文章编号:1004-0609(2013)04-1152-08
酯化改性麦糟对Pb(Ⅱ)的吸附特性
李青竹1, 2, 3,覃文庆2,柴立元1, 3,王庆伟1, 2, 3
(1. 中南大学 冶金科学与工程学院,长沙 410083;
2. 中南大学 资源加工与生物工程学院,长沙 410083;
3. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083)
摘 要:以啤酒工业废弃物麦糟为原料,采用一步快速酯化改性法制备高效重金属离子吸附剂酯化改性麦糟。研究酯化改性麦糟对Pb(Ⅱ)的吸附行为、吸附动力学及吸附机制。结果表明:在较宽的pH值范围(4~8)内,酯化改性麦糟表现出对Pb(II)良好的吸附性能。由Langmuir吸附等温线方程计算得到该吸附剂对Pb(Ⅱ)的理论饱和吸附量为393.7 mg/g,高于文献报道的大多数吸附剂的吸附量。吸附反应很快在15 min内达到平衡,吸附符合拟二级动力学方程。活化能的计算表明吸附为活性化学吸附。酯化改性麦糟吸附Pb(Ⅱ)主要是羟基和羧基中C—O基团的氧原子与Pb(Ⅱ)配合的结果。
关键词:酯化改性麦糟;吸附;Pb(Ⅱ);动力学;吸附机制
中图分类号:O647.3 文献标志码:A
Characteristics of Pb(Ⅱ) adsorption on esterified spent grain
LI Qing-zhu1, 2, 3, QIN Wen-qing2, CHAI Li-yuan1, 3, WANG Qing-wei1, 2, 3
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
3. Chinese National Engineering Research Center for Control & Treatment of Heavy Metal Pollution,
Central South University, Changsha 410083, China)
Abstract: Spent grain, a by-product of brewing industry, was used to prepare an efficient heavy metal adsorbent using one-step and fast esterification method. The adsorption behaviors, adsorption kinetics and mechanism of Pb(Ⅱ) by esterified spent grain (ESG) were investigated. The results show that the esterified spent grain exhibited good adsorption performance over the wide pH range of 4-8. The maximum theoretical Pb(Ⅱ) adsorption capacity of ESG calculated from Langmuir isotherm is 393.7 mg/g, which is higher than that of other adsorbents reported. The sorption process is very fast and the equilibrium is established in 15 min. The sorption kinetics is well described by the pseudo-second order kinetic model. The calculated activation energy (Ea) implies that adsorption of Pb(Ⅱ) on ESG is a chemical adsorption. Fourier transform infrared spectroscopy (FTIR) analysis demonstrates that the oxygen atoms of hydroxyl group and the carbon-oxygen (C—O) in carboxyl group of ESG complexing with Pb(Ⅱ) leads to the adsorption.
