DOI: 10.11817/j.issn.1672-7207.2015.07.003
盐酸头孢吡肟的合成
彭东明1, 2,王晓红1,刘艳飞1,尹伟成2,刘珍宝3,张航1,张姗姗1
(1. 中南大学 化学化工学院,湖南 长沙,410083;
2. 湖南中医药大学 药学院,湖南 长沙,410208;
3. 中南大学 药学院,湖南 长沙,410013)
摘要:以7-氨基头孢烷酸(7-ACA)为原料,二氯甲烷和环己烷为溶剂,三甲基氯硅烷(TMSCl)为保护剂,与N-甲基吡咯烷(NMP)和三甲基碘硅烷(TMSI)制成的季铵盐中间体反应,脱保护后成盐得中间体7-氨基-3-(1-甲基-1-四氢吡咯)甲基-3-头孢-4-羧酸盐酸盐(7-MPCA),再与苯并噻唑硫醇活性酯反应,合成目标产物盐酸头孢吡肟。研究硅烷化反应、碘代反应和成盐反应的主要影响因素,进行工艺参数优化。采用红外光谱、元素分析、核磁共振谱和质谱对产物盐酸头孢吡肟进行结构表征。研究结果表明:该工艺原材料易得,反应条件温和,且操作简单,以7-ACA的质量计盐酸头孢吡肟的总收率为74.6%。
关键词:7-氨基头孢烷酸;盐酸头孢吡肟;药物合成;抗生素
中图分类号:R914.5 文献标志码:A 文章编号:1672-7207(2015)07-2405-07
Synthesis of cefepime hydrochloride
PENG Dongming1, 2, WANG Xiaohong1, LIU Yanfei1, YIN Weicheng2, LIU Zhenbao3,
ZHANG Hang1, ZHANG Shanshan1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, China;
3. School of Pharmacy, Central South University, Changsha 410083, China)
Abstract: The intermediate 7-MPCA was obtained by being salified after deprotection based on the reaction of the intermediate of quaternary ammonium salt produced by N-methyl pyrrolidine (NMP) and trimethyliodosilane (TMSI), and 7-aminocephalosporanic acid (7-ACA) was used as raw material, dichloromethane and cyclohexane as solvents, and trimethylchlorosilane (TMSCl) as the protective agent. Cefepime hydrochloride was synthesized from 7-amino-3-[(1-methyl-1-pyrrolidino) methyl]-3-cephem-4-carboxylic acid (7-MPCA) with benzothiazolethiol active ester. The main influencing factors of reactions such as silylation, iodization and salification were analyzed, and the process parameters were optimized. The structure of cefepime hydrochloride was confirmed by IR, elemental analysis, NMR and MS. The results show that the improved synthetic method has some advantages, such as easy availability of raw materials, mild reaction condition, and simple operation. The total yield of cefepime hydrochloride is 74.6% based on the mass fraction of 7-ACA.
