
Preparation and characterization of porous magnesium materials
LIU Xi-qin(刘希琴), LIU Zi-li(刘子利), ZHANG Xiao-hong(张晓红),
FENG Jun-dong(丰俊东), YU Ta-xi(俞挞汐)
College of Materials Science and Engineering, Nanjing University of Aeronautics and Astronautics,
Nanjing 210016, China
Received 28 July 2006; accepted 15 September 2006
Abstract: The proper spacer material and the preparation technology for biological compatible porous magnesium materials were explored by the powder metallurgy method, and microstructures, porosity and mechanical properties of sintered porous magnesium were investigated. The results show that compared with spacer materials of NH4CO3, NH3Cl and carbamide, NH4CO3 is the best one for preparation of sintered porous magnesium, and the worst one is NH3Cl. The isolated blind pores are formed mainly by the particle interval of the magnesium powders. Adding spacer material favors the formation of open pores, while has little contribution to the formation of blind pores. The overall porosity and porosity of open pore of the sintered porous magnesium increase with the increase of added spacer material, while decrease with the increase of the molding stress. The mechanical properties of sintered porous magnesium increase with decreasing addition of spacer material and increasing molding stress.
Key words: porous magnesium; powder metallurgy; spacer material; microstructure; porosity; mechanical properties
1 Introduction
Metallic materials are widely used as biomaterials in replacement or repairing the functional bone tissue, because of their unique combination of high mechanical strength and fracture toughness compared with that of ceramics or polymeric materials[1]. Currently approved and commonly used metallic materials included stainless steel, titanium and cobalt-chromium-based alloys. They are essentially neutral in vivo, but a limitation is the possible release of toxic metallic ions and/or particles through corrosion or wear process that lead to inflammatory cascades. The permanent fixtures such as screws and pins used to secure serious fracture must be removed by a second surgical procedure after the tissue has healed sufficiently, which increases costs to the health care system and further morbidity to the patient. Moreover, the elastic moduli of current metallic biomaterials are not well matched with those of natural bone tissue, resulting in stress shielding effects that lead to reduced stimulation of new bone growth and remodeling which decrease implant stability[2-4].
As the natural bone is an interconnected, porous structure, the ideal bone substitute material should be osteoconductive in order to allow as rapid as possible integration with host bone, biodegradable and bioresorbable at a prefer rate in order to eventually be replaced by newly formed natural bone, and strong enough to fulfill required load bearing functions during the implantation period[2,5-9]. As one of most important bivalent ion associated with the formation of biological apatites, magnesium has been recently recognized as a promising biomaterial for bone substitution due to its excellent properties, e.g. relatively low elastic modulus and proper strength, excellent biocompatibility, biodegradability and bioresorbability. These features make porous magnesium an ideal scaffold for bone tissue regeneration[8-16].
In this study, porous magnesium materials were prepared by the powder metallurgy method, and effects space-holder material and the preparation parameters on porosity, microstructures, and mechanical properties were investigated.
2 Experimental
Commercially available magnesium powder was used as starting material (Table 1), and the shape of Mg powder particles is quite irregular (Fig.1). NH4CO3, NH4Cl and carbamide were used as spacer materials. WEN et al[9] reported that in porous bone substitute, the optical pore size for attachment, differentiation and growth of osteoblasts and vascularization was approximately 300-400 μm or 200-500 μm, so the spacer used in experiment was in the size of 300 μm. The spacer material was selected by its chemical properties, i.e. the ability to decompose completely at relatively low temperature so as to avoid the reaction with the host powders.
Table 1 Size of magnesium powders

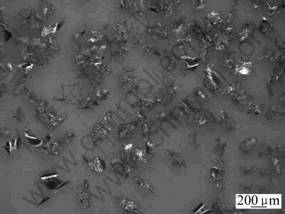
Fig.1 Shape of Mg particles
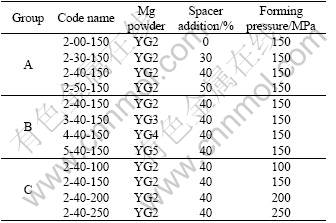
Mg powders and spacer particles were mixed homogeneously with a ball miller in a volume ratio of 30%, 40% and 50%. Powders of the mixture were uniaxially pressed at a pressure of 100, 150, 200, 250 MPa into cylindrical green compacts of d10 mm×10 mm (Table 2). The green compacts were then heat treated to burn out the spacer particles at 200 ℃ for 5 h in a DA-1A model vacuum cabinet drier, and to sinter into highly porous Mg foams in Ar protective atmosphere at 500 ℃ for 5 h.
