磷钾伴生矿中的钾在HCl-H3PO4混酸溶液中的浸出动力学
来源期刊:中国有色金属学报(英文版)2017年第8期
论文作者:马家玉 杜学兰 覃远航 吴再坤 池汝安 王存文
文章页码:1870 - 1877
关键词:动力学;浸出;钾;磷钾伴生矿;活化能;钾长石
Key words:kinetics; leaching; potassium; phosphorus-potassium associated ore; activation energy; K-feldspar
摘 要:为了减轻设备腐蚀,降低酸浸液中氯的含量及提高磷的含量,研究了磷钾伴生矿中的钾在HCl-H3PO4混酸溶液中的浸出动力学。考察了盐酸的质量分数、固液比、物料比(CaF2添加量(g)与磷钾伴生矿质量(g)比)、浸出温度等几个因素对钾浸出率的影响,结果表明:在浸出温度95 °C、盐酸浓度10%、浸出时间6 h、固液比1:6、物料比0.1的优化工艺条件下,钾浸出率可达86%以上。同时建立了基于经典缩合模型的半经验动力学模型,并用该模型成功地模拟了钾的浸出过程。结果表明:在65~95 °C范围内,钾浸出过程属于内扩散控制步骤,表观活化能为54.67 kJ/mol。
Abstract: In order to relieve the equipment corrosion, reduce chlorine and increase phosphorus contents in leaching solution, the leaching behavior of potassium from phosphorus-potassium associated ore in the mixed acids of hydrochloric acid and phosphoric acid was investigated. The effects of various factors, such as mass fraction of hydrochloric acid, solid-to-liquid ratio, material ratio (CaF2 dosage (g)/mass of ore (g)) and leaching temperature were comprehensively studied. It was found that the dissolution fraction of potassium can reach more than 86% under the optimum conditions of leaching temperature 95 °C, HCl concentration 10%, leaching time 6 h, solid/liquid ratio 1:5, and material ratio 0.1. In addition, the leaching kinetics of potassium was successfully modeled by a semi-empirical kinetic model based on the classic shrinking core model. The data showed that the leaching process of potassium was controlled by the product layer diffusion and the apparent activation energy for the process was found to be 54.67 kJ/mol over the temperature range from 65 to 95 °C.
Trans. Nonferrous Met. Soc. China 27(2017) 1870-1877
Jia-yu MA1,2, Xue-lan DU2, Yuan-hang QIN2, Zai-kun WU2, Ru-an CHI2, Cun-wen WANG2
1. State Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology, Wuhan 430081, China;
2. Key Laboratory of Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan 430205, China
Received 12 May 2016; accepted 22 December 2016
Abstract: In order to relieve the equipment corrosion, reduce chlorine and increase phosphorus contents in leaching solution, the leaching behavior of potassium from phosphorus-potassium associated ore in the mixed acids of hydrochloric acid and phosphoric acid was investigated. The effects of various factors, such as mass fraction of hydrochloric acid, solid-to-liquid ratio, material ratio (CaF2 dosage (g)/mass of ore (g)) and leaching temperature were comprehensively studied. It was found that the dissolution fraction of potassium can reach more than 86% under the optimum conditions of leaching temperature 95 °C, HCl concentration 10%, leaching time 6 h, solid/liquid ratio 1:5, and material ratio 0.1. In addition, the leaching kinetics of potassium was successfully modeled by a semi-empirical kinetic model based on the classic shrinking core model. The data showed that the leaching process of potassium was controlled by the product layer diffusion and the apparent activation energy for the process was found to be 54.67 kJ/mol over the temperature range from 65 to 95 °C.
Key words: kinetics; leaching; potassium; phosphorus-potassium associated ore; activation energy; K-feldspar
1 Introduction
Potassium is an essential ingredient in the fertilizers. At present, the global production of potash fertilizer is primarily based on the soluble potassium resources. While the soluble potassium resources is rare (<1% of world potassium storage) in China, the storage of insoluble potash ores, such as K-feldspar (KAlSi3O8), amounts to more than 10 billion tons [1]. Therefore, the economical and effective extraction of potassium from the insoluble potassium deposit has attracted many researchers’ attention in China [2].
