方锑矿在煅烧/熔解温度下和氮气气氛中的挥发动力学
来源期刊:中国有色金属学报(英文版)2016年第1期
论文作者:A. ARACENA O. JEREZ C. ANTONUCCI
文章页码:294 - 300
关键词:方锑矿;挥发速率;煅烧温度;熔解温度;动力学
Key words:senarmontite; volatilization rate; roasting temperature; melting temperature; kinetics
摘 要:采用热重分析方法,利用1.3 mL陶瓷坩埚(内径11 mm,高14 mm),在不同气体流速下研究方锑矿在中性气氛中和煅烧(550~615 °C)/熔解(660~1100 °C)温度下的挥发动力学。分析颗粒尺寸的影响。部分反应样品的失重分析和X射线衍射分析结果以及热力学研究表明,在整个温度范围内,方锑矿不形成其他化合物,直接以Sb 4O6的形式挥发。在煅烧温度下,Sb2O3的挥发动力学方程可用X=kappt描述。挥发反应受表面化学反应控制,活化能为193.0 kJ/mol。根据方锑矿在溶解温度下的挥发反应动力学方程呈线性关系的特点,确定了挥发反应的动力学常数,得到表面积为8.171×10-5 m2的挥发反应的活化能为73.9 kJ/mol。
Abstract: The volatilization kinetics of senarmontite (Sb2O3) was analyzed in a neutral atmosphere in two temperature ranges: 550-615 °C (roasting temperature) and 660-1100 °C (melting temperature) by using a thermogravimetric analysis method under various gas flow rates and using a 1.3 mL ceramic crucible (11 mm in internal diameter and 14 mm in height). The effect of particle size was also analyzed. The experimental results of mass loss data, X-ray diffraction (XRD) analysis of partially reacted samples and thermodynamic studies indicate that the senarmontite becomes volatile in the form of Sb4O6(g) without the formation of any intermediary compound in the entire temperature range. At roasting temperatures, the volatilization kinetics of Sb2O3 was analyzed using the model X=kappt. The volatilization reaction was controlled by the surface chemical reaction and an activation energy value of 193.0 kJ/mol was obtained in this temperature range. Based on the volatilization kinetics at the melting temperatures, for linear behaviour in nitrogen gas, kinetic constants were determined, and an activation energy of 73.9 kJ/mol was calculated for the volatilization reaction with a surface area of 8.171×10-5 m2.
A. ARACENA1, O. JEREZ2, C. ANTONUCCI1
1. Escuela de Ingeniería Química, Pontificia Universidad Católica de Valparaíso, General Cruz 34, Valparaíso, Chile;
2. Instituto de Geología Económica Aplicada (GEA), Universidad de Concepción, Casilla 160-C, Concepción, Chile
Received 29 January 2015; accepted 26 August 2015
Abstract: The volatilization kinetics of senarmontite (Sb2O3) was analyzed in a neutral atmosphere in two temperature ranges: 550-615 °C (roasting temperature) and 660-1100 °C (melting temperature) by using a thermogravimetric analysis method under various gas flow rates and using a 1.3 mL ceramic crucible (11 mm in internal diameter and 14 mm in height). The effect of particle size was also analyzed. The experimental results of mass loss data, X-ray diffraction (XRD) analysis of partially reacted samples and thermodynamic studies indicate that the senarmontite becomes volatile in the form of Sb4O6(g) without the formation of any intermediary compound in the entire temperature range. At roasting temperatures, the volatilization kinetics of Sb2O3 was analyzed using the model X=kappt. The volatilization reaction was controlled by the surface chemical reaction and an activation energy value of 193.0 kJ/mol was obtained in this temperature range. Based on the volatilization kinetics at the melting temperatures, for linear behaviour in nitrogen gas, kinetic constants were determined, and an activation energy of 73.9 kJ/mol was calculated for the volatilization reaction with a surface area of 8.171×10-5 m2.
