文章编号:1004-0609(2015)08-2212-06
主要载金硫化物黄铁矿的热分解动力学特性
赵留成1,孙春宝1,张舒婷1,谢文清2,郑新烟2,刘 柯3
(1.北京科技大学 金属矿山高效开采与安全教育部重点实验室,北京 100083;
2.福建省双旗山矿业有限责任公司,泉州 362509;
3. 中冶华冶(北京)国际贸易有限公司,北京 100029)
摘 要:采用TG-DSC热分析技术,在不同升温速率条件下对主要载金硫化物黄铁矿的热分解动力学进行研究,并采用SEM对反应前后的黄铁矿微观结构进行分析。结果表明:黄铁矿热分解的温度区间为400~800 ℃,质量损失率保持在26%左右;随着升温速率的增大,TG曲线向高温区偏移,即得到相同转化率所需的温度越高,其最大反应速率的温度范围为600~700 ℃。采用Kissinger法和Ozawa-Doyle法求得黄铁矿热分解反应的活化能E和指前因子A分别为259.23 kJ/mol和1×1014.67。根据Coats-Redfern方程和常见的热分解机理函数对热重分析数据进行线性拟合,采用Malek法进一步确定了黄铁矿的热分解过程符合Mample单行法则,其反应机理为随机成核和随后生长,并获得了热分解反应的微分方程。
关键词:载金硫化物;黄铁矿;热分解;动力学;热重分析;活化能
中图分类号:TD953 文献标志码:A
Characteristic of thermal decomposition kinetics of main gold-bearing sulfides pyrite
ZHAO Liu-cheng1, SUN Chun-bao1, ZHANG Shu-ting1, XIE Wen-qing2, ZHENG Xin-yan2, LIU Ke3
(1. Key Laboratory of High-Efficient Mining and Safety of Metal Mines, Ministry of Education,
University of Science and Technology Beijing, Beijing 100083, China;
2. Fujian Shuangqishan Mining Industry Limited Company, Quanzhou 362509, China;
3. MCC Huaye (Beijing) International Trade Co., Ltd., Beijing 100029, China)
Abstract: Thermal decomposition kinetics of main gold-bearing sulfides pyrite was investigated by TG-DSC thermal analysis at different heating rates. The microstructures of pyrite before and after decomposition were analyzed by SEM. The results show that the thermal decomposition temperature of pyrite is between 400-800 ℃. The mass loss rate is approximately 26%. The TG curves move in the direction of high temperature with increasing the heating rate. This means that the same conversion can be gotten at higher temperature. The temperature range of the maximum pyrite thermal decomposition reaction rate of pyrite is between 600-700 ℃. The apparent activation energy E and pre-exponential factor A were calculated by Kissinger and Ozawa-Doyle methods, and the values are 259.23 kJ/mol and 1×1014.67, respectively. According to Coats-Redfern equation, linear regressions were carried out by common mechanism functions of thermal decomposition. It is defined that the thermal decomposition process of pyrite conforms to the Mample principle by Malek method, and the corresponding mechanism is the random nucleus and the consequent growth. The differential equation of thermal decomposition reaction was deduced as well.