In recent years, the phosphorus-potassium associated ore with a reserve as much as 800 million tons has been found in Yichang City, China. The phosphorus- potassium associated ore generally contains P2O5 (6%-12%) and K2O (5%-9%), and the simultaneous extraction of phosphorus and potassium to produce PK compound fertilizer may create economic value [3]. In order to prepare PK compound fertilizer, the phosphorus-potassium associated ore should be decomposed firstly. The phosphorus from phosphorus- potassium associated ore existed in the form of fluorapatite can be easily decomposed by acid, while potassium existed in the form of K-feldspar is very difficult to be decomposed by the ordinary acidic or alkaline media due to the high stability of the Al-Si-O tetrahedron structure [4]. As so far, several processes have been developed to extract soluble potassium from K-feldspar, mainly including the following methods: high temperature roasting process [5-7], hydrothermal process [8-12], microbial decomposition process [13,14], low temperature acidic leaching process [15-17]. Among these methods, low temperature acid decomposition method has received much attention in recent decades due to its lower leaching temperature (<120 °C) and higher potassium dissolution fraction (>85%).
The previous research results have shown that the dissolution fraction of potassium from phosphorus- potassium associated ore in hydrochloric acid solution reached more than 90% by using low temperature acidic leaching process [18]. However, some problems still exist, especially the equipment corrosion, higher chlorine and lower phosphorus contents in leaching solution [3]. In order to alleviate the equipment corrosion, reduce chlorine and increase phosphorus contents in leaching solution, the use of the mixture of hydrochloric acid and phosphoric acid to leach potassium from phosphorus- potassium associated ore is of great interest. However, very limited studies involving the leaching of potassium from phosphorus-potassium associated ore in HCl-H3PO4 media have been reported. Therefore, it is necessary to investigate leaching kinetics of potassium from the ore in the mixed acids.
In this work, the leaching of potassium from phosphorus-potassium associated ore in HCl-H3PO4 media at low temperature was studied. The influence of various factors, viz., the mass fraction of hydrochloric acid (w(HCl)), leaching temperature, solid-to-liquid ratio (S/L) and material ratio (CaF2 dosage (g)/mass of ore (g), M) were systematically investigated. Moreover, the leaching kinetics of potassium was modeled by a classical shrinking core model. Then, the kinetic parameters and activation energy were obtained.
2 Experimental
2.1 Experiment materials
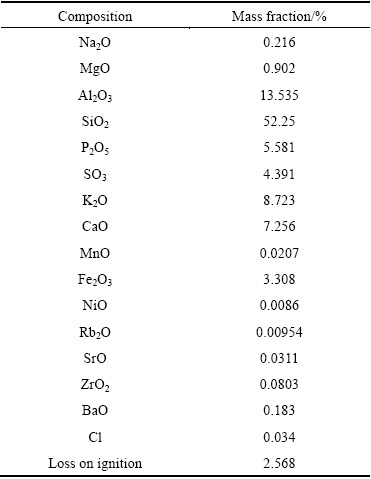
Phosphorus-potassium associated ore was obtained from Yiling district of Yichang City, Hubei Province, China. The chemical analysis of the ore performed by X-ray fluorescence (XRF) is shown in Table 1. The main minerals in the ore determined by X-ray diffractometer (XRD) and XRF characterizations are potassium feldspar (KAlSi3O8), fluorapatite (Ca5(PO4)3F), and silicon dioxide (SiO2). In this study, hydrochloric acid and phosphoric acid were mixed, and the mass fraction of phosphoric acid was kept at 30% and that of hydrochloric acid was varied.
