热解法制备碱式碳酸镁
陈白珍,江剑兵,徐 徽,石西昌,吴保安
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:以盐湖水氯镁石氨法一次沉镁后的母液经碳酸氢铵二次沉镁得到的复盐MgCO3·(NH4)2CO3·H2O为原料,采用热解法制备碱式碳酸镁。研究液固比、Mg2+用量、预氨量、热解温度和热解时间对产品质量的影响。研究结果表明:在液固比为6,Mg2+实际用量与理论用量的摩尔比为1?1,热解温度为90~100 ℃,预氨量为0.2 mL/g复盐和热解时间为4.0 h的工艺条件下得到的碱式碳酸镁质量达到HG/T 2959—2000标准,该工艺可作为盐湖水氯镁石制备碱式碳酸镁的有效方法。
关键词:碱式碳酸镁;复盐;热解;水氯镁石;碳酸氢铵;卤水
中图分类号:TF111 文献标识码:A 文章编号:1672-7207(2008)05-0907-06
Preparation of basic magnesium carbonate by pyrogenation
CHEN Bai-zhen, JIANG Jian-bing, XU Hui, SHI Xi-chang, WU Bao-an
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Basic magnesium carbonate was prepared by pyrolyzing MgCO3·(NH4)2CO3·H2O which was made from the mother liquid produced during precipitating Mg2+ from saline bischofite by ammoniation. Ammonium acid carbonate was used as the precipitation agent when MgCO3·(NH4)2CO3·H2O was prepared. The effects of liquid to solid ratio, ammonia amount used for pre-aminating, pyrogenation temperature and time on the product quality were investigated. The results show that under the condition of liquid to solid ratio 6, Mg2+ molar ratio of practical to theoretic usage 1?1, ammonia amount used for pre-aminating 0.2 mL (corresponding to 1 g of MgCO3·(NH4)2CO3·H2O obtained), pyrogenation temperature 90-100 ℃ and pyrogenation time 4 h, the products meet the standard of HG/T 2595—2000. The process can be used to prepare the basic magnesium carbonate from saline bischofite effectively.
Key words: basic magnesium carbonate; compound salt; pyrogenation; bischofite; ammonium bicarbonate; brine
碱式碳酸镁密度小且结构疏松,可作为橡胶制品的优良填充剂和增强剂,也可作绝热,耐高温的防火保温材料,颜料,油漆和日用化学品等[1-3]。目前,国内碱式碳酸镁生产方法中占主导地位的是白云石碳化法,但由于白云石为固体矿物,杂质含量高,因而,其产品质量不稳定,且设备投资大,严重影响了我国碱式碳酸镁在国际市场上的竞争力。青海省察尔汗盐湖是我国最大的钾肥生产基地,长期以来我国对青海盐湖资源的开发利用仅停留在对KCl资源的利用上,在提取KCl的过程中质量为钾肥10倍的MgCl2·6H2O重新排入盐湖中。青海盐湖集团每年排出大量的MgCl2·6H2O,这些MgCl2·6H2O如不加以利用,将给青海盐湖的生态造成严重破坏,同时造成资源的严重浪费[4-9]。为此,本文作者以青海西部镁业锡铁山实验厂生产氢氧化镁得到的母液再经碳酸氢铵二次沉镁得到的复盐MgCO3·(NH4)2CO3·H2O为原料,采用热解法制备碱式碳酸镁。