Key words: esterified spent grain; adsorption; Pb(Ⅱ); kinetics; adsorption mechanisms
基金项目:国家杰出青年科学基金资助项目(50925417);国家高技术研究发展计划资助项目(2011AA061001);中南大学博士后基金资助项目;湖南有色研究基金资助项目
收稿日期:2011-07-12;修订日期:2012-12-04
通信作者:王庆伟,讲师,博士;电话:0731-88830875;E-mail: qw_wang@csu.edu.cn
据中华人民共和国国家统计局统计,2010年全国各行业工业废水排放总量达211.86亿t,工业废水中铅的排放量达140.805 t,其中有色金属矿采选业及有色金属冶炼及压延加工业为含铅废水排放的主要源头分别占61.60%和20.27%。铅是一种具有神经毒性的重金属元素,进入人体主要损害骨髓造血系统和神经系统,引发贫血症、神经机能失调及肾损伤并对儿童造成大脑的损害[1]。含铅废水的处理方法主要包括化学沉淀法、膜分离法、离子交换法、电解法和吸附法等[2],其中吸附法处理含铅废水因其成本低廉、操作简单、不产生二次污染等优点成为研究的热点[3]。
传统吸附剂活性炭可以同时吸附多种重金属离子、吸附容量大,但价格昂贵、使用寿命短、操作费用高。近年来,使用廉价的自然资源或工农业废弃物制备生物吸附剂,因原料来源广泛,价格低廉,已经受到众多研究者的青睐[4]。使用未经处理的工农业废弃物作为吸附剂吸附容量较低,其释放的有机物导致水中COD和BOD过高,通过化学改性可以避免天然吸附剂应用时有机物的溶出并提高其吸附容量[5]。
我国产量居世界第一的啤酒行业产生大量废麦糟,没有得到合理利用,这不仅污染了环境,而且浪费了宝贵资源。除LOW等[6]和CHAI等[7]外,国内外少有研究者选用麦糟为原料制备重金属吸附剂。本文作者开发了一步快速酯化改性麦糟新方法[8],优选次亚磷酸钠(NaH2PO2·H2O)为催化剂、N, N-二甲基甲酰胺(DMF)为反应介质,以柠檬酸与麦糟反应2 h即制备了高效的重金属吸附剂酯化改性麦糟,可用于吸附Pb(Ⅱ)。本文对酯化改性麦糟吸附Pb(Ⅱ)的吸附特性、吸附动力学以及吸附机制进行系统研究,为反应器的设计和运行提供基础信息,对含铅废水处理具有重要实际意义。
1 实验
1.1 试剂
麦糟由长沙英博白沙啤酒厂提供,水洗除去表面附着的泥沙等杂质,60 ℃ 烘干,研碎,过孔径300 μm的筛,备用。未改性麦糟用RSG(Raw spent grain)表示。硝酸铅、柠檬酸、次亚磷酸钠、N, N-二甲基甲酰胺和无水乙醇均为分析纯。
1.2 酯化改性麦糟的制备与表征
取1 g 柠檬酸溶于25 mL N, N-二甲基甲酰胺(DMF)中,然后与5 g麦糟、1 g 催化剂(NaH2PO2·H2O)混合在140~150 ℃下反应2 h,自然冷却至室温。反应后的混合物先用无水乙醇洗涤去除有机溶剂,然后用大量蒸馏水冲洗,抽滤至滤液中加硝酸铅溶液无白色沉淀产生,滤渣于60℃下通风干燥12 h,研碎过孔径300 μm的筛备用。酯化改性麦糟用 ESG(Esterified spent grain)表示。大规模制备时,DMF和催化剂可分离回收,以循环利用,节省成本。通常DMF采用减压蒸馏的方法回收,次亚磷酸钠采用溶解-结晶-过滤的简单工艺回收。
酯化改性前后及吸附前后的样品采用KBr压片法在美国NICOLET公司IS10型傅立叶变换红外光谱仪上测定其红外光谱,波长范围在4 000~400 cm-1之间。
1.3 吸附实验方法
1.3.1 pH值的影响