2.2 Experimental procedure
Leaching experiments were carried out in a round-bottomed split flask with a three-necked top, equipped with a water-cooled condenser. The flask was heated by a thermostat-controlled oil batch which can control the stirring speed with a magnetic stirring apparatus. A typical experimental procedure was performed as follows: the mixed acids of known mass fraction of hydrochloric acid were put into the flask, and then the system was heated to a desired temperature. When the desired temperature reached, 20 g phosphorus-potassium associated ore (0.0385-0.105 mm) and a certain amount of CaF2 were added into the flask. In the whole experiment, the stirring speed was controlled at 1200 r/min. All the chemical reagents and samples were weighed with an uncertainly of ±0.002 g. Liquid samples (1-1.5 mL) were collected at predetermined time intervals, and filtered using 0.22 μm syringe filters. The clear filtrate was diluted and then analyzed for the concentrations of K+. The dissolution fraction of K (x) was calculated through the following expression:
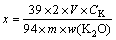 (1)
(1)
where CK is the concentration of potassium in the leached solution, V is the volume of the diluted solution, 39 and 94 denote the mole masses of K and K2O, respectively, m is the mass of the added ore, and w(K2O) is the mass fraction of K2O in the ore.
Table 1 XRF analysis of phosphorus-potassium associated ore
The mineralogical phase and morphology of the ore were characterized by XRD and scanning electron microscopy (SEM), respectively. XRD patterns were recorded on a diffractometer (using Cu Kα radiation) operating at 40 kV/30 mA. A scanning rate of 0.02 (°)/s was applied to record the patterns in the 2θ angle range from 10 to 70°. The potassium concentrations in the leached solutions were analyzed by atomic absorption spectrometry (AAS, SOLAARM6, America).
3 Results and discussion
3.1 Effect of mass fraction of hydrochloric acid
Figure 1 presents the effect of mass fraction of hydrochloric acid upon the potassium leaching as function of reaction time. It can be seen that the dissolution fraction of potassium is almost the same in the first two hours. With the increase of leaching time and mass fraction of hydrochloric acid, potassium dissolution fraction increases. The increased dissolution fraction can be attributed to the increased amount of HF resulting from increased mass fraction of hydrochloric acid [18]. It is worth noting that when the mass fraction of hydrochloric acid increases from 10% to 12%, there is no obvious increase of dissolution fraction of potassium. One possible reason is that the amount of HF will not be greatly improved because the dosage of CaF2 is only 2 g in the system.
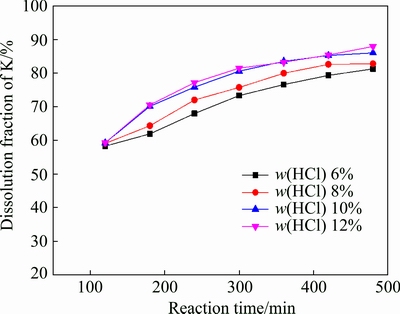
Fig. 1 Effect of mass fraction of hydrochloric acid on potassium leaching (S/L 1:5; leaching temperature 95 °C; M 0.1)
3.2 Effect of solid/liquid ratio
Figure 2 shows the effect of solid/liquid ratio on the dissolution fraction of potassium. The solid-to-liquid ratio varied from 1:3 to 1:6 g/mL was adopted to investigate the effect of solid-to-liquid ratio. These results showed that higher solid-to-liquid ratio results in lower potassium dissolution fraction, which can be partially attributed to the lower concentrations of HCl and HF in liquor resulting from higher solid-to-liquid ratio. On the other hand, when the solid-to-liquid ratio increases, the concentration of insoluble substance increases correspondingly, this may cover the ore surface, preventing the leaching reaction and thus decreasing the dissolution fraction of potassium [19].
3.3 Effect of material ratio
The influence of material ratio on the leaching of potassium was investigated, and the results are shown in Fig. 3. As can be seen, the dissolution fraction of potassium is very low (30.14%) without the addition of CaF2. The dissolution fraction of potassium increases sharply with the increase of material ratio from 0.025 to 0.1. The reason is that more HF can be produced with the increase of CaF2 dosage, which can accelerate the decomposition rate of K-feldspar. When the material ratio is 0.1, the dissolution fraction of potassium can reach 86.85%. However, further increasing the material ratio, the dissolution fraction of potassium increases smoothly[18].
