由于水化作用以—Si—OH形式存在石英表面,—Si—OH又发生解离生成—Si—O-,与带正电的Ca2+相结合生成—Si—O—Ca+,从而形成Ca2+的活化层。35 min时,开始向系统中注入一定浓度的捕收剂溶液,即箭头B标注的位置。由图4(a)中曲线4可知:加入油酸钠后,△f快速下降到-80 Hz附近,并在此后基本保持信号稳定;同时图4(a)中曲线1显示△D也快速上升到31×10-6,由于△D>5×10-6)。这说明油酸钠在石英表面吸附量较大,并且为黏弹性吸附,但达到吸附平衡的速度非常快,且从吸附开始到70 min时△f始终保持在-80 Hz,说明形成的吸附层较稳定。可以推断在这一过程中,大量的油酸根阴离子—OOR-与已经形成的活化层中的—Si—O—Ca+发生反应生成—Si—O—Ca—OOR,其中非极性—OOR基团朝向溶液,从而形成了稳定的疏水吸附层。

图4 SiO2表面Ca2+活化以及油酸钠、KS-I吸附全过程中△f和△D随时间的变化
Fig. 4 Change of △f and △D with adsorption time for Ca2+ activation and adsorption of sodium oleate and KS-I on SiO2 surface
由于KS-I吸附过程中△D和△f的变化远比油酸钠的小,因此将图4(a)中曲线2和3放大如图4(b)所示。由图4可知:在开始试验后的0~10 min向系统中通入去离子水,以及10~35 min向系统中通入Ca(OH)2饱和溶液进行活化,△D和△f的变化趋势与前面的试验结果一致。在35 min时,即箭头B处开始向系统中注入KS-I溶液,与油酸钠不同的是KS-I的△f在出现了1个较明显的波动之后,反而随着时间的延长有缓慢上升的趋势,到52 min时,△f基本保持在-2 Hz;而同时,系统的△D也在1个较明显的波动后维持在0.7×10-6。在开始注入KS-I时△f和△D出现的明显波动是因为脂肪酸根离子与SiO2表面的Ca2+发生了作用,但是脂肪酸根的吸附并不稳定,随着溶液不断通入反而逐渐从SiO2表面脱附,说明KS-I在石英表面很难形成稳定的吸附层。这一结果可以很好地解释KS-I单矿物浮选效果较差的原因。
在得到2种阴离子捕收剂吸附过程的△f和△D的基础上,通过Sauerbray模型和Voight模型分别计算得到了油酸钠和KS-I的吸附质量随时间的变化曲线,结果如图5中曲线1和2所示。由于KS-I的吸附量较小,因此将其吸附曲线放大后显示在图5右下角。由图5可知:在通入Ca(OH)2饱和溶液后,Ca2+可以迅速在SiO2 表面形成9.0×10-8 g/cm2的活化层,油酸钠在Ca2+活化后的SiO2表面可以形成吸附量为5.4×10-6 g/cm2的吸附层,且吸附层并不随溶液的通入而发生变化,说明吸附比较稳定。而KS-I在SiO2表面吸附层只有2.5×10-8 g/cm2,且随着溶液在SiO2表面的流动吸附量出现了减少的趋势,说明KS-I的吸附并不稳定。根据单矿物浮选的结果,油酸钠的浮选效果比KS-I的好,用量也比KS-I的少。结合QCM-D的结果可知:阴离子捕收剂在活化后的石英表面的吸附层性质直接影响该药剂的浮选效果,吸附量大且吸附层稳定则疏水性好,浮选效果好;反之疏水性差,浮选效果差。

图5 油酸钠和KS-I分别在Ca2+活化的SiO2表面的吸附层质量随时间的变化
Fig. 5 Change of adsorption mass of sodium oleate and KS-I on Ca2+ activated SiO2 surface with time
图4中QCM-D测定的△f和△D可以总结为图6(a)(油酸钠吸附)和6(b)(KS-I吸附)所示的△D-△f图。△D-△f图可以展示出能量耗散因子改变量随频率改变量的变化规律。图形趋势线的斜率记作K(K=|△D/△f|),可以用来说明吸附层的形成过程和结构变化。当K比较小,单位吸附量所引起的系统能量耗散较小,因此生成的是吸附牢固排列紧密的吸附层;反之,若K比较大,则吸附层黏弹性大且结构松散。若图6中只有1个K,则吸附过程中吸附层没有明显的变形;若出现多个K,说明吸附过程中有几次层结构或分子排列方向的改变。
由图6(a)可知:油酸钠在石英谐振器表面的吸附只出现1个阶段,拟合直线的斜率K=0.387 3,可知单位吸附量所产生的吸附层是黏弹性的,结构比较松散。图6(a)中左上角的小图是图6(a)虚线框中的部分曲线放大后的结果,这一阶段的表面质量变化主要是Ca2+活化过程引起的,拟合直线的斜率K1=0.059 6和K2=0.252 2,K1 <<K2<K说明生成单位质量的Ca2+活化层引起的系统能量耗散较小,所以该活化层应为致密的刚性吸附。
