J. Cent. South Univ. Technol. (2009) 16: 0032-0037
DOI: 10.1007/s11771-009-0005-7
Thermodynamic reassessment of Ga-Hg and Mg-Hg systems
FENG Yan(冯 艳), WANG Ri-chu(王日初), PENG Chao-qun(彭超群), LIU Hua-shan(刘华山)
(School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The Ga-Hg binary system was thermodynamically assessed by the CALPHAD method, but only configuration contributions were considered to the entropy of the liquid. The Mg-Hg binary system has not been assessed yet. In the assessments of the Ga-Hg and Mg-Hg binary systems, solutions including liquid and hcp (Mg) were treated as substitution solutions, of which the excess Gibbs energies were formulated with the Relich-Kister polynomial. The intermetallic phases in the Mg-Hg binary system, Mg3Hg, Mg5Hg2, Mg2Hg, Mg5Hg3, MgHg, and MgHg2, were described as stoichiometric compounds. Based on the reported experimental data and thermodynamic properties of the phase diagram, sets of self-consistent parameters describing all phases in the Ga-Hg and the Mg-Hg binary systems were obtained.
Key words: Ga-Hg system; Mg-Hg system; intermetallic phases; thermodynamic assessment; phase diagram; CALPHAD
1 Introduction
Magnesium alloys are used extensively as a sort of anode materials in the seawater battery system for its good performances such as rapid activation, high cell voltage-wide voltage range, and high power density capability [1-4]. As is known, pure magnesium electrode can be polarized easily by a protective oxide or hydroxide film. It has been reported that the alloying elements Ga and Hg can enhance the electrochemical activity of the Mg anodes because they can produce uniform dissolution of Mg anodes in environments containing aggressive ions [5-6]. Moreover, the addition of Ga in the Mg-Hg alloys can decrease the driving force of the galvanic couple corrosion and enhance the corrosion resistance and the electrode efficiency of the Mg-Hg anode materials [7]. In order to design alloy and better understand the mechanisms of Ga and Hg activating the Mg anode materials, the thermodynamic assessments of the Ga-Hg and Mg-Hg binary systems are necessary.
A review of the literature data on the Ga-Hg system, which includes publications before 1993, has been presented by GUMINSKI and ZABDYR [8]. Three thermodynamic models were published for the Ga-Hg liquid phase [9]. Using tetrahedral approximation for the Ga-Hg liquid phase, GUBBELS [9] calculated the most consistent results in extrapolation of the Ga-Hg-In ternary system, but only configuration contributions were considered to the entropy of the liquid. The newest review of the Mg-Hg binary system was reported by NAYEB-HASHEMI and CLARK [10], who summarized the publications before 1987. There has not any thermodynamic assessment on the Mg-Hg binary system yet.
In view of these, the thermodynamic reassessments of the Ga-Hg and Mg-Hg binary systems were performed in the present work.
2 Evaluation of experimental data
2.1 Experimental data of Ga-Hg binary system
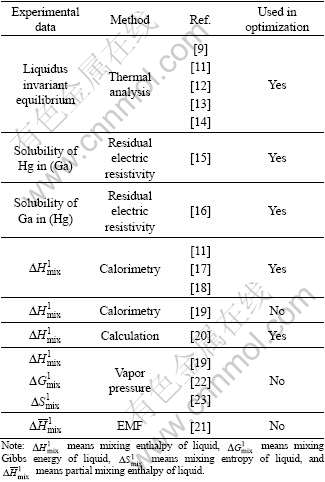
The liquidus in the Ga-Hg diagram was determined using thermal analysis [9, 11-14]. These data are consistent and used in the optimization. There is a miscibility gap in the liquid of the Ga-Hg binary system and no intermetallics exist. The largest solid solubility of Hg in orthorhombic (Ga) was 0.02%, determined by measuring the residual electric resistivity of the Hg-rich alloys [15]. The largest solid solubility of Ga in rhombohedral (Hg) was determined using the same method [16].