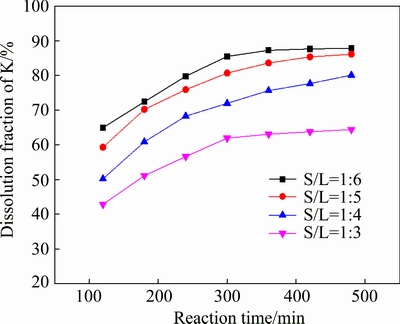
Fig. 2 Effect of solid-to-liquid ratio on dissolution fraction of potassium (w(HCl) 10%; leaching temperature 95 °C; M 0.1)
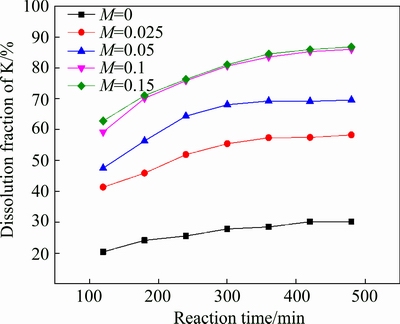
Fig. 3 Effect of material ratio on leaching of potassium (w(HCl) 10%; S/L 1:5; leaching temperature 95 °C)
3.4 Effect of leaching temperature
The leaching temperature is an important parameter in hydrometallurgy process. To study the effect of the leaching temperature on the leaching of potassium, several temperatures, viz., 65, 75, 85 and 95 °C, were selected as leaching temperature, as shown in Fig. 4. As can be observed, leaching temperature plays a major role on the potassium leaching process. The dissolution fraction of potassium is relatively low (48.72%) at 65 °C, but significantly increases as the leaching temperature rise and a high potassium dissolution fraction (86.05%) can be observed at 95 °C. The reason is that with the increase of leaching temperature, the viscosity of the liquid decreases and the diffusion coefficient increases, promoting hydrogen and fluorine ions to enter into the interior of solid particles, and thus accelerating the decomposition of K-feldspar [16,18].
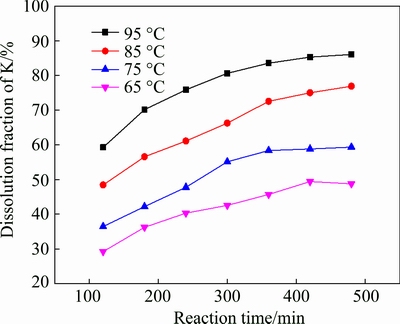
Fig. 4 Effect of leaching temperature on leaching of potassium (w(HCl) 10%; S/L 1:5; M 0.1)
3.5 Characterization of treated ore
The residue obtained after leaching at 95 °C, HCl content 10%, leaching time 6 h, solid/liquid ratio 1:5, and material ratio 0.1 was analyzed by XRD, and the result is shown in Fig. 5. As can be seen, most of K-feldspar and fluorapatite present in the raw ore disappear after leaching in the mixed acids with the addition of CaF2, and only quartz and some undecomposed K-feldspar remained in the treated ore. Hence, potassium in K-feldspar is transferred successfully from the original ore into the acidic solution via the mixed hydrochloric acid and phosphoric acid leaching at low temperature in the presence of fluoride compounds.
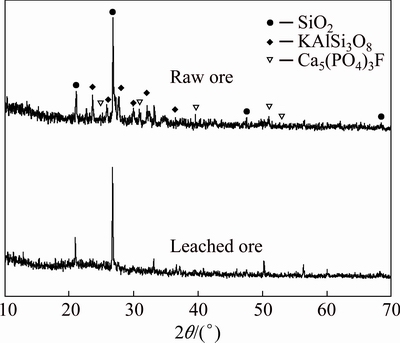
Fig. 5 XRD patterns of raw and leached ores
The morphologies of the phosphorus-potassium associated ore before and after leaching were examined by SEM (Fig. 6). The ore before leaching has a larger particle size and a compact structure. By contrast, the ore after leaching has a smaller particle size and a loose structure with fine particles deposited on the surface, which can be attributed to the decomposition of fluorapatite and K-feldspar in the ore.