Key words: senarmontite; volatilization rate; roasting temperature; melting temperature; kinetics
1 Introduction
Roasting processes are carried out at normal temperatures, allowing the minerals and/or concentrates to maintain a solid structure, without melting or becoming synthesized, in order to ensure efficient transformation of the compounds. Melting processes, on the other hand, are conducted at temperatures above the melting point, allowing the compounds to melt and combine into a single liquid phase.
Antimony is found mainly in the form of stibnite (Sb2S3) and senarmontite (Sb2O3). One of the main sources in senarmontite production is stibnite roasting. During the conventional roasting or melting/conversion of the concentrates, the antimony is eliminated through several stages, mainly in the gas phase, as the antimony and some of the oxides and sulphides are volatile at roasting and melting temperatures. In practice, retention of a significant fraction of antimony does not occur in the condensed phase. Therefore, knowledge of the chemistry and kinetics of senarmontite volatilization is very important when controlling the Sb retained in the gas phase.
Some studies have reported the mechanism of oxidation/volatilization of antimony minerals at roasting or melting temperatures. PADILLA et al [1] showed that between 350 and 500 °C, and for oxygen partial pressures between 1.01 and 21.28 kPa, the stibnite is fully oxidized to produce valentinite (Sb2O3). This oxidation occurs very rapidly even at low oxygen partial pressure. In this temperature range, the technique of thermogravimetry was used to prove that the valentinite did not become volatile, even for high oxygen partial pressure and long roasting time. This research did not explore the mechanism of volatilization and/or oxidation of the product formed, Sb2O3, at higher temperatures.
ZIVKOVIC et al [2] studied stibnite oxidation through the consecutive process of DTA-TG-DTG in air and in a temperature range of 200 to 800 °C. This research also concluded that the stibnite was oxidized to valentinite with the consecutive over-oxidation to cervantite (SbO2). Unfortunately, the oxygen concentration in the atmosphere was not varied to study the volatilization of the valentinite or cervantite. However, PADILLA et al [3] studied the volatilization of antimony sulphide and oxide compounds in nitrogen- oxygen atmospheres in a temperature range of 700 to 1150 °C using isothermal experiments. The results indicate that in the presence of oxygen, the antimony can become volatile as Sb2O3 in a temperature range of 900 to 950 °C in the gas phase with 1% to 5% oxygen. However, at high oxygen concentrations, the antimony volatilization was stopped by the formation of the non-volatile compound, SbO2. In highly oxidizing atmospheres, antimony volatilization took place only at high temperatures (above 1150 °C), where the SbO2 became an unstable compound and decomposed into SbO(g) and O2(g).
Finally, studies conducted by ASRYAN et al [4,5] through the use of Knudsen cell mass spectrometry measurements show that for the Sb-O system, specifically a composition of 70% Sb + 30% Sb2O3 (mole fraction) at a temperature of 650 °C, the mixture sublimation process leads to the production in a gas phase of 1.2×10-4 Pa Sb4 and 0.13 Pa Sb4O6. As all ionic currents (Sb4O6 partial pressure) remained constant until the complete sublimation of the Sb2O3 sample and since there was no significant amount of non-volatile residue, these results suggest that Sb2O3 sublimation produces the gaseous compound, Sb4O6(g). Experiments were also conducted with a mixture of Sb2O3+Sb2O4 at the same temperature, showing that the same compound was produced in the gas phase, i.e., Sb4O6.
Considering the above, the present research aims to study the volatilization rate of senarmontite with different particle sizes in a nitrogen atmosphere in order to determine the volatilization kinetics in a temperature range of 550 to 1100 °C.
