J. Cent. South Univ. Technol. (2008) 15: 669-673
DOI: 10.1007/s11771-008-0124-6
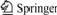
Anode oxidation of HCHO in
THPED-containing electroless copper plating solution
ZHENG Ya-jie(郑雅杰)1, XIAO Fa-xin(肖发新)1, 2, ZOU Wei-hong(邹伟红)1, WANG Yong(王 勇)1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Materials Science and Engineering, Henan University of Science and Technology,
Luoyang 471003, China)
Abstract: The electrochemical mechanism of anode oxidation of HCHO in electroless copper plating solution with N, N, N′, N′- tetrakis(2-hydroxypropyl)ethylenediamine (THPED) was investigated by measuring cyclic voltammetry curves and anodic polarization curves. Three different oxidation peaks occur at the potentials of -0.62 V (Peak 1), -0.40 V (Peak 2) and -0.17 V (Peak 3) in the anode oxidation process of THPED-containing solution. The reaction at Peak 1, a main oxidation reaction, is the irreversible reaction of adsorbed HCHO with hydrogen evolution. The reaction at Peak 2, a secondary oxidation reaction, is the quasi-reversible reaction of adsorbed HCHO without hydrogen evolution. The reaction at Peak 3 is the irreversible oxidation of anode copper. The current density of Peak 1 increases gradually, that of Peak 2 remains constant and that of Peak 3 decreases with the increase of HCHO concentration. The current density of Peak 3 increases with the increase of THPED concentration and the complexation of THPED promotes the dissolution of anode copper.
Key words: electroless copper plating; anode oxidation; THPED; HCHO; electrochemical mechanism
1 Introduction
Electroless copper plating is broadly applied in the hole-metallization of printed circuit board(PCB). At present, the potassium sodium tartrate and EDTA·2Na are usually used as chelator in electroless copper plating due to the low costs[1-3]. However, the plating speed is low and the solution is unstable[1-3]. Recently, with the rapid development of PCB industry, the depth of board increases and the diameter of hole sharply lessens, which needs high plating speed and good stability of plating solution[4-5]. Therefore, it is necessary to seek for a new plating solution to satisfy the higher requirement of PCB technology[6].
N, N, N’, N’-tetrakis(2-hydroxypropyl)ethylene- diamine (THPED) was reported as the chelator in electroless copper plating solution overseas[7-9]. The THPED- containing plating solution is stable and the depositing speed is rapid. In addition, the coating from that solution has fine electrical performance and mechanical properties[7-9]. Therefore, it is proposed to use as chelator in electroless copper plating solution in future. There is few reports concerning THPED- containing electroless plating solution in China although the solution has such advantages. And the study on its depositing mechanism has never been reported. The main traditional mechanisms of electroless copper plating include the atom hydrogen theory, hydride transmission mechanism, metal hydroxide mechanism and electrochemical mechanism. In these theories, the electrochemical mechanism based on the mixed potential has been adopted by most people. In this work, the electrochemical curves were measured to investigate the mechanism of anode oxidation of HCHO in THPED- containing electroless copper plating solution.
2 Experimental
2.1 Experimental instrument and reagents
The experimental instruments were as follows: CHI-660A electrochemical workstation, H-style cell, salt bridge (with Luggin capillaries), working electrode (copper electrode, 1.5 mm2), auxiliary electrode (Pt electrode, 234 mm2), reference electrode (Saturated Calomel Electrode, SCE).
The reagents were as follows: CuSO4·5H2O, THPED, α, α′-bipyridyl, 2-MBT, PEG-1000, HCHO, NaOH, KCl, double distilled water. All the reagents are analytical pure.
2.2 Experimental process
Prior to the experiments, copper electrode was polished by sand paper, and then immersed in acetone for for degreasing, dilute hydrochloric acid for activation and distilled water for washing. The electroless copper plating solution was placed in the H-style cell. The cyclic voltammetry(CV) curves and anode polarization curves were measured under different conditions on the CHI-660A electrochemical station.
