DOI: 10.11817/j.ysxb.1004.0609.2021-39766
硫酸铵焙烧法选择性分离锌浸出渣中的锌和铁
张杜超,任冠行,刘若麟,陈 霖,刘伟锋,杨天足
(中南大学 冶金与环境学院,长沙 410083)
摘 要:工业湿法炼锌过程中会产生大量锌浸出渣,选择性回收其中的锌成为了研究热点。本文通过硫酸铵与锌浸出渣混合焙烧后水浸实现其中的铁和锌分离,从而实现资源综合利用。通过研究确定了最优焙烧条件,即硫酸铵与锌浸出渣质量比为1.30、焙烧温度为650 ℃、焙烧时间为240 min。结果表明,硫酸铵与铁酸锌在325~425 ℃时,反应生成(NH4)2Zn(SO4)2和NH4Fe(SO4)2;然后在500~650 ℃下,(NH4)2Zn(SO4)2和NH4Fe(SO4)2各自分解生成ZnSO4和Fe2(SO4)3,其中Fe2(SO4)3继续分解生成Fe2O3和SO3。在最优条件下所得焙烧产物经水浸后,锌的浸出率达到93.53%,而铁的浸出率为5.97%,实现了锌和铁的有效选择性分离。
关键词:锌浸出渣;铁酸锌;硫酸铵焙烧;锌;铁
文章编号:1004-0609(2021)-07-1944-08 中图分类号:TF09;TF813;TD982 文献标志码:A
引文格式:张杜超, 任冠行, 刘若麟, 等. 硫酸铵焙烧法选择性分离锌浸出渣中的锌和铁[J]. 中国有色金属学报, 2021, 31(7): 1944-1951. DOI: 10.11817/j.ysxb.1004.0609.2021-39766
ZHANG Du-chao, REN Guan-xing, LIU Ruo-lin, et al. Selective separation of zinc and iron from zinc leaching residue by ammonium sulfate roasting[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(7): 1944-1951. DOI: 10.11817/j.ysxb.1004.0609.2021-39766
目前,世界上80%以上的锌是通过“焙烧-浸出-净化-电积”工艺生产的[1]。在该工艺中,硫化锌精矿经过焙烧后得到锌焙砂,再通过中性浸出工艺,使焙砂中的ZnO、ZnSO4溶解,剩下的渣称为锌浸出渣[2]。锌浸出渣中的锌含量在20%左右,主要以性质稳定的ZnFe2O4形式存在,同时由于其中还含有铅、砷、镉、铜等元素,对环境存在污染风险[3-4]。因此,清洁高效环保地处理锌浸出渣具有十分重要的意义。
关于锌浸出渣处理方法的研究早在20世纪60年代便开始了,其中比较典型的方法是还原挥发法和热酸浸出法。还原挥发法[5-6]是锌浸出渣与焦炭、粉煤等还原性物质混合后置于回转窑中,在温度高达1500 K时,渣中的ZnO和ZnFe2O4被还原为锌蒸气,然后在收尘过程中被氧化成氧化锌,以烟尘的形式回收,而铁则进入熔炼渣中,从而实现锌的回收以及锌与铁的选择性分离。该工艺虽然流程简单、处理量大、金属回收率高,但是存在能耗大、耐火材料消耗高、作业环境条件差等问题。热酸浸出法[7-8]是采用高温高酸对锌浸出渣进行浸出,以铁酸锌形态存在的锌浸出率可以达到90%以上,但此过程中大量铁、砷等杂质会进入溶液,需要在锌电积工序前进行沉铁。然而,锌浸出液经黄钾铁钒法、赤铁矿法或针铁矿法等沉铁后,会产生大量的沉铁渣,在堆存过程中会造成严重的环境污染[9-10]。