图6(b)中Ca2+和KS-I在SiO2表面的吸附共经历了3个阶段,其中阶段Ⅰ和Ⅱ为Ca2+在SiO2表面的吸附,阶段Ⅲ为KS-I在石英表面的吸附。由图6(b)可知:K1=0.063 4,K3=0.068 2,K2=0.252 4,且K1<K3<<K2,说明阶段Ⅰ中△D随△f的变化最小,吸附层最紧密;而K2约为K1的4倍,说明阶段Ⅱ中Ca2+吸附引起的能量耗散较阶段Ⅰ的大,可能有氢氧化钙沉淀生成。阶段Ⅲ显示,KS-I吸附后△f变小的同时△D也变小,可以推测KS-I在SiO2表面吸附不牢,并且通入KS-I溶液后反而使部分Ca2+从SiO2表面脱吸附。

图6 油酸钠和KS-I的△D-△f图
Fig. 6 △D-△f plots of sodium oleate and KS-I
2.3 AFM结果分析
为了更直观地了解捕收剂在石英表面的吸附状态,对表面干净的谐振器及经过QCM-D吸附测试的谐振器表面进行AFM扫描,可以直接得到油酸钠和KS-I在SiO2表面吸附前后的形貌特征。图7(a)和7(b)所示分别为干净的石英表面的AFM二维和三维图像,扫描范围为5.0 μm×5.0 μm,图像中亮度越大表示该区域高度越大。由图7(a)可知:干净的SiO2镀层表面性质均一且分布均匀,结构紧密清晰可见;由图7(b)可以看到整个表面高度差别较小,表明石英晶体谐振器为测试提供了一个均质光滑的SiO2表面。而图7(c)和7(d)所示分别为吸附有油酸根的SiO2表面的AFM二维和三维图像。从图7(c)中几乎看不到洁净表面的均匀状结构,而是出现不规则且高低起伏明显的絮状吸附质。从图7(d)可见:SiO2表面存在厚度为15.2~97.3 nm的不规则吸附层,说明SiO2表面吸附了大量的油酸根,且吸附并不均匀。图7中局部的吸附层高度甚至超过了测量范围,由此可以推断此处形成的吸附量最大,因此很可能是油酸钙在表面的沉淀。图7(e)和7(f)所示分别为吸附有KS-I的SiO2表面的AFM二维和三维图像。从图7(e)可见:石英晶体谐振器表面存在均质的SiO2镀层,说明形成的吸附层很薄,没有能覆盖整个表面;同时还可以观察到许多细小纤维状吸附质出现在表面上,该纤维状吸附质有可能是Ca(OH)2在表面析出的沉淀。从图7(f)可以看到:经过Ca2+活化和KS-I吸附之后形成了最大厚度为10.0 nm的吸附物质。由此可以看出KS-I在谐振器表面的吸附量远比油酸钠的少,且吸附层厚度薄,吸附不均匀。

图7 洁净的石英晶体谐振器表面、油酸钠及KS-I在活化后石英晶体谐振器表面作用后的AFM图
Fig. 7 AFM images of clean sensor surface and activated sensor adsorbed with sodium oleate and KS-I
2.4 Zeta电位测定结果分析
为了考察这2种阴离子捕收剂对石英表面电位的影响,对石英颗粒分别与Ca2+、油酸钠和KS-I作用前后的Zeta电位进行了测定。图8所示为石英在去离子水以及浓度分别为2.16×10-2,2.16×10-3和2.16× 10-4 mol/L的Ca(OH)2溶液中的Zeta电位随pH的变化。由图8可知:在矿浆pH为2.0~12.0范围内,石英在去离子水中的Zeta电位均为负值,且随着pH的升高,石英的Zeta电位明显降低,当pH=10.8时达到最小值-93.08 mV。加入Ca2+后,石英表面Zeta电位总体向正值方向移动,且Ca2+浓度越大石英的Zeta电位增大越明显。当pH<3.0时,石英Zeta电位随Ca2+浓度增大而升高的幅度并不大,当pH为3.0~12.0时,不同的Ca2+浓度对石英Zeta电位的影响很大,尤其是当pH为9.0~12.0时,不同Ca2+浓度对石英Zeta电位影响最大。当pH约为11.0时,纯水中石英Zeta电位为-90.91 mV,当Ca2+浓度为2.16×10-4 mol/L时石英的Zeta电位上升到-51.51 mV,而当Ca2+浓度为2.16×10-2 mol/L时石英的 Zeta电位已经变成了正值7.67 mV。这说明在pH为9.0~12.0范围内Ca2+更易吸附在石英表面,导致石英表面电负性逐渐降低并最终转变为荷正电。

图8 石英在去离子水及不同Ca2+浓度溶液中的Zeta电位
Fig. 8 Zeta potential of quartz conditioned by deionized water and Ca(OH)2 solution with different concentrations as a function of pH