By calorimetric measurements, mixing enthalpies of the Ga-Hg liquid at 478 and 513 K were determined [11,17-19]. These data are consistent except the larger values on the Ga-rich region in Refs.[11,17-18], which were confirmed by ZHANG and GUO [20] using calculation. So the symmetric mixing enthalpy of the Ga-Hg liquid in the whole composition range was not accepted in this optimization [19]. Due to lack of other new experimental data, no temperature dependence of the mixing enthalpy of the Ga-Hg liquid was considered in this optimization.
The partial molar enthalpies of mixing of the Ga-Hg liquid at 483-523 K were determined by calorimetry [9] and calculation from EMF (electromotive force) data [21]. Due to the large deviation of the data, they were not used in this work. The vapor pressures of Hg over the alloys in the temperature range from 460 to 700 K were measured [19, 22-23], and some thermodynamic data such as activities of the components in liquid phase were calculated. The representations of the results make quantitative comparison difficult, so they were not used in this optimization.
The experimental data of Ga-Hg binary system are summarized in Table 1.
Table 1 Experimental data of Ga-Hg binary system
2.2 Experimental data of Mg-Hg binary system
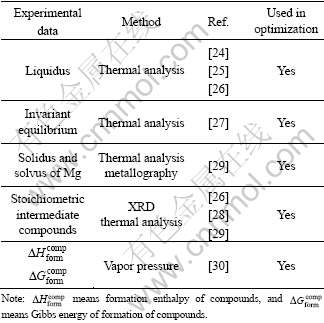
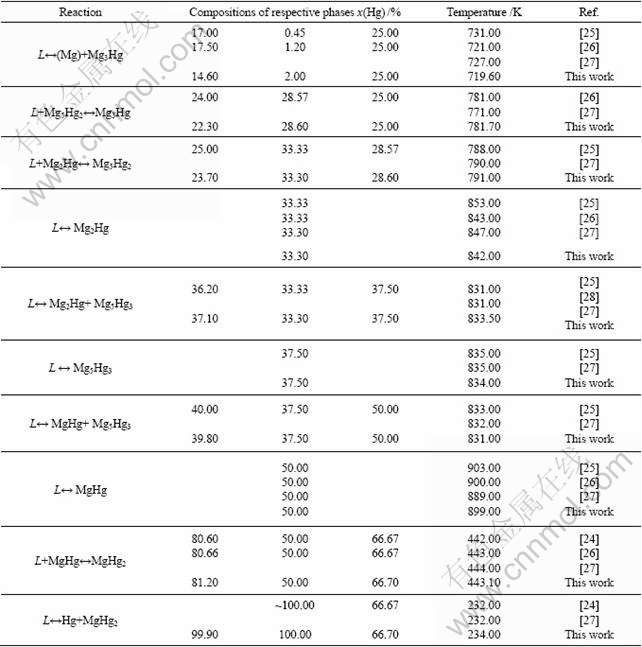
The liquidus and invariant reactions of the Mg-Hg binary system were determined by thermal analysis [24-27]. Six compounds, Mg3Hg, Mg5Hg2, Mg2Hg, Mg5Hg3, MgHg, and MgHg2, were detected using the same method [25-26]. BRAUER et al [28] determined the solid solubility of the six compounds to be minimum using X-ray diffraction method, so these compounds were considered to be stoichiometric compounds in this optimization. The largest solid solubility of Hg in hcp (Mg) was firstly measured as low as 0.43% (mole fraction) Hg by thermal analysis [25-26] and revised to be at least 1.165% (mole fraction) Hg by lattice parameter measurements [29]. The latter datum was used in the present optimization due to its accuracy.
By measuring the vapor pressure of Hg, the formation enthalpies of the Mg-Hg compounds at 298 K and the Gibbs energy of formation of these compounds at 550 and 356 K were deduced by HILPERT [30]. These data were used in the assessment. Because of lack of the thermodynamic data of the liquid, the dependence of the mixing enthalpy of the Mg-Hg liquid is not considered in this assessment.