近年来,除对上述热酸浸出法和还原挥发法处理锌浸出渣进行了深入研究外,一些研究者开始转向控制还原气氛或加入碱性氧化物等实现铁酸锌的物相转化。YAN等[11]和PENG等[12]提出通过控制CO通入比例和温度将ZnFe2O4转化为ZnO和Fe3O4,接着采用酸性浸出ZnO后,Fe3O4通过磁选进行分离,在最佳条件下,锌的回收率为61.38%,铁的回收率为80.90%。PRESTON等[13]通过在铁酸锌中加入Na2CO3、CaCO3和MnCO3等进行高温焙烧达到将铁酸锌转化为NaFeO2、ZnO、CaFe2O4、MnFe2O4的目的,从而实现锌的有效浸出。上述两种工艺尽管存在锌回收率不完全和熔炼温度高等问题,但由于两者较好地解决了锌和铁的分离问题,仍对锌浸出渣新工艺的开发具有一定的借鉴意义。
目前,硫酸铵焙烧法被广泛应用于有色金属冶炼工业中,通过利用硫酸铵与原料中的主要金属化合物反应,实现金属化合物的物相转变,进而采用浸出法实现金属的分离和提取。周国彪等[14]通过对硫酸铵与高钛型高炉渣混合焙烧的研究分析,发现了焙烧过程中反应产物的变化和形成机理,结果表明该方法不仅提取了其中的Al2O3,也富集了TiO2,为高钛型高炉渣的资源化利用和环境保护提供一种新工艺。LI等[15]对红土镍矿进行硫酸铵焙烧-水浸实验,对其中的Ni、Co和Mn等金属进行提取,其浸出率分别可以达到90.8%、85.4%和86.7%,而铁为9.98%,实现了选择性提取。这些研究表明,硫酸铵焙烧法可以实现铝、镍等金属氧化物的物相转变。在锌浸出渣的处理上,硫酸铵焙烧法具有焙烧温度低、焙烧过程中不引进杂质离子以及以水为浸出剂对设备腐蚀小等优点。彭兵等[16]通过两段式硫酸铵焙烧法来处理锌浸出渣,先后在450 ℃和650 ℃的温度下进行焙烧,最终锌的浸出率可以达到92.63%,而铁的浸出率为2.04%,但是并未对反应过程中物相变化以及反应机理进行系统分析。
基于此,本文采用硫酸铵焙烧法来分离提取锌浸出渣中的铁和锌,并对反应过程中的机理进行分析。实验中以锌和铁的浸出率作为考察指标,对焙烧过程中硫酸铵用量、焙烧温度、焙烧时间进行研究,得到最佳焙烧条件,同时查明焙烧反应过程中的物相变化规律,从而为锌浸出渣的回收处理提供一种新工艺。
1 实验
1.1 实验原料
实验所使用的原料来自于某锌冶炼企业提供的锌浸出渣。实验前,将原料在105 ℃下干燥12 h,再研磨至粒径425 μm左右。原料经过XRF分析,其结果如表1所示。由表1可知,原料主要金属元素为锌(Zn)、铁(Fe)、铅(Pb)、钙(Ca)。经过化学滴定法分析以及锌元素的化学物相分析,铁和锌含量分别为19.75%和17.95%,物相分析结果如表2所示。从表2中可以看出,锌浸出渣中的锌主要以铁酸锌存在,占总量的66.24%,其次为硫酸锌,还具有少量的氧化锌、硅酸锌。
表1 锌浸出渣主要化学组成XRF分析结果
Table 1 Main chemical composition of zinc leaching residue by XRF (mass fraction, %)
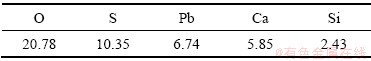