图9所示为石英分别在去离子水、3 mg/L油酸钠溶液以及30 mg/L KS-I溶液中的Zeta电位随pH的变化。由图9可知:在3 mg/L的油酸钠溶液中,当pH<7.5时,石英的Zeta电位比去离子水中的稍高,这有可能是因为当pH<7.5时油酸钠在水溶液中主要以分子及离子—分子缔合物状态存在[10],因此,油酸分子缔合物在石英表面会有少量的吸附,从而改变了石英表面的Zeta电位;而当pH>7.5时,此时油酸钠在水溶液中主要以油酸根负离子的形式存在,石英在油酸钠溶液中的Zeta电位与在去离子水中的几乎相近,表明油酸根负离子在未经Ca2+活化的石英表面没有发生吸附作用。石英在KS-I溶液中的Zeta电位与在去离子水中的Zeta电位随pH的整体变化趋势基本一致,表明KS-I并不与未经活化的石英发生吸附作用。

图9 石英分别在去离子水、3 mg/L油酸钠溶液及30 mg/L KS-I溶液中的Zeta电位
Fig. 9 Zeta potential of quartz in deionized water, 3mg/L sodium oleate solution and 30 mg/L KS-I solution as a function of pH
图10所示为分别在去离子水、2.16×10-2 mol/L的Ca(OH)2溶液以及先被Ca2+(2.16×10-2 mol/L的Ca(OH)2溶液)活化后再分别与3 mg/L油酸钠溶液和30 mg/L KS-I溶液混合并充分作用的石英表面Zeta电位随pH的变化。由图10可知:当矿浆pH为 2.0~12.0时,石英Zeta电位为负值,此时不利于阴离子捕收剂的吸附。而当加入了Ca(OH)2后,石英表面的Zeta电位朝正方向移动,且随着pH的增大这种趋势更加明显。先被Ca2+活化后再分别与油酸钠、KS-I作用的石英的Zeta电位比在去离子水中的电位高,但低于Ca(OH)2溶液中的Zeta电位。当矿浆pH为10.9~12.0时,与Ca(OH)2溶液作用后的石英表面Zeta电位已经转变为正值,分别与油酸钠和KS-I作用后,Zeta电位出现了较大幅度的下降,在这个pH范围内,油酸钠主要以油酸根及油酸根二聚物的形式存在[10],油酸根及其二聚物大量吸附在了经过Ca2+活化的石英表面,中和掉了表面的部分正电离子,造成表面Zeta电位下降,但同时也形成了疏水性好的吸附膜。刘亚川等[24]认为经Ca2+活化后的石英虽然在整体上仍显示负电性,但就局部而言,存在荷正电性的情况,因此阴离子捕收剂仍然可以在矿物表面发生吸附作用。因此,当矿浆pH为2.0~10.9时,虽然经Ca2+活化的石英的Zeta电位为负值,但油酸根和脂肪酸根阴离子仍在石英表面发生了吸附。当矿浆pH为10.5~12.0时,石英表面的Zeta电位向正值方向的移动幅度最大,因此,在该条件下的活化及吸附作用最强,且浮选效果也是最好的。在整个测试的pH范围内,油酸钠的吸附导致活化后的石英的Zeta电位较KS-I的低,说明油酸钠在活化后的石英表面的吸附量较KS-I的吸附量大。

图10 石英分别在去离子水、Ca(OH)2溶液中以及先被Ca2+活化后分别与油酸钠、KS-I作用后的Zeta电位
Fig.10 Zeta potential of quartz conditioned with deionized water, Ca(OH)2 solution, as well as oleate solution and KS-I after activated by Ca2+ as a function of pH
尽管Zeta电位的结果显示Ca2+在石英表面的活化过程主要是静电吸附作用,但从图6所示的△D-△f图可以看到:Ca2+的活化过程经历2个阶段,K1=0.063 4,K2=0.252 4,且K2>K1,说明除了K1阶段的静电吸附之外,还可能有一定量的Ca(OH)2在表面发生沉积作用,使得静电吸附的表面出现了K2的吸附阶段。且在AFM的图像中也观察到了纤维状的吸附质。同时可以看到,活化后再与捕收剂作用,尽管Zeta电位有所下降,但仍然高于在去离子水中的Zeta电位,说明吸附捕收剂阴离子之后的石英表面仍然存在大量正电荷,由此可以推断在该浓度条件下,吸附并没有达到饱和,且形成的吸附层是不均匀的,这与QCM-D以及AFM的结果相一致。
3 结论
1) 油酸钠和KS-I均在矿浆pH为12.0时得到最高的石英浮选回收率。当Ca(OH)2浓度为6.48×10-5 mol/L和油酸钠用量为30 mg/L时,石英回收率最高可以达到97.9%;而混合脂肪酸KS-I对石英的浮选回收率均比较低,在Ca(OH)2浓度为2.16×10-4 mol/L和KS-I用量为90 mg/L时最高只能达到78.6%,且Ca(OH)2浓度和捕收剂用量均是油酸钠浮选时的3倍。