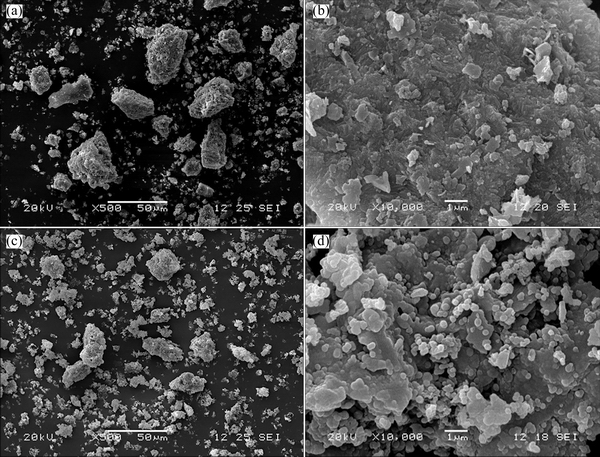
Fig. 6 SEM morphologies of raw (a, b) and leached ores (c, d)
3.6 Modeling of leaching kinetics
Fluid-solid heterogeneous reaction systems have many applications in chemical and hydrometallurgical processes. Generally, hydrometallurgical leaching operations are controlled by one of the following steps: diffusion through the fluid film, diffusion through the ash/product layer or the chemical reaction on the surface of the core of unreacted particles. The rate of the process would be controlled by the slowest of these sequential steps [19-21].
In order to establish the reaction kinetics and rate controlling step for the leaching of potassium in the mixed acid solutions, the experimental data were analyzed using the unreacted shrinking core model. According to this model, the reaction is considered to take place first at the outer surface of the particle [22-24]. The region of the reaction goes into the solid and the reacting particle shrinks during the reaction. The rate can be described by film diffusion, chemical reaction, or product layer diffusion characteristics equations, which can be written as follows:
Film diffusion: x=kt (2)
Product layer diffusion: 1-3(1-x) 2/3+2(1-x)=kt (3)
Chemical reaction: 1-(1-x) 1/3= kt (4)
where k is the kinetic constant, and t indicates the reaction time, min.
3.7 Leaching kinetics equation
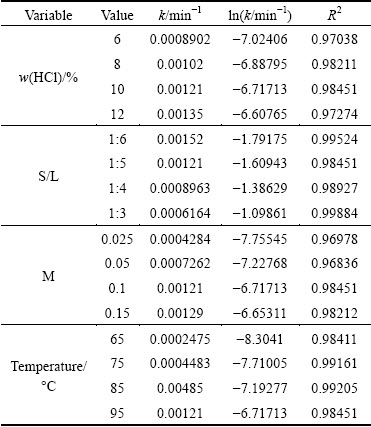
The experimental kinetic data under different conditions were correlated respectively by the film diffusion model (Eq. (2)), product layer diffusion model (Eq. (3)) and chemical reaction model (Eq. (4)). The analysis results show that Eq. (3) yields the best straight lines among the three equations (shown in Figs. 7-10), indicating that the leaching of potassium is controlled by product layer diffusion. The values of the regression coefficients and apparent rate constants calculated at various conditions are shown in Table 2.
The activation energy (Ea) of the dissolution reaction was calculated from the Arrhenius equation:
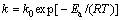 (5)
(5)
In order to determine the effects of experimental factors, viz, the mass fraction of hydrochloric acid, solid- to-liquid ratio, material ratio and leaching temperature, a semi-empirical kinetic model for dissolution of potassium feldspar was established (shown in Eq. (6)).