1 工艺流程及其理论分析
1.1 碱式碳酸镁制备工艺流程
图1所示为水氯镁石(俗称卤水)制备碱式碳酸镁工艺流程图。这里主要讨论二次沉镁后得到的含镁复盐热解法制备碱式碳酸镁的工艺条件,以便为盐湖水氯镁石资源综合利用提供有效方法。
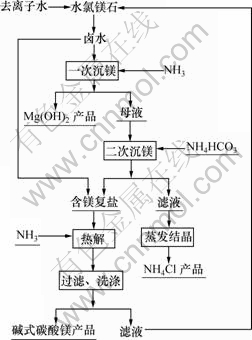
图1 碱式碳酸镁制备工艺流程图
Fig.1 Flow chart for preparation of basic magnesium carbonate
1.2 碱式碳酸镁生成机理
盐湖水氯镁石在氨的作用下,一次沉镁生成Mg(OH)2和NH4Cl:
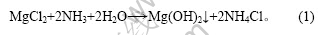
一次沉镁后得到的母液再经碳酸氢铵二次沉镁得到含镁复盐:

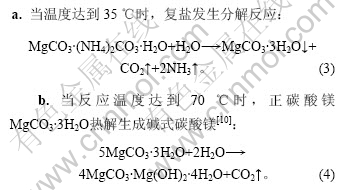
复盐热解法制备碱式碳酸镁发生的主要反应 如下。
由式(3)可知,复盐热解放出CO2和NH3。为了在此工艺中提高复盐的利用率,在热解过程中采用卤水吸收CO2和NH3,用氨水吸收多余的CO2:

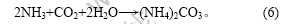
生成的Mg(HCO3)2发生热解生成MgCO3·3H2O:
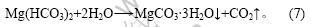
此外,卤水还可能发生如下反应:

式(7)~(9)生成的MgCO3·3H2O再按式(4)热解生成碱式碳酸镁。
2 实 验
主要原料有水氯镁石(取自青海省察尔汗盐湖)、含镁复盐(MgCO3·(NH4)2CO3·H2O,青海西部镁业锡铁山实验厂生产)、氨水(28%)。
主要实验仪器有电热恒温水浴锅、三口烧瓶、分液漏斗、恒温干燥箱、电动搅拌器。
取一定量的含镁复盐放入三口烧瓶中,并将三口烧瓶放在恒温水浴锅中加热,按一定的液固比加入去离子水,开启搅拌器同时加入预定的氨水,待温度到达40 ℃时,滴加卤水继续升温至90 ℃以上热解,热解一段时间后过滤洗涤,滤饼在120 ℃干燥得到碱式碳酸镁产品,碱式碳酸镁产品用标准AgNO3滴定Cl-含量,用EDTA滴定Mg含量来评价其质量的优劣:Cl-含量越高,产品中夹杂的NH4Cl或MgCl2越多,产品质量越差。
3 结果与讨论
3.1 液固比对碱式碳酸镁质量的影响
图2所示为液固比与碱式碳酸镁质量的关系。由图2可以看出,随着体系中液固比的增加,碱式碳酸镁中镁含量增加而Cl-含量则降低。这是因为热解时,碱式碳酸镁发生体积膨胀,若体系中液固比太小,水量不足,则最终生成的碱式碳酸镁结晶成胶体状态难过滤,从而产品中夹杂大量NH4Cl,导致Cl-含量增加,镁含量降低;另外,当整个体系中液固比比较小时,体系中NH4+的浓度过高,使碱式碳酸镁溶解度加大,增加液固比会降低体系中NH4+的浓度,使镁的利用率提高,但水量过大会造成热解能耗增大,脱水时动力消耗增加。实验结果表明,液固比为6时比较合适。

图2 液固比与碱式碳酸镁质量的关系
Fig.2 Relationship between liquid solid ratio and quality of basic magnesium carbonate
3.2 Mg2+用量对碱式碳酸镁质量的影响
图3所示为Mg2+用量(实际用量与理论用量的摩尔比)和碱式碳酸镁质量的关系,Mg2+用量通过加入卤水(MgCl2)的用量来改变。由图3可看出,随着Mg2+用量的增加,碱式碳酸镁中镁含量降低而Cl-含量增加,由反应式(1),(2),(5)和(8)可知,加入卤水起2方面的作用:一方面吸收氨;另一方面吸收CO2,提高了复盐的利用率和碱式碳酸镁的产量。但与此同时,随着卤水加入量的增加,导致碱式碳酸镁产品夹杂的NH4Cl量增加,增加洗涤难度,使碱式碳酸镁中镁含量降低,Cl-含量增加,影响产品质量。实验结果表明,镁用量为1?1较合适。