Key words: 7-aminocephalosporanic acid; cefepime hydrochloride; pharmaceutical synthesis; antibiotics
盐酸头孢吡肟(cefepime),商品名为马斯平(maxipime),化学名为氯化1-[[(6R,7R)-7-[(2Z)-(2-氨 基-4-噻唑基)-2-(甲氧亚氨基)乙酰氨基]-2-羧基-8-氧 代-5-硫杂-1-氮杂双环[4,2,0]-辛-2-烯-3-基]甲基]-1-甲基吡咯烷鎓一盐酸盐一水合物。1993年在瑞典首次上市,盐酸头孢吡肟作为第4代氨噻肟型头孢菌素类抗生素,与第3代头孢菌素相比具有更广的抗菌谱[1-2],对革兰氏阳性菌、阴性菌和厌氧菌均有良好的抗菌活性,并且对链球菌、肺炎链球菌的抗菌活性有所增强,能够较好地治疗急性呼吸道感染[3-5],人们对其进行了广泛研究[6-8]。已报道的盐酸头孢吡肟的合成路线较多,如Aburaki等[9-10]以7-ACA为原料,得到的产物为两性离子,此两性离子在室温下不稳定,且最后一步缩合的产率较低,中间体的分离必须过柱,操作繁琐,进行工业化生产成本过高。Walker等[11]以7-ACA为原料, 氟利昂(Freon TF)为溶剂, 经双三甲基硅化物中间体“一勺烩”得头孢吡肟关键中间体7-氨基-3-(1-甲基-1-四氢吡咯)甲基-3-头孢-4-羧酸盐酸盐(7-MPCA),收率为38%(以7-ACA计),化合物7-MPCA再与苯并噻唑硫醇活性酯进行7位酰化反应制得头孢吡肟的盐酸盐,总收率为30.8%。该合成路线条件温和,反应选择性较好,但反应产率不高,使用氟利昂不符合环保要求。Lim等[12-14]对盐酸头孢吡肟的合成工艺进行研究,均以7-ACA为原料,经“一勺烩”合成中间体7-MPCA再与活性酯进行反应制得头孢吡肟的盐酸盐,其中宫平等[13]所得总收率为24.7%,安明等[14]所得总收率为59.4%。本文作者在文献[11]的基础上,参考有关文献[12-18],对其合成工艺进行了优化和改进:1) 以7-ACA为原料,用三甲基氯硅烷(TMSCl)代替传统的六甲基二硅胺烷(HMDS)作为7-ACA的羧基和氨基的保护剂;2) 用二氯甲烷和环己烷代替氟利昂为反应溶剂;3) 将N-甲基吡咯烷(NMP)和三甲基碘硅烷(TMSI)制成季铵盐中间体再与保护好的7-ACA反应制得关键中间体7-MPCA。
1 实验部分
1.1 试剂
实验所用试剂为:7-氨基头孢烷酸(河北中润制药有限公司);三甲基氯硅烷(CP,武汉宝龙化工有限公司);N-甲基吡咯烷(AR,浙江台州清泉医药化工有限公司);环己烷(AR,天津市大茂化学试剂厂);二氯甲烷(AR,湖南师大化学制剂厂);三乙胺(AR,天津市泰兴试剂厂);甲醇(AR,天津市泰兴试剂厂);苯并噻唑硫醇活性酯(AR,国产工业品);丙酮(AR,长沙市湘科精细化工厂);薄层层析硅胶(CP,青岛海洋化工有限公司)。
1.2 合成路线
以7-ACA为原料改进后的合成盐酸头孢吡肟的反应路线如图1所示。
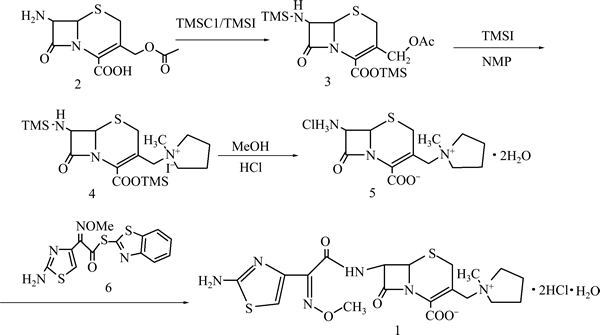
图1 盐酸头孢吡肟的合成路线
Fig. 1 Synthesis route of cefepime hydrochloride
1.3 操作步骤
1.3.1 化合物3的合成
往装有回流冷凝管、干燥管和温度计的150 mL三口烧瓶中加入7-ACA 5.0 g (18.4 mmol)、无水溶剂二氯甲烷或环己烷40 mL,于35 ℃下通入氮气5 min,再加入TMSCl 5.41 g (49.7 mmol)和TMSI 0.11 g (0.6 mmol),在氮气保护的条件下保持该温度反应,采用薄层色谱(TLC)监测反应完全后,减压蒸馏蒸出溶剂,得化合物3(见图1,下同)。