2) Ca2+的活化过程经历2个阶段,且K2>K1,说明除了K1阶段的静电吸附之外,还应该有一定量的Ca(OH)2在表面发生沉积作用。当pH为12.0时,Ca2+在SiO2表面形成了吸附量为9.0×10-8 g/cm2的吸附活化层。油酸钠在Ca2+活化后的SiO2表面可以形成5.4×10-6 g/cm2的吸附层,吸附层黏弹性较高,厚度较大;相比之下, KS-I在经Ca2+活化的SiO2表面吸附量很低,只有2.5×10-8 g/cm2。
3) 油酸钠在石英晶体谐振器表面形成厚度为15.2~97.3 nm的吸附层,吸附层表面并不均一且厚度起伏变化较大,而KS-I在谐振器表面吸附量很低,吸附层厚度在10.0 nm以下且分布不均匀。带正电的Ca2+在带负电的石英表面发生了静电吸附,从而使得带负电的油酸根离子及KS-I吸附在了石英表面,改变了石英表面的疏水性,且油酸钠在活化后的石英表面的吸附量较KS-I的吸附量大。用该结果可以很好地解释浮选过程中油酸钠效果优于KS-I的原因。
参考文献:
[1] 邱廷省, 张卫星, 方夕辉, 等. 铁矿石阳离子反浮选技术研究进展及应用现状[J]. 金属矿山, 2012, 41(2): 89-93.
QIU Tingsheng, ZHANG Weixing, FANG Xihui, et al. Research progress and application status on cationic reverse flotation technology for iron ore[J]. Metal Mine, 2012, 41(2): 89-93.
[2] LIU Yachuan, GONG Huangao. Adsorption effect mechanism of sodium oleate on feldspar and quartz[J]. Transactions of Nonferrous Metals Society of China, 1994, 4(3): 53-56.
[3] Fornasiero D, Ralston J. Cu(II) and Ni(II) activation in the flotation of quartz, lizardite and chlorite[J]. Int J Miner Process, 2005, 76: 75-81.
[4] 邱继存, 郭永文. 多价金属阳离子对石英浮游的活化机理[J]. 有色金属, 1965(6): 35-43.
QIU Jicun, GUO Yongwen. Activation mechanism of quartz by polyvalent metal cation[J]. Nonferrous Metals, 1965(6): 35-43.
[5] 石云良. 石英活化、浮选的溶液和表面化学[J]. 有色金属, 1992, 44(2): 35-40.
SHI Yunliang. Solution and surface chemistry of flotation and activation of quartz[J]. Nonferrous Metals, 1992, 44(2): 35-40.
[6] 石云良, 陈淳, 邱冠周. 石英的油酸盐浮选化学[J]. 有色金属, 1999, 51(1): 31-34.
SHI Yunliang, CHEN Chun, QIU Guanzhou. Flotation chemistry of quartz with oleat[J]. Nonferrous Metals, 1999, 51(1): 31-34.
[7] Fuerstenau M C, Miller J D, Pray R E. Metal ion activation in xanthate flotation of quartz[J]. Trans Am Inst Min Eng, 1965, 232: 359-364.
[8] CAO Zhao, ZHANG Yahui, CAO Yongdan. Reverse flotation of quartz from magnetite ore with modified sodium oleate[J]. Mineral Processing and Extractive Metallurgy Review, 2013, 34: 320-332.