2 Thermodynamic considerations
The Gibbs free energy values used for all antimony compounds were obtained from the HSC Chemistry database [6]. The antimony in the senarmontite can become volatile through thermal decomposition in an inert atmosphere, mainly in the form of the compounds: Sb4O6, SbO, Sb, Sb2, Sb3 and Sb4 [6]. The stability of each one depends on the activity of the antimony in the gas phase. The following global reactions are given for the thermal decomposition of the senarmontite in a neutral atmosphere:
2Sb2O3=Sb4O6(g) (1)
1/2Sb2O3=SbO(g)+1/4O2(g) (2)
1/2Sb2O3=Sb(g)+3/4O2(g) (3)
Sb2O3=Sb2(g)+3/2O2(g) (4)
3/2Sb2O3=Sb3(g)+9/4O2(g) (5)
2Sb2O3=Sb4(g)+3O2(g) (6)
The equilibrium constants for Reactions (1) to (6), calculated using the Gibbs free energy data, are shown in Fig. 1. It can be seen that the least thermodynamically favourable reaction is Reaction (6), while Reaction (1), which promotes antimony volatilization as Sb4O6, predominates the other reactions. Therefore, Reaction (1) is the thermodynamically favourable reaction of senarmontite decomposition in the temperature range of 550 to 1100 °C. This mechanism was also proposed by ASRYAN et al [4].
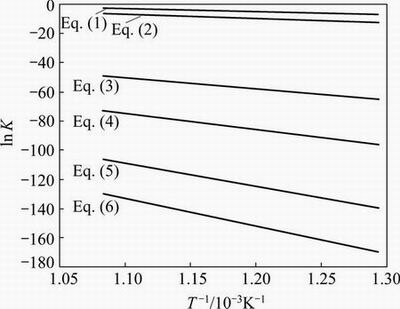
Fig. 1 Equilibrium constants in Sb-O system
3 Experimental
The synthetic senarmontite samples used for all the experiments in this study were obtained from Sigma Aldrich, and consisted of a fine powder (below 5 μm) with a purity of 98%. Pelleting (through the use of controlled pressure) was used for the experiments with larger particle sizes, thus obtaining spheres measuring 12, 16 and 24 μm.
The experiments were carried out in a conventional thermogravimetry apparatus, consisting of a quartz reaction tube (45 mm in internal diameter) mounted vertically within an oven, an electronic balance with a data acquisition system mounted on the reaction tube, and a gas distribution system to provide the necessary nitrogen atmosphere. The sample temperature was measured using a chromel-alumel thermocouple placed in the area of constant temperature within the reaction tube, which is around 90 mm in length in the centre of the tube. Prior to the volatilization experiments, the thermogravimetric system was tested to verify its correct functioning by heating active carbon of a known chemical composition in pure nitrogen and in oxidizing atmospheres.
The experiments were carried out with a sample of senarmontite of approximately 50 mg in a 1.3 mL ceramic crucible (11 mm in internal diameter and 14 mm in height). The experiments began by heating the reaction tube to the desired temperature and filling the oven with nitrogen gas to ensure the neutral atmosphere. Once the temperature had stabilized, the crucible with the sample was placed inside the reaction tube suspended from the balance by a quartz chain. The instantaneous mass loss of the sample was registered as a function of time every 3 s. The total reaction system pressure was maintained at atmospheric pressure (101.3 kPa). The reaction solids were analyzed by XRD to identify the solid reaction products. In some cases, partially reacted samples were obtained at certain reaction time in order to identify possible intermediary compounds formed during the senarmontite volatilization process. The sampling procedure consisted of rapidly raising the crucible to the upper part of the reaction tube where the sample was interrupted with a nitrogen flow. The cooling time in the upper part of the tube was around 20 s. The sample was then quickly moved from the reaction tube to be cooled in a desiccator.
4 Results and discussion
4.1 Preliminary experiments
The senarmontite volatilization kinetics results are discussed below. These results were obtained in experiments carried out under conditions in which the global reaction was not controlled by internal mass transfer. In order to achieve this, the kinetics parameters were determined directly in experiments using small 50 mg sample of Sb2O3 powder at different gas velocities. Figure 2 shows the results obtained at different gas flow rates in the range of 0.6-1.5 L/min. The data are graphed as sample mass loss fraction, defined as (mo-mt)/mo, where mo is the initial mass of the sample and mt is the mass at time t.