化合物3的1H-NMR(DMSO,500 MHz)化学位移δ:0.23(s,9H,N-Si(CH3)3),0.38(s,9H,COOSi(CH3)3),1.51(d,1H,J=13.6 Hz,NH),2.09(s,3H,COCH3),3.41(q,2H,J=18.3 Hz,SCH2),3.61(q,2H,J=18.3 Hz,SCH2),4.80(dd,1H,J=4.5,13.6 Hz,C-7β-lactam),4.83(q,2H,J=13.2 Hz,CH2OAc),5.11(q,2H,J=13.2 Hz,CH2OAc),4.91(d,1H,J=4.5 Hz,C-6β-lactam)。
1.3.2 化合物5的合成
往干燥的三口烧瓶中加入10 mL二氯甲烷,缓慢加入NMP 2.92 g (29.4 mmol)和TMSI 5.52 g (27.6 mmol),于25 ℃下搅拌2 h,在氮气保护下冰水浴冷却至5 ℃以下,缓慢加入化合物3,不断搅拌并控制反应体系温度低于10 ℃,随后加入5 mL N,N-二乙基苯胺在5 ℃下继续反应2 h,得化合物4。反应结束后,缓慢滴加2.5 mL无水甲醇,控制滴加速度使反应体系温度低于10 ℃,滴加完毕后于5 ℃搅拌10 min,加入浓度为3.21 mol/L的盐酸20 mL,加完后快速升温至25 ℃,快速搅拌至完全分层;静置后,分出水相,往水相中加入适量的活性炭于5 ℃搅拌下脱色30 min,过滤。量取滤液体积并转入250 mL三口烧瓶中,快速搅拌下滴加5倍滤液体积的丙酮,在冰箱中放置过夜,析晶后过滤,并收集析出的固体;用预先冷却的混合液丙酮与水的体积比即V(丙酮):V(水)=5:1洗涤,得淡黄色粉末,真空干燥后称量,得5.80 g淡黄色晶体,收率为85.3%,HPLC测得其纯度为95.3%。
化合物5的1H-NMR(500 MHz,D2O)化学位移δ:2.14~2.32 (m,4H,+N(CH3)CH2CH2CH2CH2),3.00 (s,3H,+NCH3),3.46~3.67(m,5H,+N(CH3)CH2CH2CH2- CH2,SCH2),3.96(q,1H,J=16.9 Hz,SCH2),4.09(q,2H,J=13.9 Hz,—CH2-+N(CH3)CH2CH2CH2CH2)、4.73(q,2H,J=13.9 Hz,—CH2-+N(CH3)CH2CH2-CH2- CH2),5.21(d,1H,J=5.1 Hz,C-7β-lactam),5.41(d,1H,J=5.1 Hz,C-6β-lactam)。
1.3.3 盐酸头孢吡肟(1)的合成
在250 mL的三口烧瓶中加入5.80 g (17.3 mmol)化合物5和116 mL混合溶剂,在氮气保护下搅拌,保持温度在5 ℃以下,滴加三乙胺1.93 g (19.0 mmol);待固体全部溶解,控制pH在6.5左右,分次加入苯并噻唑硫醇活性酯6.10 g (12.5 mmol),于5 ℃下搅拌,TLC跟踪至反应完全。
将反应液转移至分液漏斗中,用蒸馏水萃取后合并水相并加入适量活性炭脱色30 min。将水相过滤并将滤液转入体积为2 L的三口瓶中冰水浴冷却至0~5 ℃,在搅拌下滴加盐酸调节pH至1.0~2.0,量取滤液体积,于5 ℃下加入10倍滤液体积的丙酮并搅拌1 h,冷却过夜,过滤并用混合溶液V(丙酮):V(水)=5:1洗涤,得微黄色固体粉末,真空干燥后称质量,得7.82 g淡黄色晶体,收率为87.4%(以7-ACA为原料计总收率为74.6%),HPLC测其纯度为99.3%。
盐酸头孢吡肟(1):1H-NMR(500 MHz,D2O)化学位移δ:2.07~2.14 (m,4H,+N(CH3)CH2CH2CH2CH2),2.95(s,3H,+NCH3),3.43~3.66(m,5H,+N(CH3)- CH2CH2CH2CH2,SCH2),3.93(s,3H,OCH3),4.02 (q,1H,J=17.5 Hz,SCH2),4.32(q,2H,J=13.5 Hz,—CH2-+N(CH3)CH2CH2CH2CH2),4.61(q,2H,J=13.5 Hz,—CH2-+N(CH3)CH2CH2CH2CH2),5.33(d,1H,J=5.0 Hz,C-7β-lactam),5.87(d,1H,J=5.0 Hz,C-6β-lactam),6.87(s,1H,SCH),7.40~9.96(s,2H,NH2),9.78(d,1H,J=8.0 Hz,NH)