表2 浸出渣中锌的物相组成及含量
Table 2 Phase composition and content of zinc in leaching residue

锌浸出渣的XRD谱如图1所示。从图1可以知道,锌浸出渣中的主要物相是铁酸锌(ZnFe2O4),此外还有少量硫酸锌(ZnSO4)、硫酸铅(PbSO4)和硫酸钙(CaSO4)。实验采用的硫酸铵((NH4)2SO4)和铁酸锌(ZnFe2O4)铁酸锌均为分析纯。所用的去离子水,其pH为6.5。
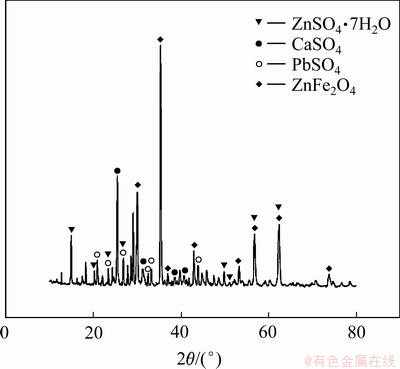
图1 锌浸出渣的XRD谱
Fig. 1 XRD pattern of zinc leaching residue
1.2 方法和步骤
每次实验,取15 g锌浸出渣与所需的硫酸铵混合,通过玛瑙坩埚研磨均匀,然后平铺置于石英舟中,并放入炉管中。焙烧实验在卧式管式炉中进行,并在炉管末端接上废气收集装置。首先,向炉管中持续通入氮气,待其中的空气排除完全后,然后再开始升温焙烧。在焙烧实验结束后,对焙烧渣进行水浸实验,以溶解渣中可溶性硫酸盐。水浸实验后,对溶液进行抽滤过滤,并对滤渣水洗3次,收集滤渣并分析其中的锌和铁含量,确定浸出率。锌和铁的浸出率(ε)计算式如下:
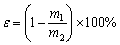
式中:m1为水浸渣中的锌或铁的质量,g;m2为原料中的锌或铁的质量,g。
1.3 检测方法
采用XRD设备(Rigaku,TTRⅢ型)分析原料、焙烧渣和水浸渣的物相。测定条件为:Cu靶Kα射线,电压40 kV,电流250 mA,扫描范围为10°~80°,扫描速度为5 (°)/min。采用化学物相法分析原料中锌的物相(铁酸锌、硫酸锌、氧化锌和硅酸锌)含量[17]。采用化学滴定法分析原料、水浸渣中锌和铁的含量[18]。
2 结果与讨论
2.1 硫酸铵用量对锌铁浸出率的影响
硫酸铵用量对锌铁浸出率的影响如图2所示。其他焙烧实验条件如下:焙烧温度600 ℃,焙烧时间120 min;水浸条件如下:液固比5:1,浸出温度30 ℃,浸出时间60 min。由图2可知,锌和铁的浸出率随着硫酸铵用量而增加。硫酸铵用量的增加会促进反应的发生,产生大量的硫酸铁和硫酸锌。同时,焙烧过程中所产生的硫酸铁也会分解生成Fe2O3和SO3,但是由于分解速率小于其生成速率,最后导致了铁的浸出率增加。
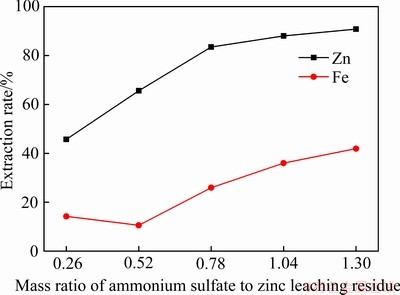
图2 硫酸铵与锌浸出渣质量比对锌铁浸出率的影响
Fig. 2 Effect of mass ration of ammonium sulfate and zinc leaching residue on extraction rate of zinc and iron

图3 硫酸铵与锌浸出渣质量比对焙烧渣和水浸渣物相的影响
Fig. 3 Effect of mass ratio of ammonium sulfate and zinc leaching residue on phase of roasting residue(a) and water-leaching residue(b)