The porosity of the porous magnesium was measured by Archimedes method. The sample was dipped under vacuum to fasten water in the sintered porous magnesium. There are two type pores in the sintered porous magnesium, which are interconnected open pores and isolated blind pores. The porosity of open pores (Po), the porosity of blind pores (Pb) and the overall porosity (P) are calculated by the following formulas:
Po=[(V-Vd+md-m)/V]×100% (1)
Pb=[(Vd-md+m-m/ρ)/V]×100% (2)
P=Po+Pb=[(V-m/ρ) /V]×100% (3)
where m is the mass of the porous magnesium sample, md is the mass of the dipped sample, V is the volume of the sample (Vaseline is coated on sample surface to avoid water entering into the sample from surface pores), Vb is drained water volume by the sample, ρ is the density of magnesium.
Table 2 Processing parameters for tested samples
The transverse section of sintered porous magnesium sample was used to observe the microstructure by VHX-100 microscope. The compression tests were carried out on the specimens with CMT5105 material tester at a strain rate of 0.5 mm/min.
3 Results and discussion
3.1 Evaporation experiment for spacer
Table 3 shows the evaporation results of different spacer materials at 200 ℃ for 30 min. From Table 3, it can be seen that at the spacer removal temperature of 200 ℃, the evaporation effect of NH4Cl is not ideal, and carbamide is in the state of liquid when it is carried out and most part are not evaporated. So, NH4CO3 is the best spacer for preparation of porous magnesium, while NH4Cl is the worst one. If it was not mentioned specially, NH4CO3 is the spacer material used in preparation of cylindrical green compacts.
Table 3 Evaporation results of different spacer materials at 200 ℃ for 30 min
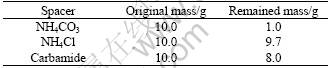
3.2 Microstructures of sintered porous magnesium
Fig.2 shows the XRD pattern of the sintered porous magnesium with NH4CO3 as spacer material. No MgO is found in the sample, which indicates that NH4CO3 is a effective spacer material for preparation in the experiment.
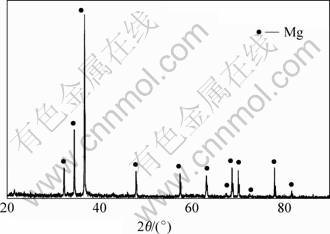
Fig.2 XRD pattern of sintered porous magnesium with NH4CO3 as spacer material
Microstructures of sintered porous magnesium samples are shown in Fig.3. The irregular Mg powder particles are fused together at the large contact surface, while the neighboring particles with small contact surface often form blind pore. There are pores that are in a size of about 100 μm. The pores are formed by the removal of spacer material, they are quite likely to contact with blind pore, and make a connected open pore. From Figs.3(a) and (b), it is found that the amount of pores, especially the open pores, increases with the increase of spacer material addition. The size of open pores is decreased with decreasing size of Mg powder, and the amount of blind pores maybe increases (Figs.3(b) and (c)). The sintered porous magnesium becomes denser with the increase of mold stress, and the size and amount of open pores decrease greatly (Figs.3(b) and (d)).
3.3 Porosity of sintered porous magnesium
For group A tested specimen(Table 2), Fig.4 shows the effect of spacer addition on the porosity of sintered porous magnesium prepared at 150 MPa molding pressure and with YG2 magnesium powder. The overall porosity and the porosity of open pores increase with increasing addition of spacer, while the effect of spacer addition on the porosity of blind pores is very little (no more than 6%), and even decrease slightly with increasing addition of spacer. The reason lies in that most of the blind pores are formed from the gap between Mg powder particles during compaction. As the size of added spacer is larger than that of Mg powder particles, with increasing addition of spacer, it is quite likely for pores formed during spacer removal process to contact with the blind pore, and become interconnected open pore. This explanation is also certified by the experimental results that the porosity of open pores is very similar to the amount of spacer addition (Fig.4).