The experimental data of Mg-Hg binary system are summarized in Table 2.
Table 2 Experimental data of Mg-Hg binary system
3 Thermodynamic modeling
The lattice stabilities of pure elements Mg, Ga, and Hg are taken from Ref.[31].
Three phases are involved in the optimization of the Ga-Hg binary system, which are the solution phases of liquid, the terminal phase of orthorhombic (Ga) and rhombohedral (Hg). Nine phases involved in the optimization of the Mg-Hg binary system, which are the solution phases of liquid and HCP (Mg), the solution compounds of Mg3Hg, Mg5Hg2, Mg2Hg, Mg5Hg3, MgHg and MgHg2, and the terminal phase of rhombohedral (Hg).
In the Ga-Hg binary system, the solution phase liquid was described as substitution solution, and its Gibbs energy is expressed as follows:
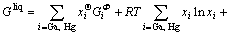
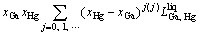 (1)
(1)
where xi (i=Ga, Hg) are the mole fractions of components Ga and Hg, respectively; 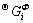 is the mole Gibbs energy of pure element i in the standard state. The interaction parameter of the solution is given below:
is the mole Gibbs energy of pure element i in the standard state. The interaction parameter of the solution is given below:
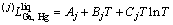 (2)
(2)
where 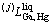 means the j-order interaction between Ga and Hg atoms in the liquid state; Aj, Bj, and Cj are to be optimized. When j=0,
means the j-order interaction between Ga and Hg atoms in the liquid state; Aj, Bj, and Cj are to be optimized. When j=0, 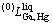 stands for the nearest-neighbor interaction between atoms Ga and Hg. As no temperature dependence of the mixing enthalpy of the liquid phase in this system is considered, Cj should be zero.
stands for the nearest-neighbor interaction between atoms Ga and Hg. As no temperature dependence of the mixing enthalpy of the liquid phase in this system is considered, Cj should be zero.
The solid solubility of Hg in orthorhombic (Ga), and that of Ga in rhombohedral (Hg) are negligible, thus they are not considered here.
In the Mg-Hg binary system, the solution phases including liquid and HCP (Mg) were all described as substitution solutions, and their Gibbs energies are expressed as follows:
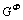 =
=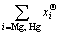
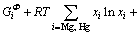
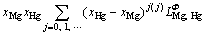 (3)
(3)
where Φ denotes all the solution phases; xi (i=Mg, Hg) are the mole fractions of components Mg and Hg, respectively; and 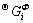 is the mole Gibbs energy of pure element i in the standard state. The interaction parameter of the solution is given below:
is the mole Gibbs energy of pure element i in the standard state. The interaction parameter of the solution is given below:
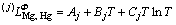 (4)
(4)
where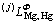 means the j-order interaction between
means the j-order interaction between
Mg and Hg atoms in Φ state, and Aj, Bj and Cj are to be optimized. When j=0, 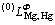 stands for the nearest-neighbor interaction between atomic Mg and Hg. As no temperature dependence of the mixing enthalpy of the liquid phase in this system is considered, Cj should be zero.
stands for the nearest-neighbor interaction between atomic Mg and Hg. As no temperature dependence of the mixing enthalpy of the liquid phase in this system is considered, Cj should be zero.
All intermediate phases in Mg-Hg binary system are treated as stoichiometric compounds because of their narrow homogeneity ranges. So the Gibbs energies of these phases are formulated as
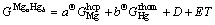 (5)
(5)
where a and b are the mole fractions in these compounds per one mole, and D and E are also parameters to be optimized.
The solid solubility of Hg in HCP (Mg) is negligible, thus it is not considered here.
The model parameters of the Mg-Hg and Ga-Hg binary systems obtained in these optimizations are shown in Tables 3 and 4, respectively.