Key words: gold-bearing sulfide; pyrite; thermal decomposition; kinetics; thermogravimetric analysis; activation energy
矿石中金呈细粒或次显微粒状被包裹或浸染于硫化物黄铁矿中,这种被包裹的金用细磨方法也很难解离,金不能与浸出试剂有效接触,导致金的浸出率降低,通常采用氧化焙烧法消除黄铁矿包裹的影响[1-3]。目前,对该类金矿的研究主要集中在对黄铁矿的预处理工艺和氧化焙烧过程中物相的转变上,而对黄铁矿热分解动力学的研究较少。王锐等[4]对某含15.03 g/t Au的金赋存于黄铁矿和毒砂中的金矿进行研究,采用氧化焙烧-氰化浸出工艺处理后金的浸出率由15.06%提高到86.21%。李峰等[5]采用氧化焙烧-硫代硫酸盐浸出某难处理含金硫精矿,硫的去除率为94.7%,金的浸出率为71.2%。BOYABAT等[6]和范博文[7]认为黄铁矿受热分解转化的产物为磁黄铁矿。HU等[8]指出在惰性气氛下黄铁矿在900~1000 ℃时,按照黄铁矿→磁黄铁矿→硫铁矿→铁进行顺序转变。胡慧萍等[9]对机械活化后的黄铁矿进行了热分解动力学研究,发现黄铁矿经机械活化后热分解活化能降低。由于黄铁矿是主要的载金硫化矿物,研究其热分解动力学对包裹型难处理金矿的预处理具有重大意义。
热分解动力学的研究多利用热分析方法[10-11],其中以热重-差示扫描量热法(TG-DSC)和热重-差热分析法(TG-DTA)为主。鲁仁予等[12]利用热重分析法研究了氮气气氛下菱镁矿的热分解动力学。宋力等[13]采用热分析方法(TG-DTA)分析了无水硫酸铝铵在氩气中的热分解动力学。张声春等[14]采用热重分析法对聚苯硫醚的热分解动力学进行了研究,得到了聚苯硫醚热分解动力学参数平均活化能E、指前因子A和对应的热分解动力学方程。
本文作者使用TA Q600热重分析仪,测得黄铁矿在不同升温速率下的多条TG-DSC曲线,应用Kissinger法和Ozawa-Doyle法计算出了黄铁矿热分解的反应活化能和指前因子,利用Coats-Redfern方程和Malek法,确定了热分解机理函数和反应机理,并采用SEM对焙烧前后黄铁矿的微观结构进行分析,进一步验证其反应机理。
1 实验
1.1 实验原料
所用的黄铁矿购于中国地质博物馆,经人工挑选后,用玛瑙研钵研磨至粒径小于0.074 mm,将其在105 ℃条件下烘干2 h后作为实验试样。试样含Fe 47.78%(质量分数),含S 51.12%,纯度为98.9%。
1.2 实验方法
采用TA Q600热重分析仪对试样进行不同升温速率的TG-DSC分析。每次称取样品质量约为20 mg,在氮气气氛中,流量为40 mL/min的条件下,分别以5、10、15、20 ℃/min的升温速率将试样从25 ℃加热至1000 ℃,使热分析曲线达到稳定状态,获得TG-DSC曲线。
采用XD-1200NT真空管式炉对试样进行焙烧试验。称取10 g试样,在氮气气氛中,流量为1.5 L/min的条件下,以10 ℃/min的升温速率将试样从25 ℃加热至700 ℃,在氮气气氛下冷却后制成光片。对焙烧前后的试样采用德国卡尔蔡司EVO18扫描电镜(SEM)观察其微观结构的变化,进一步分析黄铁矿热分解动力学机理。
2 结果与讨论
2.1 黄铁矿热分解的TG-DSC曲线
图1所示为黄铁矿在氮气气氛下不同升温速率的TG和DSC曲线。从图1可知,黄铁矿热分解的温度区间为400~800 ℃,与HU等[15]和李平等[16]的研究结果一致。不同升温速率的TG曲线变化趋势相同,质量损失率基本一致,保持在26%左右,而纯FeS2热分解的理论值为26.67%,由此说明黄铁矿纯度较高,热分解反应进行的很完全。随着升温速率的增大,TG曲线向高温区偏移,即在相同转化率条件下,升温速率越大对应的反应温度就越高。DSC曲线上出现向下的峰是由黄铁矿热分解需要吸热所致,可认为峰顶位置是黄铁矿热分解达到最大反应速率时的温度,其温度范围为600~700 ℃。过峰顶位置后DSC曲线本应向零点回归,但曲线却呈向下趋势,可能是生成的硫对测试条件造成一定的干扰,但是对DSC曲线的吸热峰没有影响。