分别取50 mL初始浓度为0.2 mmol/L的Pb(Ⅱ)溶液,加入0.1 g 吸附剂,用0.1 mol/L HNO3或0.1 mol/L NaOH调节溶液的pH值在2~10之间,置于HH-6型恒温水浴磁力搅拌器(江苏常州澳华仪器有限公司)中在25 ℃、转速200 r/min的条件下搅拌吸附20 min。过滤,取上清液,滤液用WFX-120型原子吸收分光光度计(北京瑞利分析仪器总厂生产)检测分析Pb(Ⅱ)浓度,并计算相应的去除率和吸附量。RSG按照上述同样实验条件进行实验与ESG进行对照。
金属离子的去除率R(%)和吸附剂的吸附量q(mg·g-1)分别按式(1)和(2)计算:
R=100(c0-c)/c0 (1)
q=(c0-c)V/m (2)
式中:c0为吸附前溶液中金属离子的质量浓度(mg/L);c为吸附后溶液中金属离子的质量浓度(mg/L);V为反应溶液体积(L);m为吸附剂干质量(g)。
1.3.2 吸附动力学实验
向一系列50 mL浓度为0.5 mmol/L的Pb(Ⅱ)溶液中投加0.1 g ESG,在转速200 r/min,温度分别为5、25和45 ℃的恒温水浴磁力搅拌器中吸附一定时间,在不同时间点取样,过滤,取上清液,用原子吸收分光光度计测定其中Pb(Ⅱ)的浓度,并计算相应的吸附量,用于动力学方程的拟合。
1.3.3 吸附平衡等温线的测定
向一系列50 mL浓度分别在0.2~20 mmol/L的Pb(Ⅱ)溶液中投加0.1 g ESG,在转速200 r/min,温度为25 ℃的恒温水浴磁力搅拌器中吸附20 min,过滤,取上清液,用原子吸收分光光度计测定其中Pb(Ⅱ)浓度,并计算相应的吸附量,用于吸附平衡等温线的 拟合。
2 结果与讨论
2.1 酯化改性麦糟的制备原理及表征
麦糟作为木质纤维素类物质的一种,其表面富含羟基,表现出多元醇的性质,在强酸液中羟基可被亲核基团或亲核化合物所取代,发生亲核取代反应,生成相应的酯,从而提高其吸附能力。传统酯化改性木质纤维素类物质的方法由MARSHALL等[9]在1999年提出,用于柠檬酸酯化改性大豆壳以吸附金属离子,此方法将柠檬酸于50 ℃加热12 h,脱水形成反应活 性较高的柠檬酸酐,进而活性酸酐同大豆壳(经过NaOH 溶胀预处理)中的纤维素类羟基在120 ℃、浓硫酸作催化剂的条件下反应90 min制备酯化改性大豆壳,其对Cu(Ⅱ)的吸附量达1.7 mmol/g。此后,有许多学者使用以上方法改性木质纤维素类物质制备高效吸附剂。但该方法存在原料需溶胀预处理、两步反应时间长、浓硫酸作催化剂腐蚀性强等缺点。因此,拟探索简便、高效、快速酯化改性的新方法。木质纤维素类物质通常采用NaOH溶液活化、溶胀,以提高其反应活性,本研究综合考虑选用DMF作为麦糟的活化剂,同时可作为柠檬酸的溶剂,使麦糟的前处理过程和有机反应过程同时发生,大大节省了改性时间。羧酸跟醇的酯化反应是可逆的且反应缓慢,常用浓硫酸作催化剂,腐蚀性强,在本研究中,选择温和、高效的次亚磷酸钠作为酯化反应的催化剂,大大降低了反应的活化能,提高了反应速率。柠檬酸和麦糟的酯化反应如式(3)所示。可以看出,通过使用合适的反应介质,控制一定的反应温度,在催化剂作用下,柠檬酸的羟基能够和麦糟中羟基上的氢脱水生成酯,制备酯化改性麦糟。
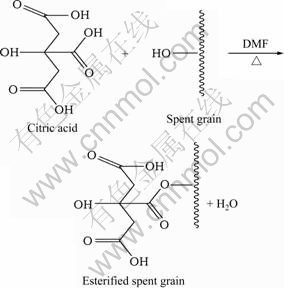 (3)
(3)
酯化改性前后麦糟的红外光谱如图1所示。对于未改性麦糟红外光谱图谱带归属如下:3 301.87 cm-1处较宽的吸收带可归属为碳水化合物中结合水的羟基O—H的伸缩振动与仲酰胺(RNHCOCH3)中N—H的伸缩振动[10];2 925.96和2 862.16 cm-1处的双峰为 —CH2的不对称伸缩振动峰。蛋白质是麦糟的主要成分之一,1 652.21 cm-1处的谱峰来自仲酰胺Ⅰ带,是C—O的伸缩振动引起的;1 531.10 cm-1处的谱峰来自仲酰胺Ⅱ带,是N—H的弯曲振动和C—N的伸缩振动的叠加引起的,这两个峰是蛋白质的特征谱带[11]。1 246.71 cm-1处的谱峰来自酰胺Ⅲ带,是C—N的伸缩振动和N—H的弯曲振动引起的。1 449.80 cm-1处的谱峰来自—CH3和—CH2—的弯曲振动。1 043.70 cm-1处的谱峰来自糖类的C—O(H)的伸缩振动[12]。