Table 3 Model parameters of Mg-Hg binary systems obtained in this work
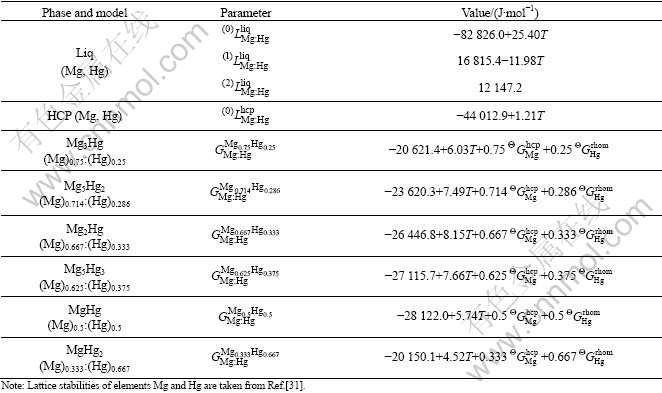
Table 4 Model parameters of Ga-Hg binary systems obtained in this work

4 Results and discussion
4.1 Calculated Ga-Hg binary system
Fig.1 illustrates the calculated phase diagram of the Ga-Hg binary system, together with the experimental boundaries. All invariant reactions in the system are listed in Table 5. Satisfactory agreement is obtained between the calculated and experimental phase diagram data.
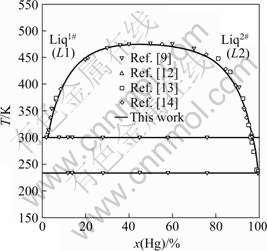
Fig.1 Calculated phase diagram of Ga-Hg binary system, together with experimental data
Table 5 Invariant reactions in Ga-Hg binary system
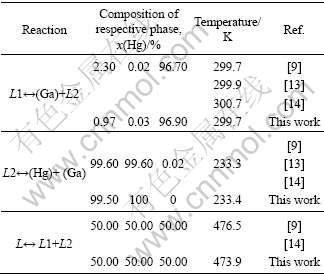
Fig.2 shows the calculated mixing enthalpies of the Ga-Hg liquid at 478 K and the experimental values [11]. The calculated results are consistent with the experimental results.
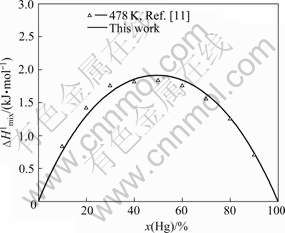
Fig.2 Comparison of calculated mixing enthalpies of Ga-Hg liquid at 478 K with experimental data
4.2 Calculated Mg-Hg binary system
The calculated phase diagram of Mg-Hg binary system with experimental phase boundaries is illustrated in Fig.3. Combined with Table 6 that lists all invariant reactions and congruent transformations in Mg-Hg binary system, it is clear that reasonable agreement is realized between the calculated and experimental phase diagrams.
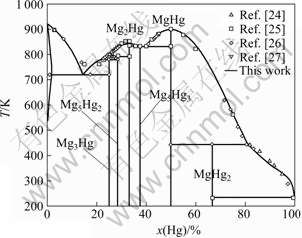
Fig.3 Comparison of calculated phase diagram of Mg-Hg binary system with experimental data
Table 6 Invariant reactions and congruent transformations in Mg-Hg binary system
The calculated and experimental formation enthalpies of the Mg-Hg intermetallic compounds at 298 K are shown in Fig.4. Fig.5 shows the calculated Gibbs energies of formation of the Mg-Hg intermetallic compounds at 550 and 356 K in comparison with experimental data. Obviously, the measured enthalpies and Gibbs energies of formation are reasonably reproduced except the values of MgHg. Considering the deviation of experiment, the measured enthalpy and Gibbs energy of formation of the MgHg compound can be accepted.