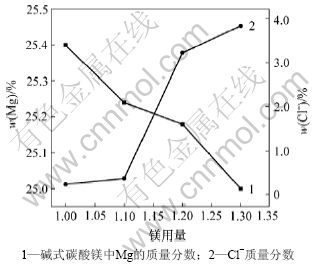
图3 Mg2+用量和碱式碳酸镁质量的关系
Fig.3 Relationship between Mg2+ usage and quality of basic magnesium carbonate
3.3 热解温度对碱式碳酸镁质量的影响
图4所示为热解温度与碱式碳酸镁质量的关系。由图4可以看出,随着热解温度的升高,碱式碳酸镁中镁含量升高,Cl-含量降低,在温度达到90 ℃时,碱式碳酸镁中镁含量最高,Cl-含量最低。这是因为热解反应是吸热反应,温度越高,化学反应速率越快,复盐热解越充分,碱式碳酸镁中镁含量越高,Cl-含量越低。正碳酸镁MgCO3·3H2O热解为碱式碳酸镁的温度为90~95 ℃[11-15],同时,为保证碱式碳酸镁煅烧得到MgO的活性和较高的视比容,热解温度控制在90~ 100 ℃,但由于复盐在35 ℃时就开始分解,因此,开始时,升温速度不应太快,温度升到40 ℃后停留10 min再升温到90~100 ℃为宜。
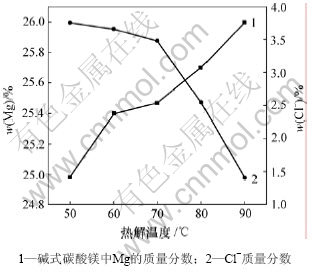
图4 热解温度与碱式碳酸镁质量的关系
Fig.4 Relationship between pyrogenation temperature and quality of basic magnesium carbonate
3.4 预氨量对碱式碳酸镁质量的影响
图5所示为预氨量与碱式碳酸镁质量的关系。由图5可以看出,随着预氨量的增加,碱式碳酸镁中镁含量增加,Cl-含量降低,由反应式(1),(4)~(6)和(8)可知:一方面NH3与MgCl2反应生成的少量Mg(OH)2吸收复盐热解出CO2;另一方面多余的NH3可能与复盐分解出的CO2在水溶液中发生酸碱中和反应,再与MgCl2反应生成MgCO3·3H2O后热解生成碱式碳酸镁。经过以上循环利用,复盐的利用率大大提高,产量也大大提高。但若预氨量加入过多,则生成较多的Mg(OH)2胶体,影响碱式碳酸镁的质量,且造成过滤洗涤困难[8],产品中夹杂的NH4Cl或MgCl2增加,导致Cl-含量增加。实验结果表明,适宜的预氨量为0.2 mL/g复盐,此时,溶液的pH值为8.3~8.4。
图5 预氨量与碱式碳酸镁质量的关系
Fig.5 Relationship between pre-added ammonia amount and quality of basic magnesium carbonate
3.5 热解时间对碱式碳酸镁质量的影响
图6所示为热解时间与碱式碳酸镁质量的关系。由图6可以看出,热解时间越长,碱式碳酸镁中镁含量越高,Cl-含量越低,这是因为热解时间越长,复盐热解越充分。Cl-含量越低,碱式碳酸镁中夹杂的NH4Cl或MgCl2越少,所以碱式碳酸镁中镁含量越高。但时间越长,热解能耗越高。综合成本和产品质量考虑选择反应时间为4.0 h。
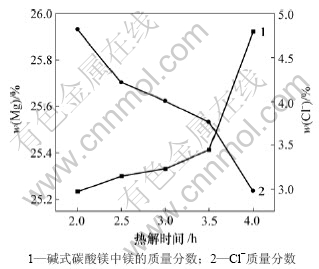
图6 热解时间与碱式碳酸镁质量的关系
Fig.6 Relationship between pyrogenation time and quality of basic magnesium carbonate
3.6 碱式碳酸镁制备正交实验
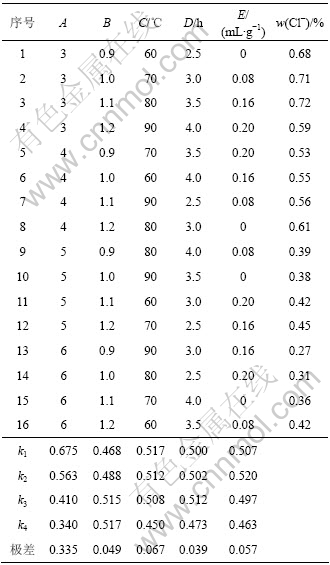
在单因素实验基础上,考察液固比(A)、镁用量(B)、热解温度(C)、热解时间(D)和预氨量(E)5个因素对产品质量的影响。选用正交表L16(45),结果如表1所示。由结果分析可知,各因素对产品质量的影响由大至小的顺序为:液固比,热解温度,预氨量,镁用量,热解时间;各因素最优水平分别为A4,B1,C4,D4和E4,这与单因素实验结果基本一致。
表1 碱式碳酸镁制备正交实验结果
Table 1 Orthogonal test results of preparation of basic magnesium carbonate