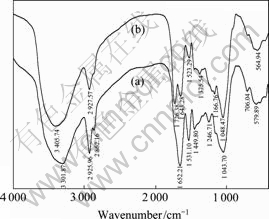
图1 未改性麦糟和酯化改性麦糟的红外光谱
Fig. 1 Infrared spectra of RSG(a) and ESG(b)
酯化改性后麦糟在1 726.38和1 166.76 cm-1处出现两个新的吸收峰,前者是酯基C=O键的特征峰,后者是酯基C—O键的特征峰[13],这说明酯化改性是成功的。而且,麦糟酯化改性后位于3 301.87 cm-1处的宽峰紫移至3 405.74 cm-1处,这说明麦糟中富含的羟基确实参与到酯化反应过程中。通过柠檬酸和麦糟中羟基的酯化反应,形成了新的酯键并引入了更多的羧基官能团,这有利于提高麦糟吸附重金属离子的能力。
2.2 pH对吸附效果的影响
pH值对麦糟酯化改性前后吸附Pb(Ⅱ)的影响如图2所示。由图2可知:未改性麦糟吸附Pb(Ⅱ)在pH值4左右去除率达到最大值,pH值低于或高于此值,去除效果均不佳;酯化改性麦糟吸附Pb(Ⅱ)的最佳pH值范围较宽,pH值在4~8之间Pb(Ⅱ)去除率均较高。当pH值小于上述最佳pH值范围时,吸附效率随pH值增大而增大;当pH值大于该范围时,吸附率随pH增大而减小。其原因如下:低pH值时,一方面H3O+与重金属离子争夺吸附位点,同时阻碍活性基团的解离,致使吸附量低,吸附效果不佳;另一方面,麦糟表面的—OH被质子化带正电荷,重金属离子表面也带正电荷,静电斥力抑制吸附的顺利进行[14]。重金属离子在水溶液中的存在形态对其吸附效果也有一定影响,利用visual MINTEQ, ver. 2.53 软件计算pH值对0.2 mmol/L铅离子在水溶液中形态的影响如图3所示。可以看出,当pH<7时,Pb主要以Pb(Ⅱ)形式存在;当pH>7时,开始形成Pb(OH)2沉淀;当pH>9时,Pb以Pb(Ⅱ)形式存在的比例仅为3%,主要以PbOH+和Pb(OH)2(aq)的形式存在,从而阻碍吸附的顺利进行,这很好地解释了高pH值不利于吸附的原因。
整个吸附过程中酯化改性麦糟对Pb(Ⅱ)的吸附效果均优于未改性麦糟的,更重要的是酯化改性后吸附Pb(Ⅱ)的最佳pH范围更宽。由于酯化改性麦糟和铅溶液接触后的初始pH为4.19,在最佳吸附pH范围内,吸附反应可以在初始pH下进行,从而省去了调节pH值这一工序。另外,较宽的最佳pH值范围保证了酯化改性麦糟处理不同种类不同性质含铅废水的能力。
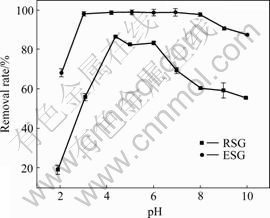
图2 pH值对酯化改性前后麦糟吸附Pb(Ⅱ)的影响
Fig. 2 Effect of pH on removal rate of Pb(Ⅱ) by ESG and RSG
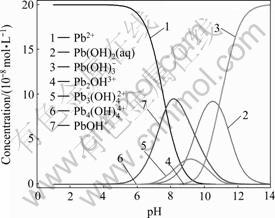
图3 pH值对铅离子在水溶液中形态的影响
Fig. 3 Effect of pH on states of Pb(Ⅱ) in aqueous solution
2.3 吸附动力学
吸附时间是影响吸附效果的一个非常重要的因素,吸附时间不足使吸附不能达到平衡,从而影响处理效果,吸附时间对酯化改性麦糟吸附Pb(Ⅱ)的影响如图4所示。结果表明:随着吸附时间的延长,Pb(Ⅱ)的去除率逐渐增大,酯化改性麦糟吸附Pb(Ⅱ)速率很快,前2 min内去除率达90%以上,随后缓慢增加至15 min吸附达到平衡。

图4 不同温度下吸附时间对酯化改性麦糟吸附Pb(Ⅱ)的影响
Fig. 4 Effect of time on Pb(Ⅱ) adsorption by ESG at various temperatures
为全面研究酯化改性麦糟吸附Pb(Ⅱ)的动力学特性,分别采用拟一级动力学模型(式(4))、拟二级动力学模型(式(5))和内扩散模型(式(6))进行拟合[15],结果见图5。