图1 黄铁矿在氮气气氛下不同升温速率的TG和DSC曲线
Fig. 1 TG and DSC curves of pyrite at different heating rates in nitrogen
2.2 动力学参数
在氮气气氛下,黄铁矿发生热分解反应如式(1)所示:
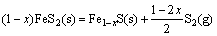 (1)
(1)
根据非等温过程固相反应动力学理论,在描述黄铁矿固相热分解反应的动力学问题时,通常用式(2)表示:
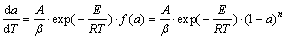 (2)
(2)
式中:a为黄铁矿热分解过程中的转化率;f(a)为热分解动力学机理函数;β为升温速率,℃/min;A为指前因子;E为反应活化能,kJ/mol;R为摩尔气体常数;T为反应温度,K。
采用Kissinger法和Ozawa-Doyle法分别求解式(2)中的动力学参数。Kissinger方程[17-19]不考虑机理函数的具体形式,只需要不同升温速率下DSC曲线上的峰顶温度Tm,即可求得反应活化能E。假设DSC曲线峰顶处为最大反应速率发生的位置,与之相对应的温度为Tm,根据式(2),并设n=1,在Tm处有 ,可得
,可得
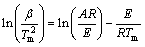 (3)
(3)
由式(3)作图可得到一条直线,从直线的斜率求活化能E,从截距求指前因子A。
Ozawa-Doyle法是一种多曲线积分法求解反应动力学参数的方法,其方程如式(4)所示:
 (4)
(4)
式中:G(a)为反应机理函数的积分形式,由于不同升温速率β下各DSC曲线峰顶温度Tm处各a值近似相等,即G(a)近似相等。因此lg β与1/Tm呈线性关系,便可求得反应的活化能E。
根据式(3)和(4),结合DSC曲线峰顶温度(见表1)进行线性拟合,计算得到黄铁矿热分解活化能和指前因子(见表2)。结果表明:Kissinger法和Ozawa-Doyle法拟合均具有较好的线性相关性,相关系数均大于0.99,两者计算得到的黄铁矿热分解活化能E也基本一致,分别为258.49和259.96 kJ/mol,平均值为 259.23 kJ/mol。
表1 不同升温速率下黄铁矿DSC曲线上的峰顶温度
Table 1 Peak temperatures in DSC curves of pyrite at different heating rates

表2 采用不同方法计算得到的黄铁矿的热分解动力学参数
Table 2 Kinetic parameters of pyrite thermal decomposition calculated by different methods
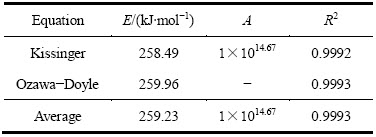

图2 ln(β/Tm2)与1/Tm的关系
Fig. 2 Relationship between ln(β/Tm2) and 1/Tm
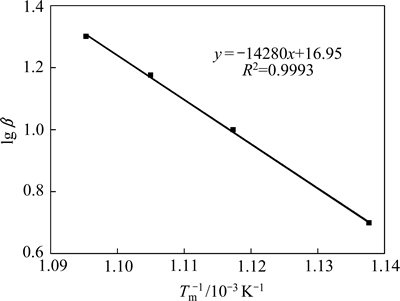
图3 lg β与1/Tm的关系
Fig. 3 Relationship between lg β and 1/Tm
2.3 动力学机理函数
实验采用Coats-Redfern法[20]对式(2)进行积分求解, ,可到黄铁矿热分解反应非等
,可到黄铁矿热分解反应非等
温动力学方程为
 (5)
(5)
由式(5)可知,如果反应机理函数G(a)选择正确, 与1/T应为线性关系。结合10种常见的机
与1/T应为线性关系。结合10种常见的机
理函数[21-22],将不同升温速率下的TG数据代入式(5)进行线性拟合,由线性相关性系数R2可以判断机理函数的可靠性。表3所列为不同升温速率下黄铁矿热分解转化率a与反应温度T的关系,表4所列为动力学参数回归计算结果。
机理函数的表达式如式(6)~(15)所示:
D1(a)=a2 (6)
D2(a)= (7)
(7)
D3(a)= (8)
(8)
D4(a)=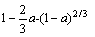 (9)
(9)
3D(a)= (10)
(10)