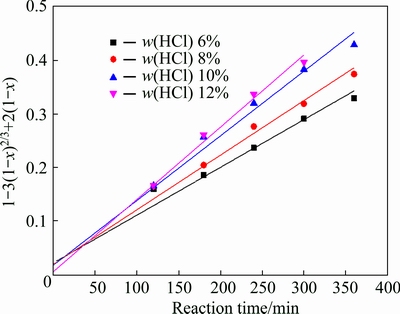
Fig. 7 Variation of 1-3(1-x)2/3+2(1-x) with time for different mass fractions of HCl
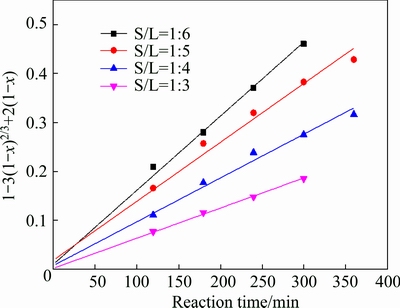
Fig. 8 Variation of 1-3(1-x)2/3+2(1-x) with time for different solid-to-liquid ratios
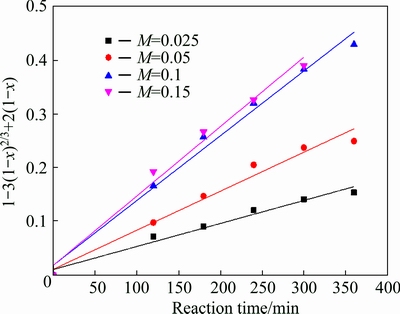
Fig. 9 Variation of 1-3(1-x)2/3+2(1-x) with time for different material ratios
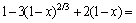
 (6)
(6)
where a, b, c are the parameters of the above model.
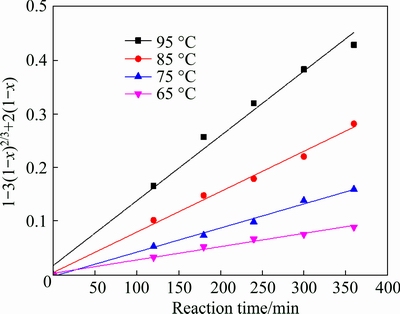
Fig. 10 Variation of 1-3(1-x)2/3+2(1-x) with time at different leaching temperatures
Table 2 Values of k calculated by Eq. (3) at different operation conditions
By simultaneous multiple regressions with the aid of Matlab software, the equation can be written as
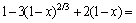
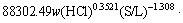
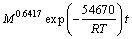 (7)
(7)
From the equation, the apparent activation energy of the leaching process was found to be 54.67 kJ/mol. Generally speaking, the activation energy of a diffusion-controlled process is typically from 4-12 kJ/mol, while for a chemically controlled process, it is usually greater than 40 kJ/mol [22]. According to this standard, the leaching of potassium from phosphorus- potassium associated ore in the mixed acids is controlled by chemical reaction. While some research results have shown that diffusion-controlled process may also have higher activation energy [25,26]. It is generally believed that the kinetic equation is more reliable than the activation energy in determining the controlling step of heterogeneous reaction. Therefore, the leaching of potassium in the mixed acids could be classified as a product layer diffusion-controlled process.
To assess the agreement between experimental dissolution fractions and the values calculated from the empirical equation, the graph of xexp vs xcal was plotted as shown in Fig. 11. As can be observed, the agreement between the experimental and calculated results is good with a correlation coefficient of 0.95 and a standard error of the estimate of 0.0217.
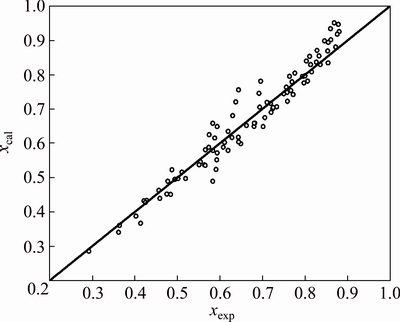
Fig. 11 Agreement between experimental and calculated dissolution fractions
4 Conclusions
1) The potassium dissolution fraction increases with the increase of hydrochloric mass fraction, leaching temperature, material ratio and the decrease of solid-to-liquid ratio. Under the optimum operation conditions, more than 86% of the potassium from phosphorus-potassium associated ore used herein can be dissolved in the acidic liquor.
2) The leaching kinetics of potassium with the mixed acids can be fitted by a semi-empirical kinetics model based on the classical shrinking core model with the product layer diffusion as the rate-controlling step and the activation energy is 54.67 kJ/mol.