ln (qe-qt)=ln qe-k1t (4)
t/qt=1/(k2qe2)+t/qe (5)
qt=kintt0.5+x (6)
式中:qt (mg·g-1)为t时刻吸附量;qe(mg·g-1)为平衡时吸附量;k1(min-1)、k2(g/(mg?min))和kint(mg/(g?min0.5))为动力学参数。
拟合得到的动力学参数如表1~3所示。采用拟一级动力学模型拟合得到的理论饱和吸附量与实验值相差很大。因此,酯化改性麦糟吸附Pb(Ⅱ)不符合拟一级动力学方程。拟二级动力学方程拟合的相关系数均很高,接近1。通过拟二级动力学方程得到的理论饱和吸附量与实验值能够很好地吻合。因此,酯化改性麦糟吸附Pb(Ⅱ)符合拟二级动力学方程,k2为吸附速率常数。吸附速率常数随温度的升高而增大表明升高温度有利于吸附的进行。另外,内扩散模型拟合分两段呈线性关系且不过原点,说明整个吸附过程中存在内扩散作用但内扩散并非唯一的速率控制步骤[16]。综合来看,拟二级动力学模型拟合的线性相关系数最高,因拟二级动力学模型以化学吸附为主,同时包含外部液膜扩散、表面吸附和颗粒内扩散等[15],所以,拟二级动力学模型更真实、全面地反映吸附剂吸附重金属离子的动力学机制,特别是酯化改性引入的功能基团与Pb(Ⅱ)配合的化学吸附机制。
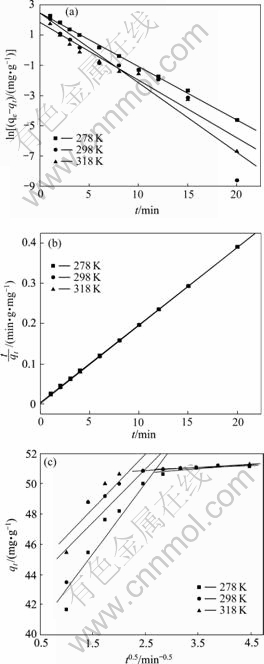
图5 不同温度下酯化改性麦糟吸附Pb(Ⅱ)的动力学曲线
Fig. 5 Kinetic plots of Pb(Ⅱ) adsorption on ESG at various temperatures: (a) Pseudo-first-order; (b) Pseudo-second-order; (c) Intraparticle diffusion
由以上动力学研究可知,k2为吸附速率常数即kads,Arrhenius方程如下:
表1 不同温度下改性麦糟吸附Pb(Ⅱ)的拟一级动力学参数
Table 1 Pseudo-first-order kinetic parameters for adsorption of Pb(Ⅱ) on ESG at different temperatures
的拟一级动力学参数.jpg)
表2 不同温度下改性麦糟吸附Pb(Ⅱ)的拟二级动力学参数
Table 2 Pseudo-second-order kinetic parameters for adsorption of Pb(Ⅱ) on ESG at different temperatures
的拟二级动力学参数.jpg)
表3 不同温度下改性麦糟吸附Pb(Ⅱ)的内扩散模型参数
Table 3 Intraparticle diffusion parameters for adsorption of Pb(Ⅱ) on ESG at different temperatures
的内扩散模型参数.jpg)
ln kads=ln A-Ea/(RT) (7)
由式(7)计算反应的活化能Ea。化学吸附力比物理吸附力要强,通常有两种化学吸附类型,活性和非活性化学吸附。活性化学吸附限定活化能在8.4~83.7 kJ/mol之间,非活性化学吸附的活化能接近0[17]。在本研究中,酯化改性麦糟吸附Pb(Ⅱ)的活化能为16.94 kJ/mol,说明酯化改性麦糟吸附Pb(Ⅱ)是活性化学吸附,这与上述研究结果一致。
2.4 吸附等温线
25 ℃时,酯化改性麦糟对Pb(Ⅱ)的吸附能力随平衡浓度的增大呈增加趋势,当初始浓度为20 mmol/L时吸附基本到达饱和。平衡吸附量(qe)对重金属离子的平衡浓度(ce)的关系用Langmuir(式(8))和Freundlich(式(9))等温线方程进行拟合,结果如图6所示。
ce/qe=ce/qm+1/(bqm) (8)
lg qe=(1/n)lg ce+lg Kf (9)
式中:b为Langmuir方程表示吸附剂表面覆盖度的经验常数(L/mg)(1/b越大,吸附剂对吸附质的吸附量越大);Kf(mg/g)和n分别为Freundlich方程与吸附量和吸附强度有关的经验常数。