Figure 2 shows that the gas flow rate does not affect the senarmontite volatilization rate. This result clearly indicates that within the gas flow range used, no mass transfer restrictions are present in the volatilization when using a shallow senarmontite sample. Therefore, the subsequent experiments used 50 mg samples of Sb2O3 and a gas flow rate of 1.0 L/min. All experiments were also isothermal and in a neutral atmosphere (with nitrogen).
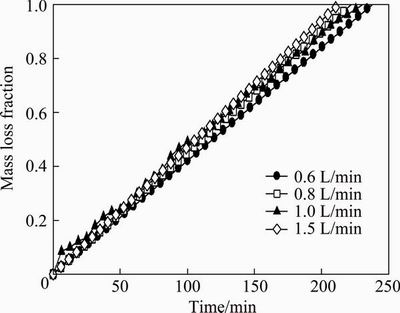
Fig. 2 Senarmontite mass loss fraction as function of time at different gas flow rates
In studying gas-solid reactions, we concerned with four phenomena (external mass transfer, pore diffusion, adsorption/desorption, and chemical reaction) as well as with several other phenomena that may affect the progress of reaction. In addition, knowledge of the effect of each phenomenon involves the development and performance of industrial equipment in which gas-solid reactions are carried out.
4.2 Effect of reaction temperature
The analysis then turned to the effect of temperature on the senarmontite volatilization rate in a nitrogen atmosphere and in two temperature ranges: 550 to 615 °C and 660 to 1100 °C. These temperature ranges were considered to be appropriate as the senarmontite melted at 655 °C [7,8]. Therefore, the first range is roasting temperature and the second is melting temperature.
The experimental results are shown in the form of sample mass loss fraction versus reaction time. Figure 3 shows the results for the temperature range of 550- 615 °C, while Fig. 4 shows the results for 660-1100 °C. These figures show that the volatilization rate increases considerably as the temperature increases. For both figures, the volatilization rate is rapid and then reaches a maximum mass loss, i.e., at 600 °C, the maximum mass loss is reached at 150 min, while at 800 °C, the maximum mass loss fraction is reached at 10 min. It should also be noted that although the Sb2O3 sample melts at 655 °C, the mass loss curves obtained at temperatures above the melting point show behaviour that is analogous to the curves below the melting point. Therefore, it seems that the reaction mechanism is the same regardless of the state of the Sb2O3 (i.e., whether the Sb2O3 is in a liquid or solid state). The strong dependence of volatilization rate on the reaction temperature suggests that the volatilization of senarmontite is controlled by a chemical reaction on the surface.
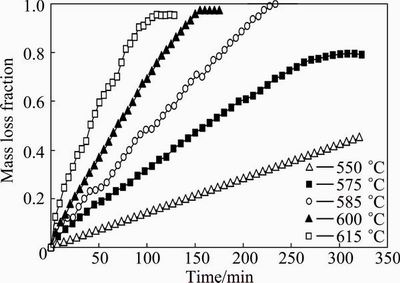
Fig. 3 Senarmontite volatilization as function of time for particle size of 5 μm and temperature range of 550-615 °C
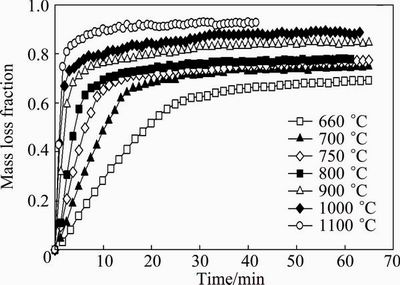
Fig. 4 Senarmontite volatilization as function of time for particle size of 5 μm and temperature range of 660-1100 °C
In addition, Figs. 3 and 4 show similar straight line behaviour for the senarmontite volatilization. In order to check whether any compound is formed by the thermal decomposition of the senarmontite, a partially reacted sample was taken from the experiment conducted at 600 °C and a time of 70 min. This sample was subjected to XRD analysis. The results are shown in Fig. 5, where senarmontite diffraction lines can be seen. Therefore, these results confirm that the volatilization of senarmontite takes place in accordance with Reaction (1). The progression of the reaction can therefore be determined with precision using the experimental mass loss fraction data.