2 结果与讨论
2.1 硅烷化反应
2.1.1 硅烷化试剂的选择
在合成头孢菌素类化合物时,通常要对7-ACA,ACLE及其类似物的4位羧基或7位氨基的活泼氢进行保护。常见保护羧基的方法是将其制成酯类,常用的保护氨基的方法是将其转化成胺的硅衍生物,但需在无水条件下制备。由于7-ACA需同时对氨基和羧基进行保护,故一般选择硅烷化试剂。传统的保护剂为六甲基二硅胺烷(HMDS),虽可有效保护氨基和羧基,但其活性较低,一般要在60~80 ℃下回流8~13 h,反应温度较高,反应时间较长,容易发生副反应。本实验采用价格较低且活泼性较强的三甲基氯硅烷代替传统的六甲基硅烷对7-ACA氨基和羧基同时进行保护,在降低生产成本的同时,大大缩短了反应时间,有利于实现大规模工业化生产。
2.1.2 硅烷化试剂物质的量对反应的影响
在回流反应中保护剂TMSCl会伴随溶剂的挥发逸出反应体系,为保证反应完全,TMSCl的物质的量n要超过理论值。为确定TMSCl与7-ACA的物质的量之比,在其他条件不变的情况下,实验仅改变TMSCl的物质的量,观察反应3 h后7-ACA转化率的情况,结果如表1所示。
表1 n(TMSCl):n(7-ACA)对7-ACA转化率的影响
Table 1 Effect of n(TMSCl):n(7-ACA) on conversion rate of 7-ACA
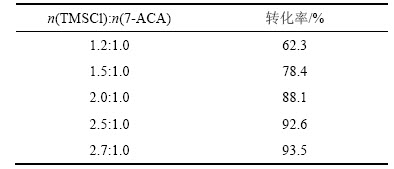
由表1可知:在其他条件相同时,不同物质的量的TMSCl对该步反应影响较大,当n(TMSCl): n(7-ACA)=2.7:1时,其转化率最高,可达93.0%以上。
2.1.3 硅烷化溶剂对反应的影响
选用环己烷和二氯甲烷作溶剂来代替对环境有破坏作用的Freon TF,在节约成本的同时有效降低了对环境的破坏。在其他条件相同的情况下,加入5.0 g 7-ACA,考察不同溶剂对7-ACA转化的影响,结果如表2所示。
表2 不同溶剂对7-ACA转化率的影响
Table 2 Effect of different solvent on conversion rate of 7-ACA

由表2可以看出:该步反应采用二氯甲烷或环己烷为溶剂与采用Freon TF为溶剂的转化率相近,但更经济、环保。
2.2 碘代反应
为提高产品收率,实验中将TMSI与NMP先制成季铵盐再与化合物3进行反应。因此,TMSI与NMP的物质的量之比为影响该步反应的关键因素,在其他条件不变的情况下,用不同物质的量比下制得的季铵盐与化合物3反应,结果如表3所示。
由表3可知:在其他条件相同时,随NMP物质的量的增加,化合物3的转化率也增加,当n(TMSI): n(NMP)=1:1.1时,该步反应转化率达90.2%,继续增加NMP的物质的量,化合物3的转化率并没有显著提高。所以,当n(TMSCl):n(NMP)=1.0:1.1时,其转化率较高,可达90.0%以上。
表3 n(TMSI):n(NMP)对化合物3转化率的影响
Table 3 Effect of n(TMSI):n(NMP) on conversion rate of compound 3

2.3 水解和成盐反应
反应中一般需要对氨基和羧基进行保护,一般采用醇解和酸解来解除保护。先用甲醇对反应物进行水解,再选择一定浓度的盐酸进行水解,得中间体7-MPCA的盐酸盐。而中间体7-MPCA结构中的β-内酰胺环在酸碱环境下都易开环,因此,中间体的盐酸水溶液饱和程度对其有较大影响。若盐酸的浓度太小,则不能使其成盐的pH满足要求;若盐酸的浓度太大,则会破坏中间体的结构。因此,盐酸的浓度对其成盐有较大影响。研究发现:当盐酸浓度为1.07 mol/L和2.14 mol/L时,加入盐酸搅拌后均有固体残留,中间体不能完全成盐酸盐;当盐酸的浓度为3.21 mol/L时,加入盐酸搅拌后固体完全溶解,中间体可以完全形成盐酸盐。