[9] 朱玉霜. 浮选药剂的化学原理(修订版)[M]. 长沙: 中南工业大学出版社, 1996: 56-61.
ZHU Yushuang. The chemical principle of flotation reagents (revised edition)[M]. Changsha: Central South University of Technology Press, 1996: 56-61.
[10] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988: 132-148.
WANG Dianzuo, HU Yuehua. Solution chemistry of flotation[M]. Changsha: Hunan Science & Technology Press, 1988: 132-148.
[11] 石云良, 邱冠周, 胡岳华, 等. 石英浮选中的表面化学反应[J]. 矿冶工程, 2001, 21(3): 43-46.
SHI Yunliang, QIU Guangzhou, HU Yuehua, et al. Surface chemical reactions in oleate flotation of quartz[M]. Mining and Metallurgical Engineering, 2001, 21(3): 43-46.
[12] Gutig C, Grady B P, Striolo A. Experimental studies on the adsorption of two surfactants on solid-aqueous interfaces: adsorption isotherms and kinetics[J]. Langmuir, 2008, 24: 4806-4816.
[13] Sauerbrey G. Verwendung von schwingquarzen zur wagung dunner schichten und zur mikrowagung[J]. Zeitschrift Fur Physik, 1959, 155: 206-222.
Sauerbrey G. Application of piezoelectric effect in quartz crystal microbalance[J]. Journal of Physics, 1959, 155: 206-222.
[14] Paul S, Paul D, Basova T, et al. Studies of adsorption and viscoelastic properties of proteins onto liquid crystal phthalocyanine surface using quartz crystal microbalance with dissipation technique[J]. The Journal of Physical Chemistry C, 2008, 112: 11822-11830.
[15] Sedeva I G, Fetzer R, Fornasiero D, et al. Adsorption of modified dextrins to a hydrophobic surface: QCM-D studies, AFM imaging, and dynamic contact angle measurements [J]. Journal of Colloid and Interface Science, 2010, 345: 417-426.
[16] Buttry D A, Ward M D. Measurement of interfacial processes at electrode surfaces with the electrochemical quartz crystal microbalance[J]. Chem Rev, 1992, 92(6): 1355-1379.
[17] TENG Fucheng, LIU Qingxia, ZENG Hongbo. In situ kinetic study of zinc sulfide activation using a quartz crystal microbalance with dissipation (QCM-D)[J]. Journal of Colloid and Interface Science, 2012, 368: 512-520.
[18] TENG Fucheng. Understanding zinc sulfide activation mechanism and impact of calcium sulfate in sphalerite flotation[D]. Edmonton: The University of Alberta, 2012: 59-78.
[19] KOU Jue, TAO Danie, XU Guangyang. A study of adsorption of dodecylamine on quartz surface using quartz crystal microbalance with dissipation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 368: 75-83.
[20] KOU Jue, TAO Danie, XU Guangyang. Fatty acid collectors for phosphate flotation and their adsorption behavior using QCM-D[J]. International Journal of Mineral Processing, 2010, 95: 1-9.
[21] Voinova M V, Rodahl M, Jonson M, et al. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: continuum mechanics approach[J]. Physica Scripta, 1999, 59: 391-396.
[22] Vogt B D, Lin E K, WU Wen-li, et al. Effect of film thickness on the validity of the sauerbrey equation for hydrated polyelectrolyte films[J]. J Phys Chem B, 2004, 108: 12685-12690
[23] DENG Meijiao, LIU Qingxia, XU Zhenghe. Impact of gypsum supersaturated solution on surface properties of silica and sphalerite minerals[J]. Mineral Engineering, 2013, 46/47: 6-15.
[24] 刘亚川, 龚焕高, 张克仁. 油酸钠和十二胺盐酸盐在长石和石英表面的吸附[J]. 矿冶工程, 1993, 13(2): 27-31.
LIU Yachuan, GONG Huangao, ZHANG Keren. The adsorption of sodium oleate and dodecylamine hydrochloride on quartz surface[J]. Mining and Metallurgical Engineering, 1993, 13(2): 27-31.
(编辑 杨幼平)
收稿日期:2014-11-07;修回日期:2015-02-20
基金项目(Foundation item):国家自然科学基金资助项目(51204012) (Project(51204012) supported by the National Natural Science Foundation of China)
通信作者:寇珏,博士,讲师,从事矿物浮选、浮选药剂性能及作用机理,复杂难处理铁矿石直接还原焙烧新工艺及理论研究;E-mail: koujue@ustb.edu.cn