3 Results and discussion
3.1 Characteristic of cyclic voltammetry curves
The CV tests were performed at the scan rate of 10 mV/s in the solution of 16.8 g/L THPED, 5 mg/L α, α′- bipyridyl, 1 g/L PEG-1000(polyethylene glycol) and 2 mg/L 2-MBT(2-mercaptobenzo-thiazole) at the pH value of 13.0. The result is shown in Fig.1. The CV curve of sample with 5.0 g/L HCHO is shown in Fig.2.
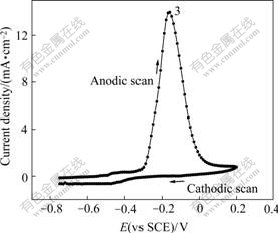
Fig.1 CV curve of sample without HCHO
Fig.2 CV curve of sample with 5 g/L HCHO
It can be seen from Fig.1 that there is a sharp oxidation peak (Peak 3) at -0.17 V approximately in the CV curve of solution without HCHO. The same peak occurs in the anode curve of solution with 5 g/L HCHO in Fig.2. Obviously, Peak 3 is generated by anode copper. The corresponding reduction peak of Peak 3 does not appear at the potential from -0.75 V to +0.2 V, which indicates that Peak 3 is an irreversible oxidation peak of anode copper. The peak current of Peak 3 (14 mA/cm2) decreases to 5 mA/cm2 approximately after the HCHO is added, which indicates that HCHO affects the oxidation of copper.
It can be seen from Fig.2 that three different oxidation peaks occur at the anode potential of -0.62 V (Peak 1), -0.40 V (Peak 2) and -0.17 V (Peak 3) respectively in the oxidation process of THPED- containing electroless plating solution. Obviously, the Peak 1 and Peak 2 are relevant with HCHO according to Fig.1. However the effect of Peak 1 on the anode curve is more distinct than that of Peak 2.
The cathode curve (reverse scan curve) of Fig.2 shows that there are two peaks at the cathode potentials of -0.45 V (Peak 4) and -0.80 V (Peak 2′), respectively. According to Fig.1, the reduction peak of copper does not occur in the cathode curve. Therefore, it can be concluded that Peak 2′ is the corresponding reduction peak of product (HCOOH) at peak 2. Peak 4 is the reduction peak of HCHO accompanying the reduction reaction of copper oxide. VASKELIS et al[6] reported that Peak 4 is generated by the reduction reaction of copper oxide (Cu2O) on the appearance of electrode with the oxidation reaction of HCHO. The peak potential shifts to negative direction for 0.2 V because the reduction of copper oxide needs a period of time[6].
3.2 Characteristics of Peak 1
In order to research the oxidation reaction mechanism of Peak 1, the effects of scan rate on the characteristics of Peak 1 were investigated in this work. The results are listed in Table 1.
The curves of I1 vs v and I1/v1/2 vs v of Peak 1 are shown in Fig.3 and Fig.4, respectively. It can be seen from Fig.3 that the peak current I1 is positively proportional with scan rate v under high scan speed. It can be seen from Fig.4 that peak current function I1/v1/2 increases with the increase of v, which is different from the eight instances described in “Electrochemical spectrum”[10]. GU[11] and PAUNOVIC[12] reported that it is the characteristic of surface adsorption of electronic active material.
