熔盐电解制备四元Mg-Zn-Li-Ca合金
来源期刊:中国有色金属学报(英文版)2013年第3期
论文作者:曹 鹏 张密林 韩 伟 颜永得 陈丽军
文章页码:861 - 865
Key words:Mg-Zn-Li-Ca alloys; electrolysis; cyclic voltammetry; molten salt
摘 要:在LiCl-KCl-MgCl2-ZnCl2-CaCl2熔盐体系中,以钼为惰性电极,在温度为943 K时,直接电解制备Mg-Zn-Li-Ca四元合金。循环伏安研究表明,在LiCl-KCl熔盐体系中,添加MgCl2、ZnCl2 和CaCl2后,Li的析出电位明显正移。计时电位研究表明,当阴极电流密度等于或者更负于-1.55 A/cm2时,Mg、Li/Zn和Ca能够实现四元沉积。X射线衍射研究表明,恒电流电解可以制备出由不同相组成的Mg-Zn-Li-Ca合金。采用金相显微镜和电子扫描显微镜对合金样品进行表征。能谱分析结果表明,Mg元素和Ca元素在合金中分布均匀,而Zn元素主要分布在基体的边缘。
Abstract: Direct electrodeposition of quarternary Mg-Zn-Li-Ca alloys on a molybdenum electrode from LiCl-KCl-MgCl2- ZnCl2-CaCl2 melts at 943 K was investigated. Cyclic voltammograms (CVs) show that the deposition potential of Li shifts in a positive direction after adding MgCl2, ZnCl2 and CaCl2. Chronopotentiometric measurements indicate that the codepositon of Mg, Li, Zn, and Ca occurs at current densities lower than -1.55 A/cm2. X-ray diffraction (XRD) indicates that Mg-Zn-Li-Ca alloys with different phases were prepared via galvanostatic electrolysis. The microstructures of typical phase of Mg-Zn-Li-Ca alloys were characterized by optical microscopy (OM) and scanning electron microscopy (SEM). The analysis of energy dispersive spectrometry (EDS) shows that elements of Mg and Ca distribute homogeneously in the Mg-Zn-Li-Ca alloy. However, element Zn mainly locates at the edges of the domain.
Trans. Nonferrous Met. Soc. China 23(2013) 861-865
Peng CAO, Mi-lin ZHANG, Wei HAN, Yong-de YAN, Li-jun CHEN
Key Laboratory of Superlight Materials and Surface Technology, Harbin Engineering University, Harbin 150001, China
Received 14 December 2011; accepted 11 June 2012
Abstract: Direct electrodeposition of quarternary Mg-Zn-Li-Ca alloys on a molybdenum electrode from LiCl-KCl-MgCl2- ZnCl2-CaCl2 melts at 943 K was investigated. Cyclic voltammograms (CVs) show that the deposition potential of Li shifts in a positive direction after adding MgCl2, ZnCl2 and CaCl2. Chronopotentiometric measurements indicate that the codepositon of Mg, Li, Zn, and Ca occurs at current densities lower than -1.55 A/cm2. X-ray diffraction (XRD) indicates that Mg-Zn-Li-Ca alloys with different phases were prepared via galvanostatic electrolysis. The microstructures of typical phase of Mg-Zn-Li-Ca alloys were characterized by optical microscopy (OM) and scanning electron microscopy (SEM). The analysis of energy dispersive spectrometry (EDS) shows that elements of Mg and Ca distribute homogeneously in the Mg-Zn-Li-Ca alloy. However, element Zn mainly locates at the edges of the domain.