F1(a)= (11)
(11)
A2(a)=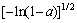 (12)
(12)
A3(a)=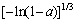 (13)
(13)
R2(a)= (14)
(14)
R3(a)=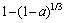 (15)
(15)
由表4可知,线性相关性系数R2较高且接近的机理函数为3D和F1。在此情况下,采用Malek法[17-18]做最概然机理函数的进一步判断。
机理函数的标准曲线方程如式(16)所示:
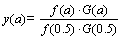 (16)
(16)
实验曲线方程如式(17)所示:
 (17)
(17)
式中:f(a)和G(a)分别为动力学机理函数的微分形式和积分形式;da/dt由TG曲线求得。
若实验曲线与标准曲线重叠或实验数据点全部落在某一标准曲线上,则判定该标准曲线为最概然的动力学机理函数。图4所示为升温速率为15 ℃/min的黄铁矿热分解y(a)-a曲线P。由图4可知,实验曲线与10种常用的动力学机理函数的标准曲线对比,可以判定黄铁矿热分解反应符合F1机理函数,即Mample单行法则,反应机理为随机成核和随后生长,其机理函数的积分形式为G(a)=-ln(1-a),微分形式为
f(a)=1-a,相应的动力学微分方程为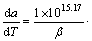

 。
。
2.4 黄铁矿热分解动力学机理分析
图5所示为黄铁矿热分解前和温度为700 ℃时的SEM像。由图5(a)可知,热分解前黄铁矿为自形晶、半自形晶和他形晶粒状结构,呈致密块状,颗粒的表面无孔隙和孔洞。700 ℃时黄铁矿发生热分解反应生成磁黄铁矿,原本致密的颗粒变得松散多孔,颗粒内出现大量的空洞,而未热分解部分颗粒依然是致密状(见图5(b)),黄铁矿的热分解反应首先发生在结晶程度较差处,即晶体中存在晶格缺陷、位错或含杂质的地方,在这些部位形成热分解的活性点,随后这些相邻近的点上的分解产物聚积成新物相的核,随着反应进行,产物的核数目增多,核慢慢长大并向内部延伸,由此可以验证黄铁矿热分解受成核与生长过程控制。
表3 不同升温速率下黄铁矿的转化率与反应温度的关系
Table 3 Relationship between pyrite transformation rate and reaction temperature at different heating rates

表4 动力学参数回归计算结果
Table 4 Regression results of kinetic parameters

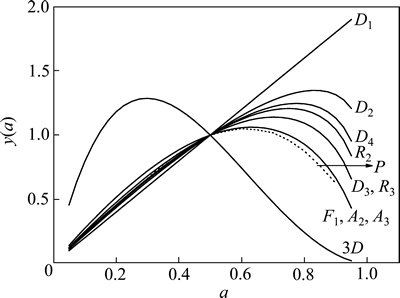
图4 黄铁矿热分解y(a)-a曲线
Fig. 4 Curve of y(a) vs a of pyrite thermal decomposition
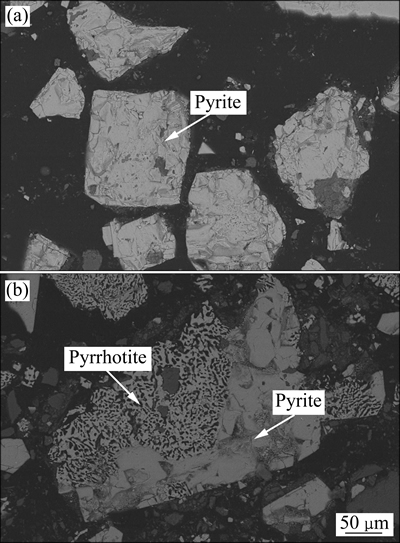
图5 黄铁矿热分解前和700 ℃分解的SEM像
Fig. 5 SEM images of pyrite before(a) and after thermal decomposition at 700 ℃(b)
3 结论
1) 采用TG-DSC热分析法对主要载金硫化物黄铁矿热分解过程进行研究,其热分解温度区间为400~800 ℃,质量损失率保持在26%左右;随着升温速率的增大,TG曲线向高温区偏移,即得到相同转化率所需的温度越高。黄铁矿热分解最大反应速率的温度范围为600~700℃。
2) 采用Kissinger法和Ozawa-Doyle法确定的黄铁矿热分解反应活化能E和指前因子A分别为259.23 kJ/mol和1×1014.67。
3) 黄铁矿的热分解过程符合Mample单行法则,其反应机理为随机成核和随后生长,并得到相应的动力学微分方程。
REFERENCES
[1] 雷占昌, 虞 洁, 马红蕊. 难处理金矿预处理技术现状及进展[J]. 现代矿业, 2014(5): 23-24, 33.