3.7 碱式碳酸镁的XDR分析
对在最佳工艺条件下所制得的碱式碳酸镁样品进行X射线衍射分析(XRD)检测,结果如图7所示。检测结果表明,得到的碱式碳酸镁产品纯度高,其组成为4MgCO3·Mg(OH)2·4H2O,这与文献[8,11]中结果吻合。
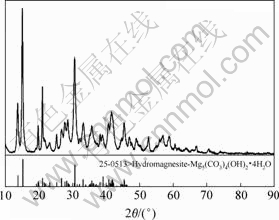
图7 碱式碳酸镁XRD图谱
Fig.7 XRD pattern of basic magnesium carbonate
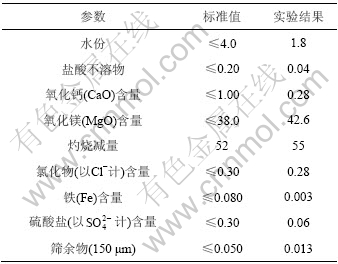
3.8 碱式碳酸镁产品质量与HG/T 2959—2000标准中要求的质量的比较
表2所列为在该工艺条件下制备的碱式碳酸镁质量分析检测结果和HG/T 2959—2000标准中要求的质量的对比。由表2可知,碱式碳酸镁产品质量达到了HG/T 2959—2000标准。
表2 碱式碳酸镁质量分析结果与HT/T 2959—2000中标准值的比较
Table 2 Comparison of test results of basic magnesium carbonate and stardard values in HG/T 2959—2000 %
4 结 论
a. 以盐湖水氯镁石氨法一次沉镁后的母液再经碳酸氢铵二次沉镁得到的复盐MgCO3·(NH4)2CO3·H2O为原料,采用热解法制备碱式碳酸镁。在液固比为6,Mg2+实际用量与理论用量的摩尔比为1?1,热解温度为90~100 ℃,预氨量为0.2 mL/g复盐,热解时间为4.0 h的工艺条件下,制得的碱式碳酸镁达到HG/T 2959—2000标准。
b. 此工艺路线制备碱式碳酸镁,工艺简单,流程短,容易实现产业化。同时,具有原材料消耗少、镁资源利用率高、产品质量稳定等优点,可作为开发盐湖镁资源的有效途径。
参考文献:
[1] 胡庆福. 我国轻质碳酸镁、轻质氧化镁生产现状及其发展[J]. 化工科技市场, 2001(6): 19-22
HU Qing-fu. Production actualities and developments of light magnesium carbonate and light magnesium oxide[J]. Market of Science and Technology of Engineering Chemistry, 2001(6): 19-22.
[2] 王万平, 张 懿. 碳酸盐热解法制备氧化镁晶须[J]. 硅酸盐学报, 2002, 30(增刊): 93-95.
WANG Wan-ping, ZHANG Yi. Preparation of magnesia whisker by the pyrolysis of magnesium carbonate[J]. Journal of the Chinese Ceramic Society, 2002, 30(Suppl): 93-95.
[3] 伍上元, 周向阳, 李 劼, 等. 水氯镁石的丁醇络合蒸馏脱水工艺[J]. 中南大学学报: 自然科学版, 2006, 37(1): 47-51.
WU Shang-yuan, ZHOU Xiang-yang, LI Jie, et al. Dehydration technology of bischofite by butanol complexation distillation[J]. Journal of Central South University: Science and Technology, 2006, 37(1): 47-51.
[4] 汪贵元. 察尔汗盐湖镁资源及开发利用[J]. 无机盐工业, 2002, 34(3): 37-38.
WANG Gui-yuan. Magnesium resource in Chaerhan Salt Lake and its exploitation and utilization[J]. Inorganic Chemicals Industry, 2002, 34(3): 37-38.