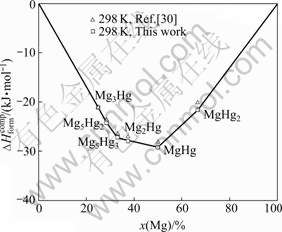
Fig.4 Calculated formation enthalpies of Mg-Hg compounds at 298 K in comparison with experimental data
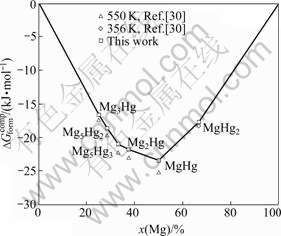
Fig.5 Comparison of calculated Gibbs energies of formation of Mg-Hg compounds at 550 and 356 K with experimental data
5 Conclusions
(1) The phase diagram of Ga-Hg binary system is modeled on the basis of experimental phase diagram and thermodynamic data from literature. A set of self- consistent model parameters describing Ga-Hg binary system are obtained, which satisfy the experimental data in a reasonable manner.
(2) The phase diagram of Mg-Hg binary system is modeled on the basis of experimental phase diagram and thermodynamic data from literature. A set of self- consistent model parameters describing Mg-Hg binary system are obtained, which satisfy the experimental data in a reasonable manner.
References
[1] DORON A, GURUKAR S S, ELENA L, ARIEL M, OREN M, ORIT C, MICHELA B. Progress in rechargeable magnesium battery technology [J]. Advanced Materials, 2007, 19: 4260-4267.
[2] RENUKA R. Influence of allotropic modifications of surphur on the cell voltage in Mg-CuI(S) seawater activated battery [J]. Materials Chemistry Physics, 1999, 59(1): 42-48.
[3] JEROME G, DANIEL C, KATIA G, MARC D, AXEL H, FRANCIS M, ANDRE H. Magnesium batteries: Towards a first use of graphite fluorides [J]. Journal of Power Sources, 2007, 173(1): 592-598.
[4] RENUKA R. AgCl and Ag2S as additives to CuI in Mg-CuI seawater activated batteries [J]. Journal of Applied Electrochemistry, 1997, 27(12): 1394-1397.
[5] FLAMINI D O, SAIDMAN S B, BESSONE J B. Aluminium activation produced by gallium [J]. Corrosion Science, 2006, 48(6): 1413-1425.
[6] FENG Yan, WANG Ri-chu, YU Kun, PENG Chao-qun, LI Wen-xian. Influence of Ga content on electrochemical behavior of Mg-5at%Hg anode materials [J]. Materials Transaction, 2008, 49(5): 1077-1080.
[7] DENG Su-hao, YI Dan-qing, ZHAO Li-hong, ZHOU Ling-ling, WANG Bin, JI Chen-nian, LAN Bo. Study on Mg alloy anode material for seawater battery [J]. Battery Technology, 2007, 131(5): 402-405. (in Chinese)
[8] GUMINSKI C, ZABDYR L. The Ga-Hg (gallium-mercury) system [J]. Journal of Phase Equilibria, 1993, 14(6): 719-725.
[9] GUBBELS G H M. Phase equilibria in the Ga-Hg-In system [J]. International Journal of Materials Research, 1990, 81: 202-208.
[10] NAYEB-HASHEMI A A, CLARK J B. The Hg-Mg (mercury- magnesium) [J]. Bulletin of Alloy Phase Diagrams, 1987, 8(1): 65-70.
[11] GAUNE-ESCARD M, BROS J P. Calorimetric determination of equilibrium phase diagrams of inorganic systems [J]. Thermochimica Acta, 1979, 31: 323-339.
[12] SPICER W M, BARTHOLOMAY H W. The mutual solubility of Hg and Ga [J]. Journal of the American Chemical Society, 1951, 73: 868-869.
[13] AMARELL G. On the Ga-Hg system [D]. Karlsruhe: Karlsruhe Technology University, 1958.