LEI Zhan-chang, YU Jie, MA Hong-rui. Statue and development of pretreatment technology for the refractory gold ore[J]. Modern Mining, 2014(5): 23-24, 33.
[2] NAN Xin-yuan, CAI Xin, KONG Jun. Pretreatment process on refractory gold ores with As[J]. ISIJ International, 2014, 54(3): 543-547.
[3] IDA D M, AGOSTINO O, STEFANO U, FRANCESCO F, FRANCESCA B, FRANCESCO V. Roasting and chlorine leaching of gold-bearing refractory concentrate: Experimental and process analysis[J]. International Journal of Mining Science and Technology, 2013, 23(5): 709-715.
[4] 王 锐, 唐道文, 杨 聪. 某难浸金矿低温焙烧预处理试验[J]. 有色金属(冶炼部分), 2013(4): 39-41.
WANG Rui, TANG Dao-wen, YANG Cong. Pretreatment of low temperature roasting for refractory gold ore[J]. Nonferrous Metals (Extractive Metallurgy), 2013(4): 39-41.
[5] 李 峰, 丁德馨, 胡 南, 彭志成, 陈 伟. 难处理含金硫精矿的焙烧氧化-硫代硫酸盐浸出[J]. 中国有色金属学报, 2014, 24(3): 831-837.
LI Feng, DING De-xin, HU Nan, PENG Zhi-cheng, CHEN Wei. Roasting oxidation-thiosulfate leaching of gold from refractory gold-bearing sulfur concentrates[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(3): 831-837.
[6] BOYABATA N,  A K, BAYRAKCEKENC S, GULABOGLU M S. Thermal decomposition of pyrite in the nitrogen atmosphere[J]. Fuel Processing Technology, 2004, 85(2/3): 179-188.
A K, BAYRAKCEKENC S, GULABOGLU M S. Thermal decomposition of pyrite in the nitrogen atmosphere[J]. Fuel Processing Technology, 2004, 85(2/3): 179-188.
[7] 范博文. 黄铁矿加热物相及其磁性特征与机理研究[D]. 成都: 成都理工大学, 2011: 32-39.
FAN Bo-wen. Heating phase of pyrite and their magnetic property[D]. Chengdu: Chengdu University of Technology, 2011: 32-39.
[8] HU Gui-lin, JOHANSEN D K, WEDEL S, HANSEN J P. Decomposition and oxidation of pyrite[J]. Progress in Energy and Combustion Science, 2006, 32(3): 295-314.
[9] 胡慧萍, 陈启元, 尹周澜, 张平民, 车洪生. 机械活化黄铁矿的热分解动力学[J]. 中国有色金属学报, 2002, 12(3): 611-614.
HU Hui-ping, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Ping-min, CHE Hong-sheng. Kinetics of thermal decomposition of mechanically activated pyrite[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(3): 611-614.
[10]  Wei-zhi, YU Dun-xi, WU Jian-qun, ZHANG Lian, XU Ming-hou. The chemical role of CO2 in pyrite thermal decomposition[J]. Proceedings of the Combustion Institute, 2014(4): 1-8.
Wei-zhi, YU Dun-xi, WU Jian-qun, ZHANG Lian, XU Ming-hou. The chemical role of CO2 in pyrite thermal decomposition[J]. Proceedings of the Combustion Institute, 2014(4): 1-8.
[11] SUPRIYA N, CATHERINE K B, RAJEEV R. DSC-TG studies on kinetics of curing and thermal decomposition of epoxy-ether amine systems[J]. Journal of Thermal Analysis and Calorimetry, 2013, 112(1): 201-208.
[12] 鲁仁予, 董 俊. 菱镁矿在氮气气氛中的热分解动力学[J]. 贵州大学学报(自然科学版), 2009, 26(2): 45-47.
LU Ren-yu, DONG Jun. Kinetics of thermal decomposition of magnesite in nitrogen[J]. Journal of Guizhou University (Natural Science), 2009, 26(2): 45-47.