Key words: Mg-Zn-Li-Ca alloys; electrolysis; cyclic voltammetry; molten salt
1 Introduction
Magnesium-lithium alloys exhibit comparable properties to other light metal alloys based on aluminum, titanium and beryllium and attract more attention in industrial application, such as in the automobile and digital gadget manufacture [1-4]. However, the further application of magnesium-lithium alloy is restricted, due to its low elevated temperature properties [5]. Researches indicated that the addition of Ca and Zn increases the strength, castability and corrosion resistance [6-8]. However, the preparation method of Mg-Zn-Li-Ca alloys has been restricted mostly to metal melting (vacuum metallurgical technique). This conventional method has many disadvantages, such as unhomogeneous alloy composition, complicated production process, serious metal oxidation, and high-energy consumption. To overcome these problems, electrochemical preparation methods for magnesium- based alloys have been investigated. Our group has successfully prepared a relatively more simple electrochemical method in which lithium was deposited and diffused to form Mg-Li alloys on a magnesium cathode from LiCl-KCl melts at 693-783 K [9,10]. Recently, we proposed a direct preparation of Mg-Zn-Li-Ca alloys via electrochemical codeposition of Mg, Li, Zn and Ca from LiCl-KCl-MgCl2-ZnCl2- CaCl2 melts. The process is simpler than previous methods. With regard to deposition and electrochemical studies of pure metallic magnesium ions, CASTRILLEJO et al [11] used voltammetric techniques to characterize the Mg (II) reduction process in the equimolar CaCl2-NaCl mixture at 550 °C. And  et al [12] investigated the electrodeposition and reduction of magnesium from halide melts [12]. However, the codeposition of Mg-Zn-Li-Ca alloys has not been investigated even though the electrochemical codeposition method has been widely used to prepare other alloys. For example, IIDA et al [13,14] investigated the electrochemical formation of Sm-Ni and Sm-Co alloy films by a Li codeposition method from corresponding chloride melts. GU [15] discussed cobalt codeposited with copper and Co-Cu alloy produced on a glassy carbon electrode (GCE) from Co-rich electrolytes.
et al [12] investigated the electrodeposition and reduction of magnesium from halide melts [12]. However, the codeposition of Mg-Zn-Li-Ca alloys has not been investigated even though the electrochemical codeposition method has been widely used to prepare other alloys. For example, IIDA et al [13,14] investigated the electrochemical formation of Sm-Ni and Sm-Co alloy films by a Li codeposition method from corresponding chloride melts. GU [15] discussed cobalt codeposited with copper and Co-Cu alloy produced on a glassy carbon electrode (GCE) from Co-rich electrolytes.
In this work, we attempt to prepare Mg-Zn-Li-Ca alloys directly from LiCl-KCl-MgCl2-ZnCl2-CaCl2 melts using a co-electrodeposition method by galvanostatic electrolysis and characterization of these alloys by ICP, XRD, SEM and EDS.
2 Experimental
A mixture of LiCl-KCl (50%:50%, analytical grade) was first dried under vacuum at 573 K for more than 72 h to remove excess water, and then melted in an alumina crucible placed in a quartz cell located in an electric furnace. The working temperature was measured with a thermocouple protected by an alumina tube inserted into the melt. Metal ion impurities in the melts were removed by pre-electrolysis at -2.00 V (vs Ag+/Ag) for 4 h. Magnesium, zinc and calcium ions were introduced into the bath in the forms of anhydrous ZnCl2, MgCl2 and CaCl2 powders. All experiments were carried out under a carefully purified and dehydrated argon atmosphere.
All electrochemical techniques were performed using an Im6eX electrochemical workstation (Zahner Co., Ltd.) with a THALES 3.08 software package. The working electrodes were d1 mm molybdenum (99.99% purity), which were polished thoroughly using SiC paper, and then cleaned ultrasonically with ethanol prior to use. The active electrode surface was determined after each experiment by measuring the immersion depth of the electrode in the molten salt. The reference electrode used was a solution of LiCl-KCl-AgCl (1%) prepared in a Pyrex tube. All potentials were referred to this Ag+/Ag couple. A graphite rod (d=1 mm, 99.99% purity) served as a counter electrode.
The samples of Mg-Zn-Li-Ca alloys were prepared by galvanostatic electrolysis under different conditions. After electrolysis, all samples were washed in hexane (99.8% purity) in an ultrasonic bath to remove salts and stored in a glove box for analysis. These deposits were analyzed by XRD (X’ Pert Pro; Philips Co., Ltd.) using Cu Kα radiation at 40 kV and 40 mA. The samples for SEM (DFC320; Leica Microsystems) were mounted in thermosetting resins using a metallographic mounting press and then mechanically polished, and finally etched with a solution of 2% HNO3 (volume fraction) in alcohol. The etching time was 30-60 s. The microstructure and micro-zone chemical analysis were also measured using SEM and EDS (JSM-6480A; JEOL Co., Ltd.). The specimen for SEM/EDS was polished and etched again using the former method and time. Each sample was dissolved in aqua regia V(HNO3):V(HCl): V(H2O)=1:3:8). The solution was diluted and analyzed using an inductively coupled plasma atomic emission spectrometer (ICP-AES, Thermo Elemental, IRIS Intrepid II XSP).