References
[1] WANG S J, LI J S. Potash fertilizer production status and its development prospect in China [J]. Chemical Minerals and Processing, 2000, 1: 1-5. (in Chinese)
[2] REN X J, XIA J P, ZI G Q, YANG C Q, ZHANG Z S, LI G B, CHAO J X. Effect of leaching conditions on dissolution rate of potassium from K-feldspar calcined sample [J]. Advanced Materials Research, 2013, 734: 911-915.
[3] ZHANG Y F, MA J Y, QIN Y H, ZHOU J F, YANG L, WU Z K, WANG T L, WANG W G, WANG C W. Ultrasound-assisted leaching of potassium from phosphorus-potassium associated ore [J]. Hydrometallurgy, 2016, 166: 237-242.
[4] MA J Y, ZHANG Y F, QIN Y H, WU Z K, WANG T L, WANG C W. The leaching kinetics of K-feldspar in sulfuric acid with the aid of ultrasound [J]. Ultrasonic Sonochemistry, 2017, 35: 304-312.
[5] QIU L H, WANG L S, JIN Z M. Leaching process of potassium sulfate from roasts of potash feldspar, gypsum and calcium carbonate [J]. Journal of Chemical Engineering of Chinese Universities, 2000, 14: 465-469.
[6] FENG W W, MA H W. Thermodynamic analysis and experiments of thermal decomposition for potassium feldspar at intermediate temperatures [J]. Journal of the Chinese Ceramic Society, 2004, 32: 789-799.
[7] GAUTIER J M, OELKERS E H, SCHOTT J. Experimental study of K-feldspar dissolution rates as a function of chemical affinity at 150 °C and pH 9 [J]. Geochimica et Cosmochimica Acta, 1994, 58: 4549-4560.
[8] YUAN B, LI C, LIANG B, LV L, YUE H R, SHENG H Y, YE L P, XIE H P. Extraction of potassium from K-feldspar via the CaCl2 calcination route [J]. Chinese Journal of Chemical Engineering, 2015, 23:1557-1564.
[9] WANG C, YUE H R, LI C, LIANG B, ZHU J H, XIE H P. Mineraliztion of CO2 using natural K-feldsapr and industrial solid waste to produce soluble potassium [J]. Industial and Engineering Chemistry Research, 2014, 53 :7971-7978.
[10] ZHU J H, XIE H P. CO2 Mineraliztion of activated K-feldspar+CaCl2 slag to fix carbon and produce soluble potash salt [J]. Industial and Engineering Chemistry Research, 2014, 53: 10557-10565.
[11] LONG G Y, YUE H R, WANG Y F, SHENG H Y, YUAN B, LU L, LI C., ZHU B L, MA H W, SU S Q, YANG J, CAI B Y, LIU M T, YAO W G, PENG H. Preparation of potassium sulfate from K-feldspar by hydrothermal alkaline method: Reaction principle and process evaluation [J]. CIESC Journal, 2014, 65: 2363-2371. (in Chinese)
[12] SU S Q, MA H W, CHUAN X Y. Hydrothermal decomposition of K-feldspar in KOH-NaOH-H2O medium [J]. Hydrometallurgy, 2015, 156: 47-52.
[13] GLOWA K, AROCENA J, MASSICOTTE H. Extraction of potassium and/or magnesium from selected soil minerals by Piloderma [J]. Geomicrobiology Journal, 2003, 20: 99-111.
[14] YI L B, PENG Q Z, HE Q Z, PENG Q J. Isolation and identification of potash feldspar solubilizing bacteria and their potassium-releasing activities [J].Chinese Journal of Microecology, 2012, 24: 773-776, 785.
[15] FOGLER H S, LUND K, MCCUNE C C. Acidization III-The kinetics of the dissolution of sodium and potassium feldspar in HF/HCl acid mixtures [J]. Chemical Engineering Science, 1975, 30: 1325-1332.