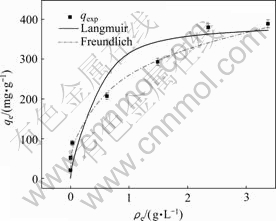
图6 酯化改性麦糟对Pb(Ⅱ)的吸附等温线
Fig. 6 Adsorption isotherms of Pb(Ⅱ) using ESG as adsorbent
表4给出了Langmuir 和Freundlich方程的特征参数值和线性相关系数。由表4可知,酯化改性麦糟吸附Pb(Ⅱ)的数据可以用Langmuir和Freundlich方程描述,Freundlich吸附等温线的线性相关系数略高。相应的Freundlich吸附等温线方程的特征常数n为3.47,一般认为n值为2~10时容易吸附[18],说明酯化改性麦糟容易吸附Pb(Ⅱ)。通过Langmuir吸附等温线方 程计算得到酯化改性麦糟对Pb(Ⅱ)的饱和吸附量为393.7 mg/g,高于文献报道的其他改性工农业废弃物对Pb(Ⅱ)的吸附能力(如表5所示),表明酯化改性麦糟是一种优越的Pb(Ⅱ)吸附剂。
表4 25 ℃时酯化改性麦糟吸附重金属离子的吸附等温线参数
Table 4 Isotherm parameters for adsorption of Pb(Ⅱ) on ESG at 25 ℃

表5 改性工农业废弃物对不同重金属离子的吸附容量
Table 5 Pb(Ⅱ) adsorption capacity of modified agricultural and industrial wastes
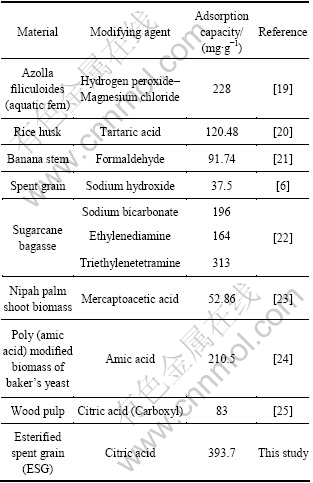
表6 麦糟酯化改性前后及其吸附Pb(Ⅱ)后的红外光谱分析
Table 6 Infrared spectra analysis of spent grain before and after esterification and ESG adsorption of Pb(Ⅱ)
后的红外光谱分析.jpg)
2.5 吸附机理
酯化改性麦糟吸附Pb(Ⅱ)前后红外光谱主要特征峰的变化如表6所示。由表6可以看出,羟基O—H吸收峰的位置偏移最大,吸附前羟基吸收峰在 3 405.71 cm-1处,吸附Pb(Ⅱ)后羟基吸收峰出现在 3 291.26 cm-1处;另外一处非常明显的变化是C—O键1 166.76 cm-1处的吸收峰在吸附Pb(Ⅱ)后消失。羟基吸收峰的位置发生偏移是因为羟基本身具有络合重金属离子的能力,由于羟基络合重金属离子导致羟基的吸收谱带发生了较大的位移。酯化改性麦糟在 1 166.76 cm-1处的C—O谱带在吸附Pb(Ⅱ)后消失,证明麦糟经酯化改性处理后生成的酯基在吸附重金属离子过程中发挥了重要作用,主要是酯基C—O键中的氧原子与重金属离子配合达到吸附重金属离子的目的。另外,C=O基团以及含氮基团N—H和C—N吸收峰在吸附Pb(Ⅱ)后也有一定程度的位移,说明这些基团有助于吸附Pb(Ⅱ)。
3 结论
1) 酯化改性麦糟对Pb(Ⅱ)有较好的吸附能力,其理论饱和吸附量达393.7 mg/g,较文献报道的其他改性工农业废弃物对Pb(Ⅱ)的吸附能力高。
2) 酯化改性麦糟吸附Pb(Ⅱ)的最佳pH范围较宽为4~8,该吸附反应很快,15 min即达到平衡,为活性化学吸附,有利于其工业应用并保证运行的稳定性和经济性。吸附剂富含的羟基与酯化改性引入的羧基在吸附Pb(Ⅱ)时发挥了重要作用,另外,C=O基团以及含氮基团N—H和C—N也有助于Pb(Ⅱ)吸附。酯化改性麦糟是一种极具应用潜力的Pb(Ⅱ)吸附剂。
REFERENCES
[1] WADANAMBI L, DUBEY B, TOWNSEND T. The leaching of lead from lead-based paint in landfill environments [J]. Journal of Hazardous Materials, 2008, 157(1): 194-200.