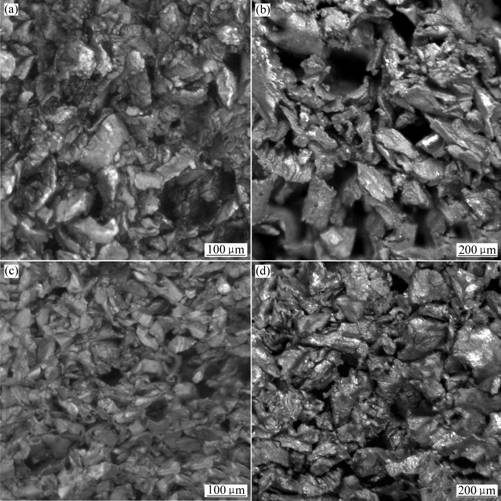
Fig.3 Microstructures of sintered porous magnesium samples: (a) 2-30-150; (b) 2-40-150; (c) 4-40-150; (d) 2-40-250
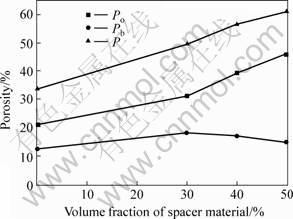
Fig.4 Effect of spacer addition on porosities of sintered porous magnesium
For group B tested specimen, effect of the size of Mg powder on the porosity of sintered porous magnesium prepared at 150 MPa molding pressure with 40% spacer addition is shown in Fig.5. The size of Mg powder has very small effect on the porosity of sintered porous magnesium, but the porosity of sintered porous magnesium prepared by the finer Mg powder particles (YG5) is much small. Tested results contradicte the general idea that the overall porosity and the porosity of open pores decrease with decreasing size of powder particle[14]. However, the tested Mg powder particle is in a quite irregular shape, which is easy to get pore in sintered porous magnesium; furthermore, the amount of pores is related to the size of different Mg powder and spacer material, therefore, under experimental conditions the porosity of sintered porous magnesium is not sensitive to powder particle size.
For group C tested specimen, effect of molding pressure on the porosity of sintered porous magnesium prepared with YG2 magnesium powder and 40% spacer addition is shown in Fig.6. The overall porosity and the porosity of open pores decrease with increasing molding pressure, while the effect of molding pressure on the porosity of blind pores is very little, and even increase slightly with increasing molding pressure. This is because with increasing molding pressure, cylindrical green compacts become denser. During the densification process some of the open pores will be compressed into blind pores and even disappear, while some blind pores become smaller and even vanish.
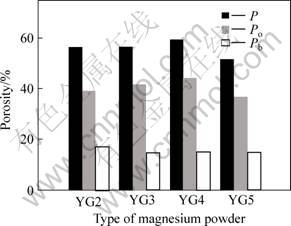
Fig.5 Porosity of porous magnesium with different sizes of magnesium powders
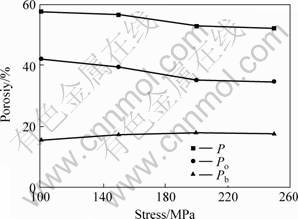
Fig.6 Effect of molding stress on porosity of sintered porous magnesium
3.4 Mechanical properties of sintered porous magnesium
With increasing addition of spacer material, the porosity, especially the porosity of open pores, of sintered porous magnesium increases, which greatly decreases the actual load-bearing area and is very easy for the crack to develop, therefore, the elastic modulus and compression stress of sintered porous magnesium decrease sharply with increasing addition of spacer (Fig.7). However, the overall porosity and the porosity of open pores decrease with increasing molding pressure and the molding stress, and then mechanical properties of sintered porous magnesium increase with increasing mold stress (Fig.8). Generally speaking, the mechanical properties of the sintered porous magnesium increase with the decrease of size of Mg powder particle (Fig.9). The reason is the same as that of molding stress, for they all lead to a denser green compacts during mold pressing process.
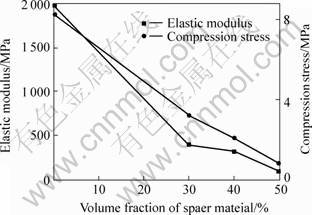
Fig.7 Effect of spacer addition on mechanic properties of sintered porous magnesium
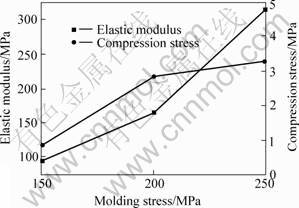
Fig.8 Effect of molding stress on mechanical properties of sintered porous magnesium
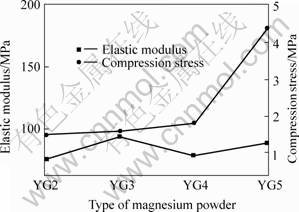
Fig.9 Mechanical properties of sintered porous magnesium with different types of magnesium powders
4 Conclusions
1) Compared with spacer materials of NH4CO3, NH4Cl and carbamide, NH4CO3 is the best spacer material for preparation of sintered porous magnesium, and the worst one is NH4Cl.