[16] CHEN T P, HAN X Z, ZHENG Y X, LI W, FANG D M, ZHENG B C. Study on ball milling reaction process based on potash feldspar-phosphate rocks-hydrochloric acid system [J]. Chemical Minerals and Processing, 2013, 1: 1-4. (in Chinese)
[17] JI H P, XU J M. Reseach progress of potassium extraction from potash feldspar [J]. Modern Chemical Industry, 2011, 31: 30-33. (in Chinese)
[18] MA J Y, ZHANG S, LV R L, WANG W G, QIN Y H, WANG C W. The leaching kinetics and mechanism of potassium from phosphorus-potassium associated ore in hydrochloric acid at low temperature [J]. Separation Science and Technology, 2017, 52: 132-141.
[19] TIAN J, YIN J Q, CHI R, RAO G H, JIANG M T, OUYANG K. Kinetics on leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution [J]. Hydrometallurgy, 2010, 101: 166-170.
[20] XIAO Q G, CHEN Y, GAO Y Y, XU H B, ZHANG Y. Leaching of silica from vanadium-bearing steel slag in sodium hydroxide soution [J]. Hydrometallurgy, 2010, 104: 216-221.
[21] CHI R, ZHU G C, TIAN J. Leaching kinetics of rare earth from black weathering mud with hydrochloric acid [J]. Transactions of Nonferrous Metals Society of China, 2000, 10: 531-533.
[22] GAO W C, WEN J K, LI Z B. Dissolution kinetics of magnesium from calcined serpentine in NH4Cl solution [J]. Industrial & Engineering Chemistry Research, 2014, 53:7947-7955.
[23] YADAV V P, SHARMA T, SAXENA V K. Dissolution kinetics of potassium from glauconitic sandstone in acid lixiviant [J]. International Journal of Mineral Processing, 2000, 60: 15-36.
[24] ARACENA A, JEREZ O, ANTONUCCI C. Senarmontite volatilization kinetics in nitrogen atmoshere at roasting/melting temperatures [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 294-300.
[25] LIU J L, YIN Z L, LI X H, WANG D, HU Q Y. Kinetics of dissolution of lithium and recovery of valuable metals from lepidolite by atmosphere leaching [J]. Transactions of Nonferrous Metals Society of China, 2016, DOI: 10.1016/S1003-6326(16)64371-6.
[26] SUN Z, ZHANG Y, ZHENG S L, ZHANG Y. A new method of potassium chromate production from chromite and KOH-KNO3- H2O binary submolten salt system [J]. AIChE Journal, 2009, 55: 2646-2656.
马家玉1,2,杜学兰2,覃远航 2,吴再坤2,池汝安2,王存文2
1. 武汉科技大学 省部共建耐火材料与冶金国家重点实验室,武汉 430081;
2. 武汉工程大学 绿色化工过程省部共建教育部重点实验室,武汉 430205
摘 要:为了减轻设备腐蚀,降低酸浸液中氯的含量及提高磷的含量,研究了磷钾伴生矿中的钾在HCl-H3PO4混酸溶液中的浸出动力学。考察了盐酸的质量分数、固液比、物料比(CaF2添加量(g)与磷钾伴生矿质量(g)比)、浸出温度等几个因素对钾浸出率的影响,结果表明:在浸出温度95 °C、盐酸浓度10%、浸出时间6 h、固液比1:6、物料比0.1的优化工艺条件下,钾浸出率可达86%以上。同时建立了基于经典缩合模型的半经验动力学模型,并用该模型成功地模拟了钾的浸出过程。结果表明:在65~95 °C范围内,钾浸出过程属于内扩散控制步骤,表观活化能为54.67 kJ/mol。
关键词:动力学;浸出;钾;磷钾伴生矿;活化能;钾长石
(Edited by Yun-bin HE)
Foundation item: Project (51274153) supported by the National Natural Science Foundation of China; Projects (2011CDA120, 2015CFB523) supported by the Natural Science Foundation of Hubei Province of China; Project (G201510) supported by the State Key Laboratory of Refractories and Metallurgy (Wuhan University of Science and Technology), China; Project (K201454) supported by the Youths Science Foundation of Wuhan Institute of Technology, China
Corresponding author: Cun-wen WANG; E-mail: wangcw0120@163.com
DOI: 10.1016/S1003-6326(17)60211-5