3 Results and discussion
3.1 Cyclic voltammetry
Figure 1 shows typical cyclic voltammograms of the LiCl-KCl melts on a molybdenum electrode before (curve 1) and after addition of 5.0% MgCl2, 0.5% ZnCl2 and 3.0% CaCl2 (curve 2). In curve 1, cyclic voltammograms in the LiCl-KCl melts, as the control, show no significant peaks within the electrochemical window, except a couple of cathodic/anodic signals (B/B') corresponding to the reduction and oxidation of Li, respectively. After the addition of 0.5% ZnCl2 and 3.0% CaCl2, another redox system found at a more positive potential (-0.75 V vs Ag+/Ag ) is characteristic of a metallic deposition and can be attributed to the reduction of Zn (II) to Zn (0) (peak A), because the deposition potential of Zn (II) ions is more positive than that of Mg(II), Li(I) and Ca (II) ions in a chloride system [16]. Peak A' shows the reoxidation of the deposited Zn metal on the molybdenum electrode. Peak C, associated with the reduction of Mg2+ ions, followed by the dissolution of the deposited Mg metal (peak C') first was detected at around -1.65 V (vs Ag/AgCl). Afterward, a cathodic current was slowly increased from approximately -1.80 V indicating that the formation of the Mg-Ca alloy or deposition of metallic Ca occurs. Since the decomposition potentials of calcium and lithium chloride are significantly close (the difference is only about 0.12 V) and deposition rate of lithium on Mg has high underpotential at relatively high temperature, an Mg-Li-Ca alloy is formed almost from the beginning of the formation of the magnesium phase on the molybdenum substrate. Consequently, the current peak for Mg-Ca alloys or deposition of metallic Ca is not detected easily. A similar phenomenon was observed in Ref. [17]. In addition, a clear anodic peak (labeled D') at a potential of -1.0 V is observed, which is thought to correspond to the dissolution of the Mg-Zn deposits.
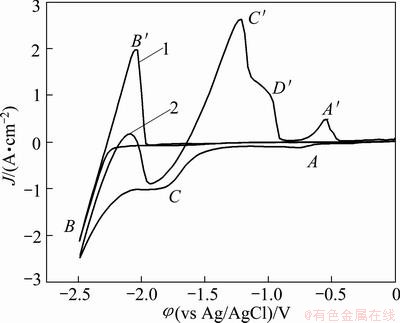
Fig. 1 Typical CVs of LiCl-KCl melts before (curve 1) and after (curve 2) addition of 5.0% MgCl2, 0.5% ZnCl2 and 3.0% CaCl2 on molybdenum electrode at 943 K (Scan rate: 0.1 V/s)
3. 2 Chronopotentiometry
A chronopotentiometric experiment was carried out to further study the formation of Mg-Zn-Li-Ca alloys. Figure 2 presents chronopotentiograms measured on a molybdenum electrode (S=0.322 cm2) in LiCl-KCl- MgCl2 (5.0%) melts containing 0.5% ZnCl2 and 3.0% CaCl2 at different current intensities. At a cathodic current lower than -50 mA (-0.16 A/cm2), only a potential plateau corresponding to the reduction of zincum occurs. At a cathodic current more negative than -60 mA (-0.19 A/cm2), the curves exhibit two potential plateaus (plateaus A and B), which are associated with the reduction of zinc and magnesium ions to the corresponding metals, respectively. At this current intensity, codeposition of Zn and Mg occurs. According to the phase diagram of Mg-Zn system [5], Mg-Zn alloy can be formed at this temperature. When the current reaches -500 mA (-1.55 A/cm2), a third plateau appears. This plateau is caused by a reduction of lithium ions. At this current intensity, codeposition of Mg, Li and Ca occurs. It is obvious that the potential ranges for deposition of Mg, Li, and Ca are the same as those observed in the cyclic voltammograms.