The 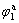 (anode potential of peak 1) increases with the increase of scan rate of υ(anode peak potential shifts to positive direction), which is the same as the characteristic of irreversible reaction of electric active adsorption state. JUSYS and VASKELIS[13] also reported
(anode potential of peak 1) increases with the increase of scan rate of υ(anode peak potential shifts to positive direction), which is the same as the characteristic of irreversible reaction of electric active adsorption state. JUSYS and VASKELIS[13] also reported
Table 1 Characteristic parameters of Peak 1
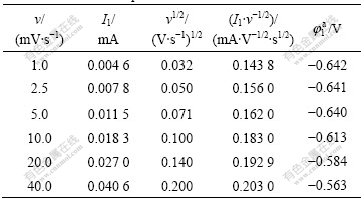
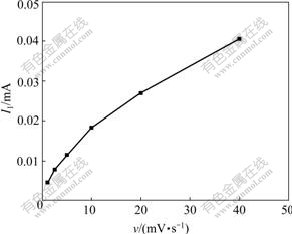
Fig.3 Curve of I1 vs v of Peak 1
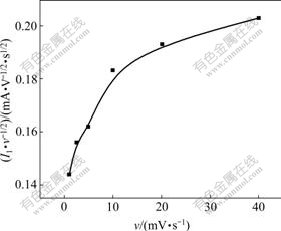
Fig.4 Curve of I1/v1/2 vs v of Peak 1
that there was a similar irreversible reaction of absorbed HCHO. Therefore, the reaction of Peak 1 is the irreversible oxidation reaction of absorbed HCHO and its reaction course is shown as follows[6]:
HCHO+OH- CH2(OH)O- (1)
CH2(OH)O- (1)
CH2(OH)O-→CH2(OH)Oad+e- (2)
CH2(OH)Oad+OH-→HCOO-+H2O+Had (3)
Had+Had→H2↑ (4)
In conclusion, whatever the reaction course of Peak 1 is, the reaction of Peak 1 is the main anode reaction of electroless copper plating. The Cu2+ ion is reduced by capturing electron and H2 is evolved. HCHO must be firstly absorbed on the appearance of electrode and then the oxidation reaction with H2 evolution occurs on the surface of electrode after activation treatment. As a result, the area and surface state of copper electrode have obvious effects on the oxidation of HCHO. Therefore, the reaction of Peak 1 is complicated and can be expressed as follows:
2HCHO+4OH-→2HCOO-+2H2O+H2↑+2e- (5)
3.3 Characteristics of Peak 2
In order to research the oxidation reaction mechanism of Peak 2, the effects of scan rate on the characteristics of Peak 2 were investigated. The results are listed in Table 2.
It can be seen from Table 2 that Δφ2 of Peak 2 is relative to scan rate of υ and its diversification rule accords to the characteristic of quasi-reversible reaction, which shows the reaction of Peak 2 is quasi-reversible reaction rather than simple complete-reversible reaction. The curves of I2 vs v and I2/υ1/2 vs v of Peak 2 according to Table 2 are shown in Fig.5 and Fig.6, respectively.
Fig.5 shows that the peak current of I2 is in direct ratio with the scan rate of v under high scan rate. It can be seen from Fig.6 that the peak current function of I2/v1/2 increases with the increase of scan rate of v, which is different from the eight circumstance of “electrochemical spectrum”[10]. The 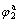 increases and
increases and 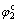 decreases with the increase of scan rate of v, which is the same as the characteristic of quasi-reversible reaction of electric active absorption state O. It can be inferred that the reaction of Peak 2 is the quasi-reversible reaction of surface absorption of HCHO and its reaction course can be listed as reaction (1) and as follows[6]:
decreases with the increase of scan rate of v, which is the same as the characteristic of quasi-reversible reaction of electric active absorption state O. It can be inferred that the reaction of Peak 2 is the quasi-reversible reaction of surface absorption of HCHO and its reaction course can be listed as reaction (1) and as follows[6]:
CH2(OH)O-
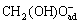 (6)
(6)
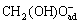 +2OH-→HCOO-+2H2O+2e- (7)
+2OH-→HCOO-+2H2O+2e- (7)
Therefore, the reaction at Peak 2, the secondary anode oxidation reaction of electroless plating, is the quasi-reversible oxidation reaction of adsorbed HCHO without hydrogen evolution. The Cu2+ ion is reduced by capturing electron without H2 evolution. The total reaction can be expressed as follows[14]:
HCHO+3OH- HCOO-+2H2O+2e- (8)
HCOO-+2H2O+2e- (8)
3.4 Influences of HCHO on anode polarization curve of THPED-containing solution
The influences of HCHO concentration on the anode polarization curve are shown in Fig.7 under pH value of 13.0 and 20 g/L THPED.