不同硫酸铵用量下得到的焙烧渣以及其水浸渣的XRD谱如图3所示。从图3(a)可以看出,硫酸铵用量的增加促进了锌浸出渣中的铁酸锌与硫酸铵反应,铁酸锌物相消失。同时,焙烧渣的XRD谱中都出现了硫酸铁物相,这是因为焙烧时间并不充足,产出的硫酸铁来不及完全分解,依然有部分残存在渣中。从图3(b)可以看出,水浸渣中存在氧化铁以及原料中含的石膏、硫酸铅等不溶于水的物质。在硫酸铵用量较低时,水浸渣中出现了铁酸锌物相,随着硫酸铵用量的提高,铁酸锌的衍射线强度降低,这是因为铁酸锌与硫酸铵的反应为固固反应,以及在600 ℃下硫酸铵会不断分解,最后导致硫酸铵量不足以与铁酸锌完全反应。为了保证锌铁选择性分离,同时确保较高的锌浸出率,所以选择硫酸铵与锌浸出渣质量比为1.30作为最优条件。
2.2 焙烧温度对锌铁浸出率的影响
焙烧温度对锌铁浸出率的影响如图4所示。其他焙烧实验条件如下:硫酸铵与锌浸出渣质量比1.3,焙烧时间120 min;水浸条件:液固比5:1,浸出温度30 ℃,浸出时间60 min。
从图4可知,锌和铁的浸出率分别在325~ 650 ℃和325~500 ℃间一直增加,并分别在650和500 ℃两个温度处开始下降。这是因为在未到硫酸锌的分解温度(680 ℃)和硫酸铁的分解温度(480 ℃)之前,提高温度有利于焙烧反应的发生,但是在达到各自的分解温度后,会由于它们的分解反应产生不溶于水的氧化物,从而导致其浸出率降低。
不同焙烧温度下得到的焙烧渣以及其水浸渣的XRD谱如图5所示。从图5(a)可以看出,在焙烧温度为425 ℃时,锌和铁以(NH4)2Zn(SO4)2·6H2O、NH4Fe(SO4)2以及少量的铁酸锌形式存在,锌和铁浸出率分别达到了82.63%和67.92%。当在温度上升到500 ℃以后,(NH4)2Zn(SO4)2·6H2O开始转换成ZnSO4、ZnSO4·H2O,而NH4Fe(SO4)2物相消失,硫酸铁物相出现,这时铁的浸出率达到最高,为68.97%。在焙烧温度为600和650 ℃时,由于硫酸铁的分解,铁的浸出率开始降低,同时出现明显的Fe2O3特征峰。在温度为700 ℃时,ZnSO4会与Fe2O3反应生成ZnFe2O4和SO3[16],所以未出现氧化锌物相,而出现了铁酸锌的特征峰。从图5(b)可以发现,在焙烧温度325~500 ℃下,水浸渣的物相基本一致,都含有铁酸锌物相;600 ℃焙烧温度下的物相与650 ℃时的基本一致,都出现了Fe2O3的特征峰;而在700 ℃时,水浸渣中重新出现铁酸锌物相。为了使锌和铁有效分离,应选择锌浸出率高而铁浸出率低的条件,因此,选择650 ℃作为最优焙烧温度。

图4 焙烧温度对锌、铁浸出率的影响
Fig. 4 Effect of roasting temperature on extraction rate of zinc and iron
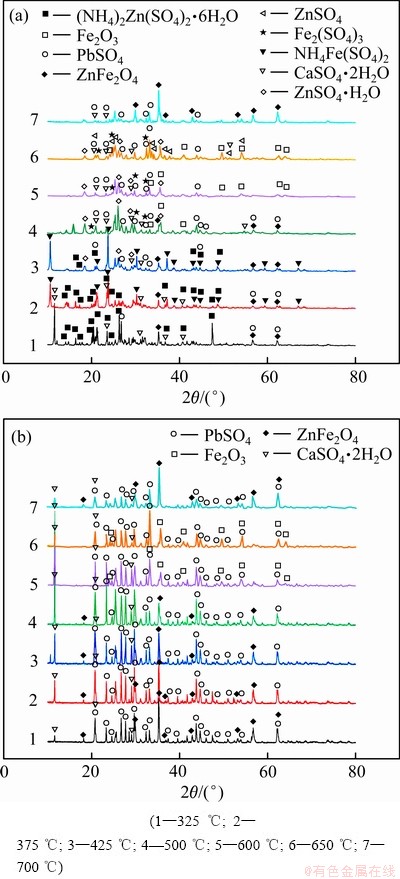
图5 焙烧温度对焙烧渣和水浸渣物相的影响
Fig. 5 Effect of roasting temperature on phases of roasting residue(a) and water-leaching residue(b)
2.3 焙烧时间对锌铁浸出率的影响
焙烧时间对锌铁浸出率的影响如图6所示。其他焙烧实验条件是:硫酸铵与锌浸出渣质量比为1.30,焙烧温度650 ℃;水浸条件为液固比5:1、浸出温度30 ℃和浸出时间60 min。