Fig. 2 Chronopotentiograms obtained at different current intensities on molybdenum electrode (0.322 cm2) in LiCl-KCl-MgCl2 (5.0%) melts containing 0.5% ZnCl2 and 3.0% CaCl2 at 943 K
3.3 Galvanostatic electrolysis and characterization of deposits
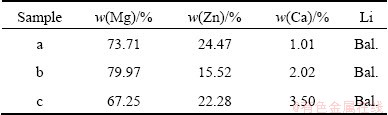
Based on the results obtained by CVs and chronopotentiometrys, further galvanostatic electrolysis experiments were carried out in LiCl-KCl melts containing different MgCl2, ZnCl2 and CaCl2 concentrations on molybdenum electrodes at 943 K. The microstructure of a typical Mg-Zn-Li-Ca alloy, obtained by codeposition from LiCl-KCl-MgCl2 (8%) melts containing 0.5% ZnCl2 and 3.0% CaCl2, shows that the alloy is composed of white α phase and black eutectic structure (Fig. 3). According to the fundamentals of solidification and the binary phase diagram of Mg-Zn system, Zn acts with Mg and distributes along grain boundaries in the solidification process, which lowers the diffusion rate of atoms, and increases the number of nuclei to restrict the growth of grains. On the other hand, the enrichment of solute atoms leads to the formation of Mg-Zn phases, which mainly distribute in the grain boundary area, and thus inhibits further grain growth. A SEM and EDS mapping analysis was used to examine the general distribution of elements in Mg-Zn-Li-Ca alloys by codeposition from LiCl-KCl-ZnCl2 (0.5%)- CaCl2 (3.0%) melts containing 8.0% MgCl2 (Fig. 4). The result confirms that the Mg and Ca elements distribute homogeneously throughout the Mg-Zn-Li- Ca alloy. However, Zn distribution is not uniform and mainly located in grain boundary. Figure 5 shows the complex XRD patterns of Mg-Zn-Li-Ca alloys obtained by galvanostatic electrolysis from LiCl-KCl melts with different concentrations of MgCl2, ZnCl2 and CaCl2 at -6.21 A/cm2 for 2.5 h, respectively. In patterns in Figs. 4(a)–(c), most of the strong peaks are identified from α Mg phase. For patterns in Figs. 4(a) and (b), peaks for a new phase, Mg7Zn3, are recognized in pattern (a) with a high concentration of ZnCl2, because enough Zn atoms concentrate at the grain boundaries to react with Mg, forming the compound, Mg7Zn3 phase, in the Mg-Zn-Li-Ca alloy. In addition, peaks for a new phase LiMgZn are recognized in pattern (c) because of the relatively high content of lithium in Mg-Zn-Li-Ca alloy. Moreover, with the decrease of MgCl2 concentration in LiCl-KCl-ZnCl2 (0.5%)-CaCl2 (3.0%) melt, the lithium content of Mg-Zn-Li-Ca alloys increases at the constant current intensity. However, for patterns (a), (b) and (c), Ca has no apparent effect on the phase structure of Mg-Zn-Li-Ca alloys because of the relatively low Ca content in the Mg-Zn Li-Ca alloys. EDS is a qualitative and semi-quantitative estimate of elemental concentration, and element Li cannot be detected in the EDS. Therefore, we carried out the ICP analysis of alloys. The ICP analyses of all samples obtained by galvanostatic electrolysis are listed in Table 1. The ICP results show that the chemical compositions of Mg-Zn-Li-Ca alloys are consistent with the phase structures of the XRD patterns, Under galvanostatic electrolysis, the lower the MgCl2 concentration in the LiCl-KCl melts with equivalent ZnCl2 and CaCl2 concentration at a constant current intensity, the higher the lithium content of Mg-Zn-Li-Ca alloys. With the increasing ZnCl2 concentration of LiCl-KCl-MgCl2 melts, the zincum content of Mg-Zn-Li-Ca alloys increases, while calcium is opposite. Based on these results, it can be concluded that the lithium, zincum and calcium contents of Mg-Zn-Li-Ca alloys can be adjusted by changing the MgCl2 and ZnCl2 concentrations.