[13] 宋 力, 朱建君, 刘启祥, 洪建和, 袁良杰, 张友祥, 张克立. 无水硫酸铝铵在氩气中的热分解动力学研究[J]. 化学世界, 2011, 52(6): 342-345.
SONG Li, ZHU Jian-jun, LIU Qi-xiang, HONG Jian-he, YUAN Liang-jie, ZHANG You-xiang, ZHANG Ke-li. Study on kinetics of thermal decomposition of anhydrous NH4Al(SO4)2 in argon[J]. Chemical World, 2011, 52(6): 342-345.
[14] 张声春, 杭祖圣, 李 栋, 应三九. 非等温热重法研究聚苯硫醚热分解动力学[J]. 应用化学, 2011, 40(12): 2189-2292.
ZHANG Sheng-chun, HANG Zu-sheng, LI Dong, YING San-jiu. Study on the thermal decomposition kinetics of polyphenylene sulfide (PPS) by non-isothermal thermogravimetry[J]. Applied Chemical Industry, 2011, 40(12): 2189-2292.
[15] HU Hui-qing, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Ping-min, ZOU Jian-peng, CHE Hong-sheng. Study on the kinetics of thermal decomposition of mechanical activated pyrites[J]. Thermochimica Acta, 2002, 389(1/2): 79-83.
[16] 李 平, 陈天虎, 杨 燕, 谢巧勤, 谢晶晶. 氮气保护下热处理胶状黄铁矿的矿物特性演化[J]. 硅酸盐学报, 2013(11): 1564-1570.
LI Ping, CHEN Tian-hu, YANG Yan, XIE Qiao-qin, XIE Jing-jing. Effect of heat treatment in nitrogen on mineralogy characteristics of colloidal pyrite[J]. Journal of the Chinese Ceramic Society, 2013(11): 1564-1570.
[17] 张 强, 何宏平, 陶 齐. 基于热重分析的菱镁矿分解动力学解析[J]. 岩石矿物学杂志, 2014, 33(2): 391-396.
ZHANG Qiang, HE Hong-pin, TAO Qi. Decomposition kinetics of magnesite deduced from thermogravimetric analysis[J]. Acta Petrologica Et Mineralogica, 2014, 33(2): 391-396.
[18] 刘欣伟, 冯雅丽, 李浩然. 菱镁矿热分解微分方程的建立[J]. 无机盐工业, 2011, 43(11): 15-18.
LIU Xin-wei, FENG Ya-li, LI Hao-ran. Establishment of differential equations of magnesite thermal decomposition[J]. Inorganic Chemicals Industry, 2011, 43(11): 15-18.
[19] 李 歌, 李增和, 马鸿文, 陈登利. 热重分析法研究氢氧化镁纳米粉体的非等温分解动力学[J]. 化工学报, 2014(2): 576-582.
LI Ge, LI Zeng-he, MA Hong-wen, CHEN Deng-li. Non-isothermal decomposition kinetics of nano-Mg(OH)2 using thermal gravimetric analysis[J]. CIESC Journal, 2014(2): 576-582.
[20] 杨华明, 杨武国, 胡岳华, 邱冠周. 一水硬铝石的热分解反应动力学[J]. 中国有色金属学报, 2003, 13(6): 1523-1527.
YANG Hua-ming, YANG Wu-guo, HU Yue-hua, QIU Guan-zhou. Kinetics analysis of thermal decomposition reaction of diaspore[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(6): 1523-1527.
[21] LIU Xin-wei, FENG Ya-li, LI Hao-ran. Thermal decomposition kinetics of magnesite from thermogravimetric data[J]. Journal of Thermal Analysis and Calorimetry, 2012, 107(1): 407-412.
[22] 胡荣祖, 高胜利, 赵凤起. 热分析动力学[M]. 2版. 北京: 科学出版社, 2008: 48.
HU Rong-zu, GAO Sheng-li, ZHAO Feng-qi. Thermal analysis kinetics[M]. 2nd Ed. Beijing: Science Press, 2008: 48.
(编辑 王 超)
收稿日期:2014-11-17;修订日期:2015-03-16
通信作者:孙春宝,教授,博士;电话:010-62334953;E-mail: suncb@ustb.edu.cn