从图6可知,随着焙烧时间的延长,锌的浸出率基本保持不变,而铁的浸出率呈现逐渐降低的趋势。焙烧温度为650 ℃时,硫酸锌(ZnSO4)达不到分解温度,但是硫酸铁(Fe2(SO4)3)会发生分解反应生成Fe2O3,随着时间延长,硫酸铁分解量增加,铁的浸出率降低。
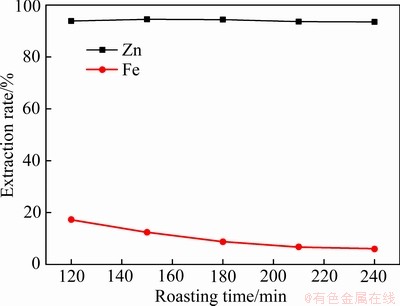
图6 焙烧时间对锌、铁浸出率的影响
Fig. 6 Effect of roasting time on extraction rate of zinc and iron
不同焙烧时间下得到的焙烧渣以及其水浸渣的XRD谱如图7所示。从图7(a)可知,焙烧时间对焙烧渣的物相影响较小,渣中的主要物相基本一致,其中除了PbSO4、CaSO4·H2O等物相外,还检测出硫酸铁物相,这说明240 min的焙烧时间并不足以使硫酸铁分解完全。从图7(b)可知,焙烧时间对水浸渣物相影响同样较小,物相也基本保持一致,主要为氧化铁、硫酸铅和石膏。尽管继续延长焙烧时间有利于降低铁浸出率,但是从节约能耗角度,最终选择240 min作为最优焙烧时间。
2.4 机理分析
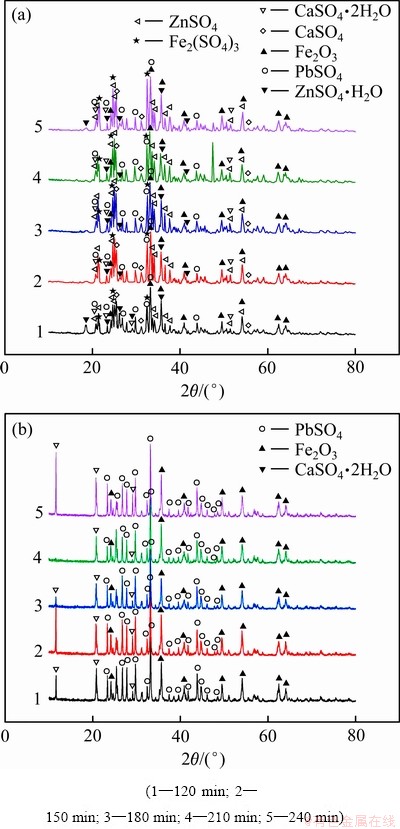
图7 焙烧时间对焙烧渣和水浸渣物相的影响
Fig. 7 Effect of roasting time on phases of roasting residue(a) and water-leaching residue(b)
硫酸铵焙烧法处理锌浸出渣,其核心是将铁酸锌转化成硫酸铁和硫酸锌。由于在合适的温度范围内,硫酸锌不分解,而硫酸铁会分解生成氧化铁,因此可基于焙烧产物的水溶性差异,使铁以氧化铁的形式保留在水浸渣中,而锌以硫酸锌的方式进入水溶液中。硫酸铵处理锌浸出渣的过程,发生的主要反应方程式如下:
ZnFe2O4+4(NH4)2SO4=ZnSO4+Fe2(SO4)3+8NH3↑+4H2O↑ (1)
反应(1)的吉布斯自由能如图8所示。在温度达到400 ℃后,反应吉布斯自由能小于0,说明反应可以发生。同时,硫酸锌和硫酸铁的分解温度分别为680和480 ℃,所以在480~680 ℃间的焙烧温度,就可以使硫酸铁分解而硫酸锌不分解。
刘科伟等[19]和范芸珠等[20]曾详细进行了硫酸铵的热分解机理研究,确定了硫酸铵分解过程化学反应方程式,如表3所示。
表3 硫酸铵的分解过程化学反应方程式
Table 3 Chemical reaction equations of decomposition process of ammonium sulfate
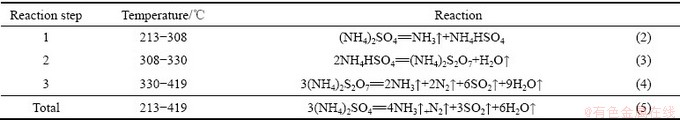
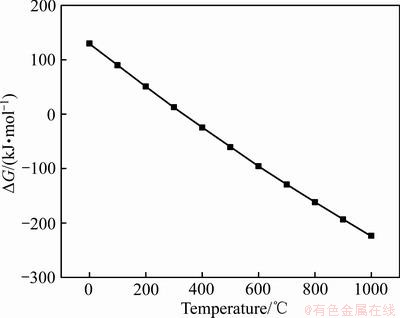
图8 反应(1)的吉布斯自由能变化图
Fig. 8 Gibbs free energy change diagram of reaction (1)
由表3可知,硫酸铵的热分解过程为分步反应。在分解温度达到213 ℃后,硫酸铵开始发生分解,产生硫酸氢铵和氨气,随着温度继续升高,NH4HSO4也开始发生分解,生成(NH4)2S2O7。在温度达到330℃后,(NH4)2S2O7分解成NH3、N2、SO2和H2O。