[5] 李陇岗, 钟 辉, 杨建元. 盐湖水氯镁石制备高纯镁砂的研究进展[J]. 盐湖研究, 2004, 12(1): 57-61.
LI Long-gang, ZHONG Hui, YANG Jian-yuan. Advances in preparation of magnesium from bischofite in salt lake[J]. Journal of Saltlake Research, 2004, 12(1): 57-61.
[6] 许荣辉, 李海民. 高纯氧化镁制备原理初探[J]. 盐湖研究, 2003, 11(4): 39-41.
XU Rong-hui, LI Hai-min. The principles in preparing of high purity magnesium oxide[J]. Journal of Saltlake Research, 2003, 11(4): 39-41.
[7] 徐日瑶, 刘宏专. 盐湖水氯镁石制取金属镁及高纯镁砂的生产技术[J]. 盐湖研究, 2003, 11(2): 46-50.
XU Ri-yao, LIU Hong-zhuan. Technology for the production of magnesium and high-purity magnesite clinker using bischofite from Salt Lake[J]. Journal of Saltlake Research, 2003, 11(2): 46-50.
[8] 徐 徽, 苏元智, 李新海, 等. 盐湖水氯镁石制取轻质氧化镁工艺[J]. 中国有色金属学报, 2004, 14(10): 1776-1781.
XU Hui, SU Yuan-zhi, LI Xin-hai, et al. Technology of preparation for light magnesium oxide from bischofite[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1776-1781.
[9] 高 洁, 狄晓亮, 李昱昀. 氧化镁的发展趋势及其生产方法[J]. 化工生产与技术, 2005, 12(5): 36-41.
GAO Jie, DI Xiao-liang, LI Yu-yun. Development trend and production methods of magnesium oxide[J]. Chemical Production and Technology, 2005, 12(5): 36-41.
[10] 王 锋, 李稳宏, 刘焕强, 等. 高活性氧化镁生产新工艺研究[J]. 石化技术与应用, 2002, 20(3): 152-154.
WANG Feng, LI Wen-hong, LIU Huan-qiang, et al. A new process for production of high active magnesium[J]. Petrochemical Technology & Application, 2002, 20(3): 152-154.
[11] 隋 升, 曹广益. 氧化镁生产工艺的改进[J]. 上海交通大学学报, 2001, 35(4): 595-598.
SUI Sheng, CAO Guang-yi. Improvement on the process of MgO manufacture[J]. Journal of Shanghai Jiaotong University, 2001, 35(4): 595-598.
[12] 徐日瑶. 镁冶金学[M]. 北京: 冶金工业出版社, 1981.
XU Ri-yao. Magnesium metallurgy[M]. Beijing: Metallurgical Industry Press, 1981.
[13] 徐 徽, 蔡 勇, 陈白珍, 等. 用低品位菱镁矿制取高纯镁砂[J]. 中南大学学报: 自然科学版, 2006, 37(4): 698-702.
XU Hui, CAI Yong, CHEN Bai-zhen, et al. Preparation of high purity magnesia from low-grade magnesite[J]. Journal of Central South University: Science and Technology, 2006, 37(4): 698-702.
[14] 高 峰, 聂祚仁, 王志宏, 等. 中国皮江法炼镁的资源消耗和环境影响分析[J]. 中国有色金属学报, 2006, 16(8): 1456-1461.
GAO Feng, NIE Zuo-ren, WAN G Zhi-hong, et al. Resource depletion and environmental impact analysis of magnesium produced using Pidgeon process in China[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(8): 1456-1461.
[15] 黄晓锋, 周 宏, 何镇明. 镁合金的防燃研究及其进展[J]. 中国有色金属学报, 2000, 10(增刊1): 271-274.
HUANG Xiao-feng, ZHOU Hong, HE Zhen-ming. Research and development on ignition proof magnesium alloys[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(Suppl. 1): 271-274.
收稿日期:2007-12-26;修回日期:2008-02-28
基金项目:国家科技攻关项目(2005BA639C)
通信作者:陈白珍(1946-),女,江苏江宁人,教授,从事盐湖卤水开发研究;电话:0731-8877352;E-mail: yhschem@csu.edu.cn