2) The isolated blind pores are formed mainly by the particle interval of the magnesium powders, and adding spacer material favors the formation of open pores, while has little contribution to the formation of blind pores. The overall porosity and porosity of open pore of the sintered porous magnesium increase with the increase of spacer material addition, while decrease with the increase of the molding stress.
3) The mechanical properties of sintered porous magnesium increase with decreasing addition of spacer material and increasing molding stress.
References
[1] NIINOMI M. Recent metallic materials for biomedical applications[J]. Met Mater Trans A, 2002, A33: 477-486.
[2] STAIGER M P, PIETAK A M, HUADMAI J, DIAS G. Magnesium and its alloys as orthopedic biomaterials: A review[J]. Biomaterials, 2006, 27: 1728-1734.
[3] LHOTKA C, SZEKERES T, STEFFAN I, ZHUBER K, ZWE- YMUELLER K. Four-year study of cobalt and chromium blood levels in patients managed with two different metal-on-metal total hip replacements[J]. J Orthop Res, 2003, 21: 189-195.
[4] WANG J Y, WICKLUND B H, GUSTILO R B, TSUKAYAMA D T. Titanium, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro[J]. Biomaterials, 1996, 17: 2233-2240.
[5] STROGANOV G B, SAVITSKY E, MIKHAILOVICH T, NINA M, TEREKHOVA V, FEDOROVNA V. Magnesium-base alloys for use in bone surgery[P]. US: 3 687 135, 1972.
[6] OKUMA T. Magnesium and bone strength[J]. Nutrition, 2001, 17: 679-680.
[7] VORMANN J. Magnesium: nutrition and metabolism[J]. Mol Aspects Med, 2003, 24: 27-37.
[8] WOLF F I, CITTADINI A. Chemistry and biochemistry of magnesium[J]. Mol Aspects Med, 2003, 24: 3-9.
[9] WEN C E, MABUCHI M, YAMADA Y, SHIMOJIMA K, CHINO Y. Processing of biocompatible porous Ti and Mg[J]. Scripta Materialia, 2001, 45: 1147-1153.
[10] KUWAHARA H, AL-ABDULLAT Y, MAZAKI N, TSUTSUMI S, AIZAWA T. Precipitation of magnesium apatite on pure magnesium surface during immersing in Hank’s solution[J]. Mater Trans, 2001, 42(7): 1317-1321.
[11] LI Long-chuan, GAO Jia-cheng, WANG Yong. Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid[J]. Surface & Coatings Technology, 2004, 185: 92-98
[12] WITTE F, KAESE V, HAFERKAMP H, SWITZER E, MEYER-LINDENBERG A, WIRTH C J, WINDHAGEN H. In vivo corrosion of four magnesium alloys and the associated bone response[J]. Biomaterials, 2005, 26: 3557-3563.
[13] WEN C E, YAMADA Y, SHIMOJIMA K, CHINO Y, HOSOKAWA H, MABUCHI M. Porous bioresorbable magnesium as bone substitute[J]. Materials Science Forum, 2003, 419-422: 1001-1006.
[14] WEN C E, YAMADA Y, SHIMOJIMA K, CHINO Y, HOSOKAWA H, MABUCHI M. Compressibility of porous magnesium foam: dependency on porosity and pore size[J]. Mater Lett, 2004, 58: 357-360.
[15] KARAGEORGIOU V, KAPLAN D. Porosity of 3D biomaterial scaffolds and osteogenesis[J]. Biomaterials, 2005, 26: 5474-5491.
[16] BANHART J. Manufacture, characterization and application of cellular metals and metal foams[J]. Prog Mater Sci, 2001, 46: 559-632.
(Edited by YANG You-ping)
Corresponding author: LIU Xi-qin; Tel: +86-25-52112626; E-mail: liuxiqin@nuaa.edu.cn