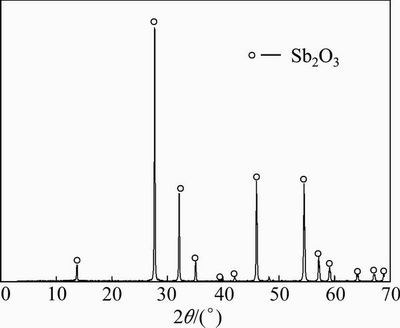
Fig. 5 XRD pattern of sample from experiment time of 70 min and temperature of 600 °C
Samples were also taken for XRD analysis at a temperature of 800 °C and a reaction time of 8 min. The results were identical to the diffractograms shown in Fig. 5, identifying only senarmontite. This clearly indicates that the Sb2O3 follows the same volatilization mechanism to form Sb4O6 in the gas phase, as shown in Reaction (1).
4.3 Effect of particle size
Figure 6 shows the effect of particle size on the volatilization rate for four different particle sizes: 5, 12, 16 and 24 μm, at a temperature of 600 °C. The results observed in the figure were expected, showing a significant effect of particle size on the volatilization rate. Thus, for a particle size of 5 μm, the mass loss fraction is half at 70 min, whereas a size of 12 μm gives the same fraction after 160 min.

Fig. 6 Effect of particle size on Sb2O3 volatilization at temperature of 600 °C
The increased senarmontite volatilization rate is mainly due to the increase in particle interface area. Therefore, these results justify the proposition that senarmontite volatilization is controlled by a chemical reaction on the surface, and not based on diffusion processes.
4.4 Sb2O3 volatilisation kinetics below 655 °C
The experimental data obtained for the senarmontite volatilization were analyzed using the well-known kinetics model shown in Eq. (7), assuming dependence on a surface chemical reaction [9].
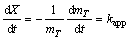 (7)
(7)
or
X=kappt (8)
with
 (9)
(9)
In these equations, X is the fractional conversion, kapp is the apparent rate constant and mt is the theoretical mass loss according to the stoichiometry of Reaction (1). The dependence of the apparent rate constant on temperature and particle size is given by the following general expression:
 (10)
(10)
where k′ is the intrinsic rate constant and ro is the initial Sb2O3 particle radius.
The experimental data shown in Fig. 3 were used to create the graph of X in Fig. 7 as a function of time in the temperature range of 550 to 615 °C for senarmontite samples with a particle size of 5 μm. This figure shows a very good linear fit for the kinetics data with regression coefficients, R2, from 0.98 to 0.99 in the entire temperature range. This good correlation fit suggests the applicability of Eq. (8). The apparent kinetics constant values at different temperatures were obtained from the gradients of the straight lines and are shown in Table 1.

Fig. 7 Senarmontite volatilization rate in temperature range of 550-615 °C at particle size of 5 μm
Table 1 Apparent rate constant for senarmontite volatilization
![说明: C:\Users\Administrator\AppData\Roaming\Tencent\Users\943171351\QQ\WinTemp\RichOle\W}LD7OVQK9Y][X`I)%D(MA8.png](/web/fileinfo/upload/magazine/12511/310847/image022.jpg)
When the volatilization rate is controlled by chemical reaction on the surface of the particle, k′ must vary linearly with the inverse of the initial particle radius (ro), as shown in Eq. (10). In order to verify this dependence, the particle size data (Fig. 6) were fitted in accordance with Eq. (8) and the result of this fit is shown in Fig. 8. A high linear fit can be seen for the four particle sizes, reaching values of R2 above 0.98.
The values of kapp obtained from this figure were then graphed in Fig. 9 as a function of the inverse of the initial Sb2O3 particle radius. As can be seen in this figure, there is adequate linear dependence for the data (R2>0.98), thus justifying the proposed kinetics model.