在焙烧温度处于325、375和425 ℃时,除了硫酸铵自身的分解外,它的分解过程中的中间物质也会与铁酸锌发生反应,化学反应方程式如下:
ZnFe2O4+6NH4HSO4=2NH4Fe(SO4)2+(NH4)2Zn(SO4)2+2NH3↑+4H2O↑ (6)
ZnFe2O4+3(NH4)2S2O7=2NH4Fe(SO4)2+(NH4)2Zn(SO4)2+2NH3↑+H2O↑ (7)
在焙烧温度处于500、600和650 ℃时,NH4Fe(SO4)2和(NH4)2Zn(SO4)2发生分解反应,同时硫酸铁也会在其分解温度下开始分解,反应方程式如下:
2NH4Fe(SO4)2=Fe2(SO4)3+2NH3↑+SO3↑+H2O↑ (8)
(NH4)2Zn(SO4)2=ZnSO4+2NH3↑+SO3↑+H2O↑ (9)
Fe2(SO4)3=Fe2O3+3SO3↑ (10)
在焙烧温度处于700 ℃时,硫酸锌会与分解产物氧化铁发生反应,生成铁酸锌,具体反应方程式如下:
ZnSO4+Fe2O3=ZnFe2O4+SO3↑ (11)
3 工艺流程提出
基于上述工艺参数优化和机理分析,提出一种硫酸铵焙烧法处理锌浸出渣的工艺流程,如图9所示。
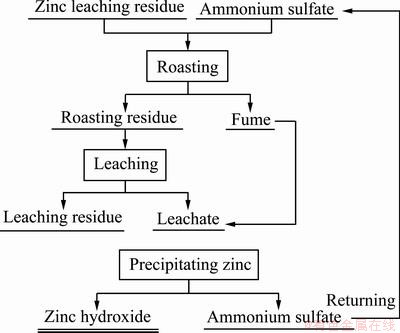
图9 硫酸铵焙烧法处理锌浸出渣的工艺流程图
Fig. 9 Process flow chart of ammonium sulfate roasting of zinc leaching residue
在焙烧阶段,锌浸出渣与硫酸铵混合焙烧产生焙烧产物,以及氨气、SO3等气体。焙烧产物再通过水浸分离其中的锌和铁,锌进入溶液中,铁保留再渣中。焙烧过程中产生的废气直接通入浸出液中,再通过调节pH进行沉锌操作,最终得到氢氧化锌以及硫酸铵溶液,而硫酸铵溶液积累至一定浓度后通过结晶法回收后可以返回焙烧过程。工艺不仅有效地分离了锌浸出渣中的锌和铁,并对锌进行了回收,也对焙烧产生的废气再利用,得到了硫酸铵,实现了资源的高效循环利用。
4 结论
1) 采用硫酸铵焙烧法来处理锌浸出渣,其焙烧最优条件如下:硫酸铵与锌浸出渣质量比1.30、焙烧温度650 ℃、焙烧时间240 min,所得焙烧产物在经水浸后,锌的浸出率达到93.53%,铁浸出率5.97%,实现了锌和铁的良好分离。最终锌进入浸出溶液中,铁以三氧化二铁的形式富集在水浸渣中。
2) 在325~425 ℃时,硫酸铵与铁酸锌反应生成(NH4)2Zn(SO4)2和NH4Fe(SO4)2,然后在500~ 650 ℃下,分解生成ZnSO4和Fe2(SO4)3,而Fe2(SO4)3会在温度达到480 ℃后分解生成Fe2O3,因此,可基于化合物水溶性性质差异,进行锌和铁的选择性分离。而当焙烧温度达到700 ℃时,ZnSO4会与Fe2O3反应生成ZnFe2O4。
3) 将锌浸出渣与硫酸铵进行混合焙烧,通过查明锌浸出渣中铁酸锌物相转化规律和优化工艺参数,进而提高锌浸出率和抑制铁的溶解,达到了锌铁分离的目的。同时,在湿法回收锌的过程中,硫酸铵可以再生,通过此闭路循环,降低传统工艺所带来的环境问题,表明该工艺具有一定的创新性和重要的学术研究价值。
REFERENCES
[1] 彭容秋. 锌冶金[M]. 长沙: 中南大学出版社, 2005.