[2] KURNIAWAN T A, CHAN G Y S, LO W H, BABEL S. Physico-chemical treatment techniques for wastewater laden with heavy metals[J]. Chemical Engineering Journal, 2006, 118(1/2): 83-98.
[3] SHEN Li, XIA Jin-lan, HE Huan, NIE Zhen-yuan. Comparative study on biosorption of Pb(Ⅱ) and Cr(Ⅵ) by Synechococcus sp.[J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1336-1342.
[4] DEMIRBAS A. Heavy metal adsorption onto agro-based waste materials: A review[J]. Journal of Hazardous Materials, 2008, 157(2/3): 220-229.
[5] WAN NGAH W S, HANAFIAH M A K M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review[J]. Bioresource Technology, 2008, 99(10): 3935-3948.
[6] LOW K S, LEE C K, LIEW S C. Sorption of cadmium and lead from aqueous solutions by spent grain[J]. Process Biochemistry, 2000, 36(1): 59-64.
[7] CHAI Li-yuan, CHEN Yun-nen, YANG Zhi-hui. Kinetics and thermodynamics of arsenate and arsenite biosorption by pretreated spent grains[J]. Water Environment Research, 2009, 81(6): 843-848.
[8] LI Qing-zhu, CHAI Li-yuan, WANG Qing-wei, YANG Zhi-hui, YAN Hu-xiang, WANG Yun-yan. Fast esterification of spent grain for enhanced heavy metal ions adsorption[J]. Bioresource Technology, 2010, 101(10): 3796-3799.
[9] MARSHALL W E, WARTELLE L H, BOLER D E, JOHNS M M, TOLES C A. Enhanced metal adsorption by soybean hulls modified with citric acid[J]. Bioresource Technology, 1999, 69(3): 263-268.
[10] DENG S, TING Y P. Characterization of PEI-modified biomass and biosorption of Cu(Ⅱ), Pb(Ⅱ) and Ni(Ⅱ)[J]. Water Research, 2005, 39(10): 2167-2177.
[11] NOGHABI K A, ZAHIRI H S, YOON S C. The production of a cold-induced extracellular biopolymer by Pseudomonas fluorescens BM07 under various growth conditions and its role in heavy metals absorption[J]. Process Biochemistry, 2007, 42(5): 847-855.