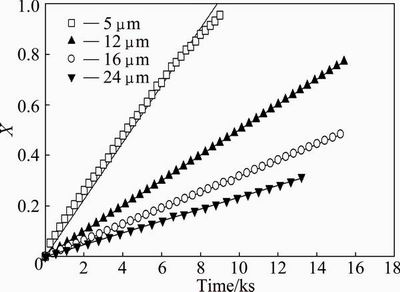
Fig. 8 Dependence of model on Sb2O3 volatilization for temperature of 600 °C
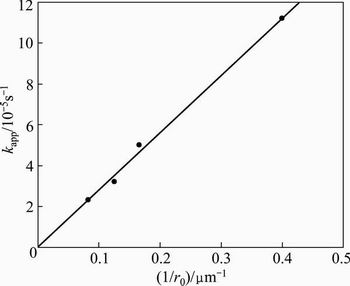
Fig. 9 Dependence of apparent rate constants on inverse of initial particle size
The intrinsic rate constant k′ at different temperatures was calculated using the apparent kinetics constants from Fig. 7 and the particle radius of 2.5 μm. Table 2 shows both the intrinsic and apparent rate constants for the temperature range in question.
Table 2 Senarmontite volatilization rate constants in temperature range of 550 to 615°C
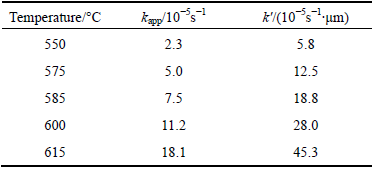
The calculated values of the intrinsic constants k′ were then used to draw the Arrhenius plot shown in Fig. 10. This figure shows a good linear fit (R2>0.99) for the intrinsic kinetics constants at each temperature. The activation energy (Ea) was calculated to be 193.0 kJ/mol in the temperature range of 550 to 615 °C. This Ea value is typical of a reaction controlled by a surface chemical reaction.
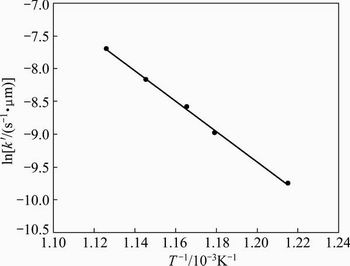
Fig. 10 Arrhenius plot for Sb2O3 volatilization at temperatures between 550 and 615 °C
Therefore, the senarmontite volatilization kinetics below the melding temperature can be represented by the following equation:
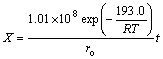 (11)
(11)
where R equals 8.314 J/(mol·K), ro is in μm, t is in s and k′ =1.01×108 μm/s.
4.5 Sb2O3 volatilization kinetics above 655 °C
In the temperature range of 660 to 1100 °C, Fig. 4 shows linear behaviour for the senarmontite volatilization, and therefore the volatilization rate can be described by the following equation [3]:
 (12)
(12)
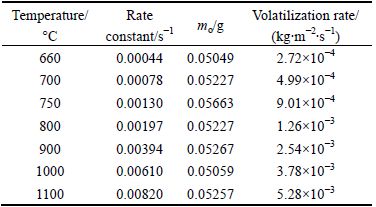
where Δm is the mass loss during interval t and S is the sample interface surface. Thus, using the gradients of the mass loss fraction curves in Fig. 4, the rate constants were calculated and are shown in Table 3. The Sb2O3 volatilization rate is also given in the table.
Using the rate constants in Table 3, the Arrhenius plot shown in Fig. 11 was created. The gradient of the straight line was in turn used to calculate the activation energy, obtaining a value of 73.9 kJ/mol for the temperature range of 660 to 1100 °C for the senarmontite volatilization in a nitrogen atmosphere in accordance with Reaction (1). The relatively low activation energy may be because some of the energy were consumed and the molecules to be melted, allowing less energy is needed for the volatilization of the species involved.
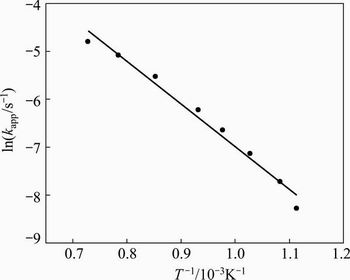
Fig. 11 Arrhenius plot for Sb2O3 volatilization at temperatures between 660 and 1100 °C
Table 3 Senarmontite volatilization rate constants in temperature range of 660-1100 °C for samples with surface area of 8.171×10-5 m2
5 Conclusions
1) Senarmontite volatilization in a neutral atmosphere in a temperature range of 550 to 1100 °C occurred by a single step with production of Sb4O6(g).