PENG Rong-qiu. Zinc metallurgy[M]. Changsha: Central South University Press, 2005.
[2] 刘 斌, 王伟涛. 浅谈湿法炼锌工艺的浸出渣问题[J]. 四川环境, 2007, 26(2): 105-108.
LIU Bin, WANG Wei-tao. On the leaching slag problem of the hydrometallurgical zinc smelting process[J]. Sichuan Environment, 2007, 26(2): 105-108.
[3] LI Mi, PENG Bing, CHAI Li-yuan, et al. Technological mineralogy and environmental activity of zinc leaching residue from zinc hydrometallurgical process[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1480-1488.
[4] MIN Xiao-bo, XIE Xian-de, CHAI Li-yuan, et al. Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(1): 208-218.
[5] SUETENS T, KLAASEN B, ACKER K V, et al. Comparison of electric arc furnace dust treatment technologies using exergy efficiency[J]. Journal of Cleaner Production, 2014, 65: 152-167.
[6] 梅 毅. 回转挥发窑在锌浸出渣处理中的应用[J]. 有色金属设计, 2003(S1): 113-117, 119.
MEI Yi. Application of rotary volatilization kiln in the treatment of zinc leaching residue[J]. Nonferrous Metals Design, 2003(S1): 113-117, 119.
[7] ZHANG Yan-juan, LI Xuan-hai, PAN Liu-ping, et al. Studies on the kinetics of zinc and indium extraction from indium-bearing zinc ferrite[J]. Hydrometallurgy, 2010, 100(3/4): 172-176.
[8] CRUELLS M, ROCA A, NUNEZ C. Electric arc furnace flue dusts: Characterization and leaching with sulphuric acid[J]. Hydrometallurgy, 1992, 31(3): 213-231.
[9] 周桂英, 刘美荣. 湿法冶金过程净化除铁的研究进展[C]// 中国采选技术十年回顾与展望. 中国冶金矿山企业协会, 2012: 69-76.
ZHOU Gui-ying, LIU Mei-rong. Research progress in purifying and removing iron from hydrometallurgical process[C]// Review and Prospect of China’s Mining Technology in Ten Years. China Metallurgical and Mining Enterprises Association, 2012: 69-76.
[10] RIVEROS P A, DUTRIZAC J E. The precipitation of hematite from ferric chloride media[J]. Hydrometallurgy, 1997, 46(1/2): 85-104.
[11] YAN H, CHAI L Y, PENG B, et al. A novel method to recover zinc and iron from zinc leaching residue[J]. Minerals Engineering, 2014, 55: 103-110.
[12] PENG N, PENG B, CHAI L Y, et al. Decomposition of zinc ferrite in zinc leaching residue by reduction roasting[J]. Procedia Environmental Sciences, 2012, 16: 705-714.
[13] HOLLOWAY P C, ETSELL T H, MURLANDU A L. Use of secondary additives to control the dissolution of iron during Na2CO3 roasting of La Oroya zinc ferrite[J]. Metallurgical and Materials Transactions B, 2007, 38: 793-808.
[14] 周国彪, 彭同江, 孙红娟, 等. 高钛型高炉渣与硫酸铵焙烧过程中反应产物的变化与形成机理研究[J].岩石矿物学杂志, 2013, 32(6): 893-898.