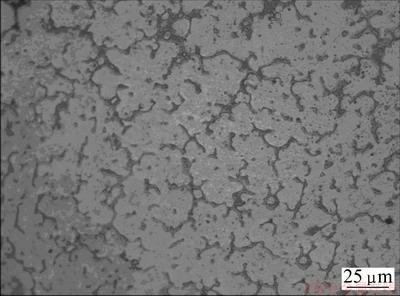
Fig. 3 Optical micrograph of Mg-Zn-Li-Ca alloy by codeposition from LiCl-KCl-MgCl2 (8%) melts containing 0.5% ZnCl2 and 3.0% CaCl2

Fig. 4 SEM image (a) and EDS mapping analysis Mg(b), Zn(c) and Ca(d) of Mg-Zn-Li-Ca alloys by codeposition from LiCl-KCl-MgCl2 (8%) melts containing 0.5% ZnCl2 and 3.0% CaCl2
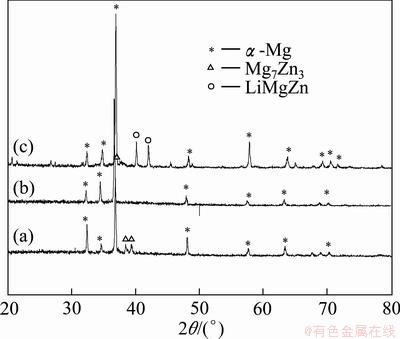
Fig. 5 XRD patterns of deposits obtained by codeposition on Mo electrodes in LiCl-KCl melts containing 0.5% ZnCl2, 3.0% CaCl2, 10.0% MgCl2 (a), 0.2% ZnCl2, 3.0% CaCl2, 10.0% MgCl2 (b) and 0.5% ZnCl2, 3.0% CaCl2 and 8.0% MgCl2 (c)
Table 1 ICP analyses of samples obtained by galvanostatic electrolysis (-2 A) on Mo electrode (S=0.322 cm2) from LiCl-KCl melts containing 0.5% ZnCl2, 3.0% CaCl2 and 10.0% MgCl2 (a), 0.2% ZnCl2, 3.0% CaCl2 and 10.0% MgCl2 (b) and 0.5% ZnCl2, 3.0% CaCl2 and 8.0% MgCl2 (c)
4 Conclusions
1) From the CVs, the deposition potential of Li shifts in a positive direction after adding MgCl2, ZnCl2, and CaCl2. The codepositon of Mg, Li, Zn and Ca occurs at current densities lower than -0.31 A/cm2 in LiCl-KCl-MgCl2 (5%) melts containing 3.0% CaCl2 and 0.5% ZnCl2.
2) When the concentration of ZnCl2 is 0.2% in LiCl-KCl-MgCl2(10.0%), Zn has no apparent effect on the phase structure of Mg-Li alloys. A new Mg7Zn3 phase occurs in Mg-Zn-Li-Ca alloy when the concentration of ZnCl2 is increased from 0.2% to 0.5% in LiCl-KCl-MgCl2 (10%) melts. A new LiMgZn phase occurs in Mg-Zn-Li-Ca alloy when the concentration of MgCl2 is decreased to 8.0%. The lithium, zinc, and calcium contents of Mg-Zn-Li-Ca alloys can be controlled by MgCl2, ZnCl2, and CaCl2 concentrations.
3) The EDS results show that element Zn concentrates at the edges of domain to react with Mg and Ca which distribute homogeneously in the Mg-Zn-Li- Ca alloy.
References
[1] SANSCHAGRIN A, TREMBLAY R, ANGERS R, DUBE D. Mechanical properties and microstructure of new magnesium- lithium base alloys [J]. Materials Science and Engineering A, 1996, 220: 69-77.
[2]  Deformation behaviour of Mg-Li alloys at elevated temperatures [J]. Materials Science and Engineering A, 2005, 410: 148-151.
Deformation behaviour of Mg-Li alloys at elevated temperatures [J]. Materials Science and Engineering A, 2005, 410: 148-151.
[3] SONG J M, WEN T X, WANG J Y. Vibration fracture properties of a lightweight Mg-Li-Zn alloy [J]. Scripta Materialia, 2007, 56: 529-532.
[4] WATANBLE H, TSUTSUI H, MUKAI T, KOHZU M, TANABE S, HIGASH K. Deformation mechanism in a coarse-grained Mg-Al-Zn alloy at elevated temperatures [J]. International Journal of Plasticity, 2001, 17: 387-397.
[5] WANG T, ZHANG M L, WU R Z. Microstructure and properties of Mg-8Li-1Al-1Ce alloy [J]. Materials Letters, 2008, 62: 1846-1848.
[6] WASIUR-RAHMAN S, MEDRAJ M. Critical assessment and thermodynamic modeling of the binary Mg-Zn, Ca-Zn and ternary Mg-Ca-Zn systems [J]. Intermetallics, 2009, 17: 847-864
[7] SOMEKAWA H, MUKAI T. High strength and fracture toughness balance on the extruded Mg-Ca-Zn alloy [J]. Materials Science and Engineering A, 2007, 459: 366-370.