2.4 盐酸头孢吡肟的合成
从中间体7-MPCA为原料,采用“一锅法”合成化合物1。该步反应是酯的氨解,为亲核-消除反应。为进一步考查溶剂对反应的影响,在相同条件下,分别采用不同溶剂进行反应。通过实验可得:当采用V(水):V(丙酮)=1:2混合溶剂时,盐酸头孢吡肟的收率为78.4%;采用二氯甲烷为溶剂时,其收率为72.2%;采用V(二氯甲烷):V(甲醇)=52:9为溶剂时,其收率最高为88.2%。
2.5 结构确证
2.5.1 元素分析
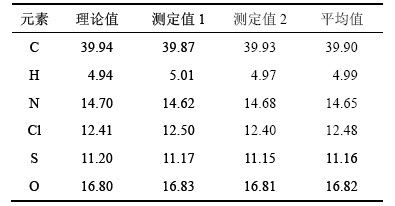
采用元素分析仪对盐酸头孢吡肟(1)样品元素的质量分数进行分析,结果如表4所示。
由表4可知:各元素的质量分数的实测值与理论值基本一致;沉淀滴定法的分析结果证实Cl以阴离子的形式存在于结构中,故合成样品的化学式可表述为C19H24N6O5S2·2HCl·H2O,与盐酸头孢吡肟的化学式一致。
表4 盐酸头孢吡肟的元素分析结果(质量分数)
Table 4 Elemental analysis of cefepime hydrochloride %
2.5.2 紫外光谱(UV)
合成的盐酸头孢吡肟(1)的紫外光谱分析结果如表5所示。
表5 盐酸头孢吡肟的紫外光谱分析结果
Table 5 UV Spectrum of cefepime hydrochloride
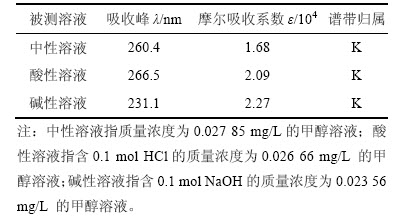
由表5可知:盐酸头孢吡肟(1)中有明显的K吸收带,且在碱性溶液中K吸收带显著紫移,这与盐酸头孢吡肟结构中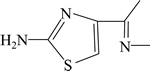 和
和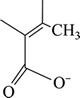 官能团的共轭吸收特征相符。
官能团的共轭吸收特征相符。
2.5.3 质谱(MS)
合成盐酸头孢吡肟(1)样品的质谱图如图2所示。盐酸头孢吡肟的化学式为C19H24N6O5S2·2HCl·H2O,相对分子质量为571.33。由图2可见:在质谱中,没有出现HCl和H2O的峰,因此,不计HCl和H2O的相对分子质量可得盐酸头孢吡肟碱基的质荷比为480。图中质荷比481为[M+H]的分子离子峰,396为[M+H]的分子离子峰失去基团( )后形成的碎片离子的质荷比。
)后形成的碎片离子的质荷比。
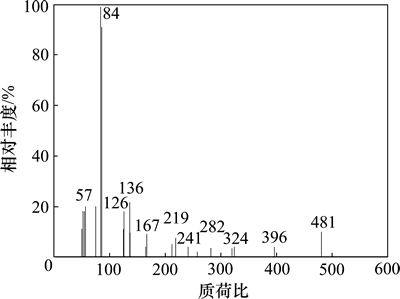
图2 化合物1的MS谱
Fig. 2 MS spectrum of compound 1
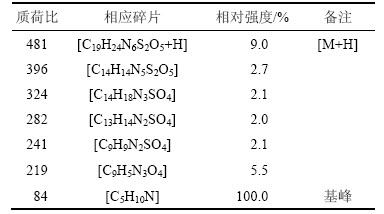
质荷比为324,282,241,219和84各碎片峰都可找到相对应的碎片离子,其中质荷比为84的碎片峰因相对丰度最大为基峰。盐酸头孢吡肟(1)的质谱分析结果如表6所示。
表6 化合物1 MS分析结果
Table 6 MS results of compound 1
2.5.4 核磁共振谱(1H-NMR)
盐酸头孢吡肟(1)的核磁共振氢谱如图3所示。
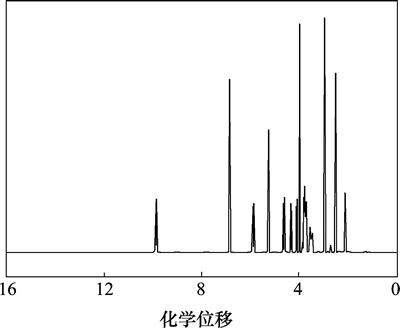
图3 化合物1的1H-NMR谱图
Fig. 3 1H-NMR spectrum of compound 1
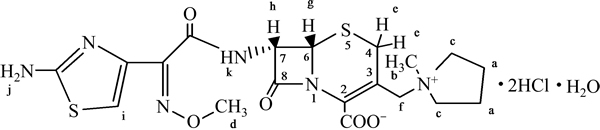
图4 化合物1的结构图
Fig. 4 Structure of compound 1
由图3可知:1H-HMR谱中有13组峰,峰面积积分比为1:1:1:1:1:1:1:1:3:1:4:3:4,除去H(NH2),H(2HCl)和H(H2O)形成极宽峰不易辨认外,其他各峰的化学位移、质子个数和耦合常数等都与盐酸头孢吡肟的结构特征相符。按结构式(如图4所示)不同位置的氢谱分析结果如表7所示。
表7 盐酸头孢吡肟的1H-NMR谱测定结果