[12] GRUBE M, LIN J G, LEE P H, KOKOREVICHA S. Evaluation of sewage sludge-based compost by FT-IR spectroscopy[J]. Geoderma, 2006, 130(3/4): 324-333.
[13] ARSLANOGLU H, ALTUNDOGAN H S, TUMEN F. Heavy metals binding properties of esterified lemon[J]. Journal of Hazardous Materials, 2009, 164(2/3): 1406-1413.
[14] 毛金浩, 刘引烽, 杨 红, 王立平. 丝瓜络的化学改性及其对金属离子的吸附[J]. 水处理技术, 2008, 34(7): 46-50.
MAO Jin-hao, LIU Yin-feng, YANG Hong, WANG Li-ping. Chemical modification of luffa sponge and its adsorption characteristic for metal ions[J]. Technology of Water Treatment, 2008, 34(7): 46-50.
[15] HO Y S, NG J C Y, MCKAY G. Kinetics of pollutant sorption by biosorbents: Review[J]. Separation & Purification Reviews, 2000, 29(2): 189-232.
[16] WEBER W J, MORRIS J C. Kinetics of adsorption on carbon from solution[J]. Journal of the Sanitary Engineering Division, American Society of Civil Engineers, 1963, 89(17): 31-60.
[17] HAN Run-ping, ZHANG Li-jun, SONG Chen, ZHANG Man-man, ZHU Hui-min, ZHANG Li-juan. Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode[J]. Carbohydrate Polymers, 2010, 79(4): 1140-1149.
[18] 陈 田, 王 涛, 王道军, 赵建庆, 丁晓春, 吴士超, 薛海荣, 何建平功能化有序介孔碳对重金属离子Cu(Ⅱ)、Cr(Ⅵ)的选择性吸附行为[J]. 物理化学学报, 2010, 26(12): 3249-3256.
CHEN Tian, WANG Tao, WANG Dao-jun, ZHAO Jian-qing, DING Xiao-chun, WU Shi-chao, XUE Hai-rong, HE Jian-ping. Selective adsorption behavior of Cu(Ⅱ) and Cr(Ⅵ) heavy metal ions by functionalized ordered mesoporous carbon[J]. Acta Physico-Chimica Sinica, 2010, 26(12): 3249-3256.
[19] GANJI M, KHOSRAVI M, RAKHSHAEI R. Biosorption of Pb, Cd, Cu and Zn from the wastewater by treated Azolla filiculoides with H2O2/MgCl2[J]. International Journal of Environmental Science and Technology, 2005, 1(4): 265-271.
[20] WONG K K, LEE C K, LOW K S, HARON M J. Removal of Cu and Pb from electroplating wastewater using tartaric acid modified rice husk[J]. Process Biochemistry, 2003, 39(4): 437-445.
[21] NOELINE B F, MANOHAR D M, ANIRUDHAN T S. Kinetic and equilibrium modelling of lead (II) sorption from water and wastewater by polymerized banana stem in a batch reactor[J]. Separation and Purification Technology, 2005, 45(2): 131-140.
[22] KARNITZ O. Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse[J]. Bioresource Technology, 2007, 98(6): 1291-1297.
[23] WANKASI D, HORSFALL JNR M, IBUTEME SPIFF A. Sorption kinetics of Pb2+ and Cu2+ ions from aqueous solution by Nipah palm (Nypa fruticans Wurmb) shoot biomass[J]. Electronic Journal of Biotechnology, 2006, 9(5): 587-592.
[24] YU Jun-xia, TONG Mi, SUN Xiao-mei, LI Bu-hai. A simple method to prepare poly (amic acid)-modified biomass for enhancement of lead and cadmium adsorption[J]. Biochemical Engineering Journal, 2007, 33(2): 126-133.
[25] LOW K S, LEE C K, MAK S M. Sorption of copper and lead by citric acid modified wood[J]. Wood Science and Technology, 2004, 38(8): 629-640.
(编辑 龙怀中)