[8] OH-ISHI K, WATANABE R, MENDIS C L, HONO K. Age-hardening response of Mg-0.3 at.% Ca alloys with different Zn contents [J]. Materials Science and Engineering A, 2009, 526: 177-184.
[9] ZHANG M L, YAN Y D, HOU Z Y, FAN L A, CHEN Z, TANG D X. An electrochemical method for the preparation of Mg-Li alloys at low temperature molten salt system [J]. Journal of Alloys and Compounds, 2007, 440: 362-366.
[10] YAN Y D, ZHANG M L, HAN W, CAO D X, YUAN Y, XUE Y, CHEN Z. Electrochemical formation of Mg-Li alloys at solid magnesium electrode from LiCl-KCl melts [J]. Electrochimica Acta, 2008, 53: 3323-3328.
[11] CASTRILLEJO Y, MARTNEZ A M, PARDO R, HAARBERG G M. Electrochemical behaviour of magnesium ions in the equimolar CaCl2-NaCl mixture at 550 °C [J]. Electrochimica Acta, 1997, 51: 1869-1876.
[12]  B, HAARBERG G M, TUNOLD R. Electrodeposition of magnesium from halide melts-charge transfer and diffusion kinetics [J]. Electrochimica Acta, 1997, 51: 1869-1876.
B, HAARBERG G M, TUNOLD R. Electrodeposition of magnesium from halide melts-charge transfer and diffusion kinetics [J]. Electrochimica Acta, 1997, 51: 1869-1876.
[13] IIDA T, NOHIRA N, ITO Y. Electrochemical formation of Sm-Co alloy films by Li codeposition method in a molten LiCl-KCl-SmCl3 system [J]. Electrochimica Acta, 2003, 48: 901-906.
[14] IIDA T, NOHIRA N, ITO Y, Electrochemical formation of Sm-Ni alloy films in a molten LiCl-KCl-SmCl3 system [J]. Electrochimica Acta, 2001, 46: 2537-2544.
[15] GU M. Initial stages of the electrocrystallization of Co-Cu alloys on GCE from the Co rich electrolytes [J]. Electrochim Acta, 2007, 52: 4443-4448.
[16] YANG Q Q, FANG B L, TONG Y X. Applied Electrochemistry [M]. Guangzhou: Sun Yat-Sen University Press, 2005: 131-134. (in Chinese)
[17] YAN Y D, ZHANG M L, XUE Y, HAN W, CAO D X, JING X Y, HE L Y, YUAN Y. Electrochemical formation of Mg-Li-Ca alloys by codeposition of Mg, Li and Ca from LiCl-KCl-MgCl2-CaCl2 melts [J]. Physical Chemistry Chemical Physics, 2009, 11: 6148-6155.
曹 鹏,张密林,韩 伟,颜永得,陈丽军
哈尔滨工程大学 教育部超轻材料与表面技术重点实验室,哈尔滨 150001
摘 要:在LiCl-KCl-MgCl2-ZnCl2-CaCl2熔盐体系中,以钼为惰性电极,在温度为943 K时,直接电解制备Mg-Zn-Li-Ca四元合金。循环伏安研究表明,在LiCl-KCl熔盐体系中,添加MgCl2、ZnCl2 和CaCl2后,Li的析出电位明显正移。计时电位研究表明,当阴极电流密度等于或者更负于-1.55 A/cm2时,Mg、Li/Zn和Ca能够实现四元沉积。X射线衍射研究表明,恒电流电解可以制备出由不同相组成的Mg-Zn-Li-Ca合金。采用金相显微镜和电子扫描显微镜对合金样品进行表征。能谱分析结果表明,Mg元素和Ca元素在合金中分布均匀,而Zn元素主要分布在基体的边缘。
关键词:Mg-Zn-Li-Ca合金; 电解; 循环伏安;熔盐
(Edited by Xiang-qun LI)
Foundation item: Project (2011AA03A409) supported by the High-tech Research and Development Program of China; Projects (21103033, 21101040, 21173060, 91226201) supported by the National Natural Science Foundation of China; Project (HEUCF20130012) supported by the Fundamental Research Funds for the Central Universities, China; Project (1253G016) supported by the Foundation for University Key Teacher of Heilongjiang Province of China
Corresponding author: Mi-lin ZHANG; Tel/Fax: +86-451-82533026; E-mail: zhangmilin2009@sina.com
DOI: 10.1016/S1003-6326(13)62540-6