Table 7 1H-NMR results of cefepime hydrochloride

3 结论
1) 本实验以7-ACA为原料,二氯甲烷和环己烷为溶剂,三甲基氯硅烷为保护剂,与N-甲基吡咯烷和三甲基碘硅烷制成季铵盐反应,脱保护后成盐合成盐酸头孢吡肟的关键中间体7-MPCA,再与苯并噻唑硫醇活性酯反应,最终生成盐酸头孢吡肟,该合成路线操作简单,对环境污染小,原料易得,产品纯度高,产品总收率能达到74.6%。
2) 以三甲基氯硅烷代替传统的HMDS作硅烷化试剂,缩短了反应时间,减少了副反应的发生,有效提高了工艺效率。
3) 将N-甲基吡咯烷和三甲基碘硅烷制成季铵盐,克服了N原子电子云密度高、反应活性强、副反应多的弊端,提高了产品的收率和纯度。
4) 通过工艺优化得到了最佳的反应条件如下:n(TMSCl):n(7-ACA)=2.7:1.0,n(TMSI):n(NMP)=1.0:1.1;硅烷化反应温度为35 ℃,反应时间3 h;由7-MPCA合成盐酸头孢吡肟,采用的溶剂为二氯甲烷与甲醇混合液的体积比即V(二氯甲烷):V(甲醇)=52:9。
参考文献:
[1] Ohki H, Kawabata K, Inamoto Y, et al. Studies on 3′-quaternary ammonium cephalospoins-Ⅲ synthesis and antibacterial activity of 3′-(3-aminopyrazolium) cephalosporins[J]. Bioorganic & Medicinal Chemistry, 1997, 5(3): 557-567.
[2] 薛雨, 陈宇瑛. 头孢菌素类抗生素的最新研究进展[J]. 中国抗生素杂志, 2011, 36(2): 86-92.
XUE Yu, CHEN Yuying. New development of cephalosporin antibiotics[J]. Chinese Journal of Antibiotics, 2011, 36(2): 86-92.
[3] 龚轶欣. 盐酸头孢吡肟治疗急性呼吸道感染的效果观察[J]. 中外医疗, 2013, 32(9): 108-110.
GONG Yixin. The effect of cefepime hydrochloride in treatment of acute respiratory tract infection[J]. China & Foreign Medical Treatment, 2013, 32(9): 108-110.
[4] 赵贾漪. 盐酸头孢吡肟治疗小儿下呼吸道感染的疗效观察[J]. 医学信息, 2014(5): 310.
ZHAO Jiayi. The effect of cefepime hydrochloride in treatment of acute the children with lower respiratory infection[J]. Medical Information, 2014(5): 310.
[5] Behin S, Punitha I S R, Krishnan S. Stability studies of cefepime hydrochloride by stability indicating RP-HPLC method[J]. International Journal of Pharmaceutical Sciences and Nanotechnology, 2013, 6(3): 2181-2186.
[6] LI Yu, XU Li, WANG Fuan, et al. Solubilities of cefepime hydrochloride in water (Ethanol, 1-Propanol, or 2-Propanol) from (278.15 to 308.15) K [J]. Journal of Chemical & Engineering Data, 2010, 55(9): 4098-4103.