2) Temperature and particle size both significantly affect the senarmontite volatilization rate.
3) In a temperature range of 550-615 °C, the senarmontite volatilization kinetics was analyzed using the model, X=kappt, identifying the dependence of the volatilization rate on temperature.
4) It was concluded that the volatilization reaction is controlled by the surface chemical reaction. The activation energy was calculated to be 193.0 kJ/mol for the temperature range of 550 to 615 °C.
5) At temperatures above the melting point of senarmontite (>655 °C), the activation energy was calculated to be 73.9 kJ/mol.
References
[1] PADILLA R, ARACENA A, RUIZ MAR?A C. Kinetics of stibnite (Sb2S3) oxidation at roasting temperaturas [J]. Journal of Mining and Metallurgy, 2014, 50: 127-132.
[2] ZIVKOVIC Z, STRBAC N, GRUJICIC D, BOYANOV B. Kinetics and mechanism of Sb2S3 oxidation process [J]. Thermochimica Acta, 2002, 383: 137-143.
[3] PADILLA R, RAMIREZ G, RUIZ MAR?A C. High-temperature volatilization mechanism of stibnite in nitrogen-oxygen atmospheres [J]. Metallurgical and Materials Transactions B, 2010, 41: 1284-1292.
[4] ASRYAN N A, ALIKHANYAN A S, NIPAN G D. p-T-x phase diagram of the Sb-O system [J]. Inorganic Materials, 2004, 40: 626-631.
[5] ASRYAN N A, ALIKHANYAN A S, NIPAN G D. Specifics of sublimation of antimony oxides [J]. Doklady Physical Chemistry, 2003, 392: 221-226.
[6] ROINE A. HSC chemistry 6.1 [M]. Pori, Finland: OutoKumpu Research Py, 1999.
[7] BARIN I. Thermochemical data of pure substances [M]. Weinheim: VCH Verlagsgesellschaft, 1995.
[8] PANKRATZ L. Bulletin No. 672 [M]. Washington: U.S. Bureau of Mines, 1982.
[9] PADILLA R, ARACENA A, RUIZ MAR?A C. Reaction mechanism and kinetics of enargite oxidation at roasting temperatures [J]. Metallurgical and Materials Transactions B, 2012, 43: 1119-1126.
A. ARACENA1, O. JEREZ2, C. ANTONUCCI1
1. Escuela de Ingeniería Química, Pontificia Universidad Católica de Valparaíso, General Cruz 34, Valparaíso, Chile;
2. Instituto de Geología Económica Aplicada (GEA), Universidad de Concepción, Casilla 160-C, Concepción, Chile
摘 要:采用热重分析方法,利用1.3 mL陶瓷坩埚(内径11 mm,高14 mm),在不同气体流速下研究方锑矿在中性气氛中和煅烧(550~615 °C)/熔解(660~1100 °C)温度下的挥发动力学。分析颗粒尺寸的影响。部分反应样品的失重分析和X射线衍射分析结果以及热力学研究表明,在整个温度范围内,方锑矿不形成其他化合物,直接以Sb 4O6的形式挥发。在煅烧温度下,Sb2O3的挥发动力学方程可用X=kappt描述。挥发反应受表面化学反应控制,活化能为193.0 kJ/mol。根据方锑矿在溶解温度下的挥发反应动力学方程呈线性关系的特点,确定了挥发反应的动力学常数,得到表面积为8.171×10-5 m2的挥发反应的活化能为73.9 kJ/mol。
关键词:方锑矿;挥发速率;煅烧温度;熔解温度;动力学
(Edited by Yun-bin HE)
Corresponding author: A. ARACENA; E-mail: alvaro.aracena@ucv.cl
DOI: 10.1016/S1003-6326(16)64117-1