ZHOU Guo-biao, PENG Tong-jiang, SUN Hong-juan, et al. Study on the change and formation mechanism of reaction products during roasting of high titanium blast furnace slag and ammonium sulfate[J]. Journal of Rock and Mineralogy, 2013, 32(6): 893-898.
[15] LI Jin-hui, CHEN Zhi-feng, SHEN Bang-po, et al. The extraction of valuable metals and phase transformation and formation mechanism in roasting-water leaching process of laterite with ammonium sulfate[J]. Journal of Cleaner Production, 2017, 140: 1148-1155.
[16] 彭 兵, 李燕春, 柴立元, 等. 锌浸渣硫酸铵焙烧-选择性浸出回收锌[J]. 中国有色金属学报, 2015, 25(9): 2596-2603.
PENG Bing, LI Yan-chun, CHAI Li-yuan, et al. Zinc leaching residue ammonium sulfate roasting-selective leaching to recover zinc[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(9): 2596-2603.
[17] 北京矿冶研究总院. 化学物相分析[M]. 北京: 冶金工业出版社, 1979.
Beijing General Research Institute of Mining and Metallurgy. Chemical phase analysis[M]. Beijing: Metallurgical Industry Press, 1979.
[18] 北京矿冶研究总院. 矿石及有色金属分析手册[M]. 北京: 冶金工业出版社, 1990.
Beijing General Research Institute of Mining and Metallurgy. Handbook of ore and non-ferrous metal analysis[M]. Beijing: Metallurgical Industry Press, 1990.
[19] 刘科伟, 陈天朗. 硫酸铵的热分解[J]. 化学研究与应用, 2002,14(6): 737-738.
LIU Ke-wei, CHNE Tian-lang. Studies on the thermal decomposition of ammonium sulfate[J]. Chemical Research and Application, 2002, 14(6): 737-738.
[20] 范芸珠, 曹发海. 硫酸铵热分解反应动力学研究[J]. 高校化学工程学报, 2011, 25(2): 341-346.
FAN Yun-zhu, CAO Fa-hai. Research on kinetics of thermal decomposition of ammonium sulfate[J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(2): 341-346.
Selective separation of zinc and iron from zinc leaching residue by ammonium sulfate roasting
ZHANG Du-chao, REN Guan-xing, LIU Ruo-lin, CHEN Lin, LIU Wei-feng, YANG Tian-zu
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: In the process of industrial hydrometallurgical zinc smelting, a large amount of zinc leaching residue was produced, and the selective recovery of zinc has become a research hotspot. In this paper, ammonium sulfate and zinc leaching residue were mixed and roasted and then water-leached to achieve the separation of iron and zinc, thereby achieving comprehensive utilization of resources. The optimal roasting conditions are determined through experiments, that is, the mass ratio of ammonium sulfate to zinc leaching residue is 1.3, the roasting temperature is 650 ℃, and the roasting time is 240 min. The results show that ammonium sulfate and zinc ferrite will react to form (NH4)2Zn(SO4)2 and NH4Fe(SO4)2 at 325-425 ℃, and then at 500-650 ℃, (NH4)2Zn(SO4)2 and NH4Fe(SO4)2 respectively decompose to produce ZnSO4 and Fe2(SO4)3, of which Fe2(SO4)3 continues to decompose to produce Fe2O3 and SO3. Under the optimal conditions, the leaching rate of zinc in the calcined product after water leaching reaches 93.53% and that of iron is 5.97%, thus realizing the effective and selective separation of zinc and iron.
Key words: zinc leaching residue; zinc ferrite; ammonium sulfate roasting; zinc; iron
Foundation item: Projects(2018JJ3678) supported by the Natural Science Foundation of Hunan Province, China; Project(2019zzts503) supported by the Fundamental Research Funds for the Central Universities of Central South University, China
Received date: 2020-09-10; Accepted date: 2021-05-30
Corresponding author: CHEN Lin, Tel: +86-15111045540; E-mail: chenlin0210@csu.edu.cn
(编辑 龙怀中)
基金项目:湖南省自然科学基金资助项目(2018JJ3678);中南大学中央高校基本科研业务费专项资金资助项目(2019zzts503)
收稿日期:2020-09-10;修订日期:2021-05-30
通信作者:陈 霖,副教授,博士;电话:15111045540;E-mail:chenlin0210@csu.edu.cn