[7] 彭东明, 王春燕, 刘艳飞, 等. 盐酸头孢吡肟缓释微球的制备与工艺优化[J]. 中南大学学报(自然科学版), 2013, 44(3): 907-913.
PENG Dongming, WANG Chunyan, LIU Yanfei, et al. Preparation and optimization of controlled release microspheres of cefepime dihydrochloride[J]. Journal of Central South University (Science and Technology), 2013, 44(3): 907-913.
[8] 郝军香, 李谦和, 彭东明. 头孢吡肟的合成进展[J]. 合成化学, 2004, 12(1): 43-48.
HAQ Junxiang, LI Qianhe, PENG Dongming. Development in synthesis of cefepime[J]. Chinese Journal of Synthetic Chemistry, 2004, 12(1): 43-48.
[9] Aburaki S, Kamachi H, Narita Y, et al. Cephalosporin: DE 3307550[P]. 1983-09-27.
[10] Aburaki S, Kamachi H, Narita Y, et al. Cephalosporins: US 4525473[P]. 1983-06-25.
[11] Walker D G, Brodfuehrer P R, et al. Use of bistrimethylisilylated intermediates in the preparation of semisynthetic 7-amino-3- substituted-cephems. Expedient synthesis of a new 3-[(1-methyl- 1-pyrrolidinio) methyl] cephalosporin[J]. Journal of Organic Chemistry, 1988, 53(5): 983-991.
[12] Lim G M F, Rouble J M. Process for the preparation of a cephalosporin antibiotic: US 15594129[P]. 1997-01-14.
[13] 宫平, 赵燕芳, 冯润良, 等. 盐酸头孢吡肟的合成[J]. 中国药物化学杂志, 2002, 12(6): 350-352.
GONG Ping, ZHAO Yanfang, FENG Runliang, et al. Synthesis of cefepime dihydrochloride[J]. Chinese Journal of Medicinal Chemistry, 2002, 12(6): 350-352.
[14] 安明, 常珍, 高雷, 等. 盐酸头孢吡肟的合成[J]. 中国医药工业杂志, 2004, 35(9): 515-516.
AN Ming, CHANG Zhen, GAO Lei, et al. Synthesis of cefepime hydrochloride [J]. Chinese Journal of Pharmaceuticals, 2004, 35(9): 515-516.
[15] 周磊, 樊会芳. 一种盐酸头孢吡肟的合成方法: CN 101735251 A[P]. 2009-12-26.
ZHOU Lei, FAN Huifang. A synthesis method of cefepime hydrochloride: CN 101735251 A[P]. 2009-12-26.
[16] Page N, Stevenson R, Powell M. Analysis of N-methylpyrrolidine in cefepime hydrochloride by ion chromatography using suppressed conductivity detection with solid-phase extraction pre-treatment[J]. The Royal Society of Chemistry, 2014, 6(4): 1248-1253.
[17] 唐双强. 第四代头孢菌素盐酸头孢吡肟及中间体合成条件的研究[D]. 石家庄: 河北科技大学化学与制药工程学院, 2008: 14-18.
TANG Shuangqiang. Study on the synthetical technnics of cefepime hydrochloride and it’s intermediate[D]. Shijiazhuang: Hebei University of Science and Technology. College of Chemistry and Pharmaceutical Engineering, 2008: 14-18.
[18] Subramanian N H, Thyagarajan S, Manigandan P, et al. An improved ion chromatography method for fast and sensitive determination of N-methylpyrrolidine in cefepime hydrochloride[J]. Journal of Chromatography Science, 2009, 47(8): 549-552.
(编辑 刘锦伟)
收稿日期:2014-11-24;修回日期:2015-01-13
基金项目(Foundation item):国家自然科学基金资助项目(81301258);湖南省科技计划项目(2012SK3134);湖南省自然科学基金资助项目(13JJ3099) (Project(81301258) supported by the National Natural Science Foundation of China; Project(2012SK3134) supported by Science and Technology Program of Hunan Province; Project(13JJ3099) supported by the Natural Science Foundation of Hunan Province)
通信作者:刘艳飞,博士,副教授,从事药物合成研究;E-mail: liuyf@csu.edu.cn