[14] PREDEL B. On the phase diagram of Ga-Bi and Ga-Hg: Comparison of coexistence curve with theory of mixing [J]. International Journal of Research in Physical Chemistry & Chemical Physics, 1960, 24: 206-216.
[15] ALEKSANDROV B N, LOMONOS O I. On the solubility of metals in solid Hg [J]. Zhurnal Fizicheskoi Khimii, 1971, 45: 3003-3006.
[16] ALEKSANDROV B N, DUKIN V V. Effects of impurities on the residual electrical resistivity of In and Ga [J]. Fizika Nizika Temperature, 1976, 2(1): 105-121.
[17] MARCO F, NAVARRO J, TORRA V. Application of flow calorimetry to the study of alloy formation: Enthalpies of solution of In, Tl, Cd, Zn, Pb, Ga, Sn and Bi in Hg at 293.15 K [J]. Journal of Chemical Thermodynamics, 1975, 7(11): 1059-1066.
[18] BROS J P, CASTANET R, LAFFITTE M, LEFEVRE M. Determination of enthalpy of formation of liquid alloys Ga-Hg and Ga-Bi from pure metals [J]. Journal de Chimie Physique, 1968, 65: 591-598.
[19] PREDEL B, MOHS R, ROTHACKER D. On thermodynamics of the systems Ga-Hg and Ga-Zn [J]. Journal of the Less-Common Metals, 1967, 12(6): 483-493.
[20] ZHANG Z C, GUO J K. Calculation of thermodynamic properties from alloy phase diagram with miscibility gap using non-random two-liquid equation [J]. CALPHAD, 2002, 26(3): 327-340.
[21] YATSENKO S P, DRUZHININA E P, DANILIN V N. Thermodynamic properties of the Ga-Hg system [J]. Russian Journal of Applied Chemistry, 1969, 42(3): 605-609.
[22] PREDEL B, ROTHACKER D. Thermodynamical investigation of the Hg-Sn and Hg-Ga system [J]. Acta Metallurgica, 1969, 17(6): 783-791.
[23] KOZIN L F, NIGMETOCA R S, DERGACHEVA M B. Thermodynamic of binary amalgam systems [M]. Alma-Ata: Nauka Press, 1977: 206-209.
[24] CAMBI L, SPERONI G. Amalgams of magnesium [J]. Atti della Accademia Nazionale del Lincei, 1915, 24(1): 734-738.
[25] BECK R P. The EMF properties of magnesium and thermal analysis of the Mg-Hg system [J]. Recueil des Travaux Chimiques des Pays-Bas, 1922, 41: 353-399.
[26] DANILTSCHENKO P T. Mg-Hg phase diagram [J]. Russian Journal of General Chemistry, 1930, 1(7): 975-988.
[27] CALVO F A, HIERRO M P. Study of the Hg-Mg phase diagram by differential thermal analysis [J]. Metallurgia International, 1987, 23(5): 333-340.
[28] BRAUER G, NOWOTNY H, RUDOLPH R. X-ray analysis of the magnesium amalgams [J]. International Journal of Materials Research, 1947, 38: 81-84.
[29] BUSK R S. Lattice parameters of magnesium alloys [J]. Transactions of the Metallurgy, 1950, 188: 1460-1464.
[30] HILPERT K. Study of the vaporization of amalgams with a new Knudsen cell-mass spectrometer system (Part Ⅱ): The compounds Mg5Hg3, Mg2Hg, Mg5Hg2, and Mg3Hg and the formation enthalpies of all compounds of the Mg-Hg system [J]. Physical Chemistry, 2002, 46: 37-41.
[31] DINSDALE A T. SGTE data for pure elements [J]. CALPHAD, 1991, 15(4): 317-425.
Received date: 2008-11-20; Accepted date: 2008-12 -30
Corresponding author: PENG Chao-qun, Professor; Tel/Fax: +86-731-8877197; E-mail: pcq2005@163.com
(Edited by CHEN Wei-ping)