Table 2 Characteristic parameters of Peak 2
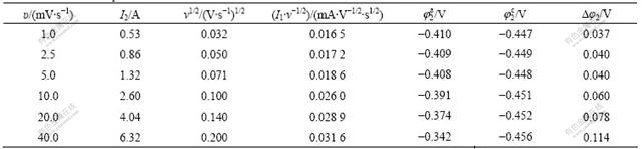
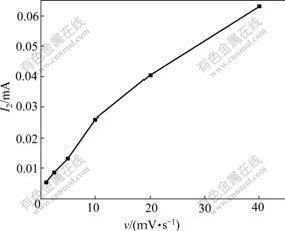
Fig.5 Curve of I2 vs v of Peak 2
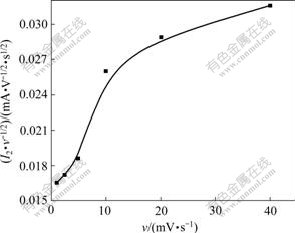
Fig.6 Curve of I2/v1/2 vs v of Peak 2
It can be seen from Fig.7 that HCHO has less effect on the potential of Peak 1, while great effects on the current density of Peak 1. The current density of Peak 1 increases from 2.30 to 2.92 mA/cm2 when HCHO concentration from 1.6 g/L to 3.1 g/L and sharply increases to 3.51 mA/cm2 at 4.7 g/L HCHO. Therefore, HCHO has distinct effect on the oxidation reaction of adsorbed HCHO with hydrogen evolution under high concentration. In addition, HCHO has less effect on the current density of Peak 2 and great effect on that of Peak 3. The current density of Peak 3 decreases with the increase of HCHO concentration.
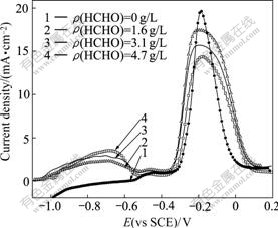
Fig.7 Influence of HCHO concentration on anode polarization curve at scan rate of 10 mV/s
3.5 Influences of THPED concentration on anode polarization curve of electroless copper plating solution
The influences of THPED concentration on the anode polarization curve are shown in Fig.8 under pH value of 13.0 and 4.7 g/L HCHO.
It can be seen from Fig.8 that THPED concentration has less effects on Peak 1 and Peak 2. The current density of Peak 1 decreases from 3.99 to 3.51 mA/cm2 and that of peak 2 remains almost constant when the concentration of THPED is 0-20 g/L. THPED reduces the current density of anode peak of HCHO at low concentration, while it is not obvious under high concentration. Therefore, the chelator of THPED has less effect on the oxidation reaction of adsorbed HCHO with hydrogen evolution. In addition, THPED has great effect on the current density of Peak 3. The current density of Peak 3 increases with the increase of HCHO concentration. Therefore, THPED promotes the dissolution of anode copper greatly.
In general, the ohm resistance of transmission of reaction particle in the solution can be ignored, therefore
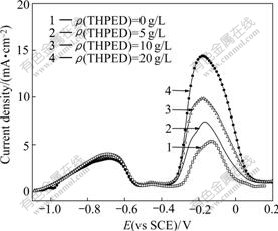
Fig.8 Influence of THPED concentration on anode polarization curve at scan rate of 10 mV/s
the control levels of anode and cathode of electroless copper plating can be computed as follows:
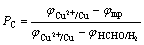 (9)
(9)
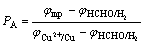 (10)
(10)
where PC and PA stand for the control level of cathode and anode of electroless copper plating, respectively; φmp is the mixed potential (stable potential) of electroless copper plating system;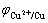 and
and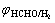 are the stable potentials of cathode and anode of electroless copper plating, respectively.
are the stable potentials of cathode and anode of electroless copper plating, respectively.
4 Conclusions
1) There are three different oxidation reactions at the anode process in the THPED-containing electroless copper plating solution. The first reaction which occurs at the potential of -0.62 V is the main anode oxidation reaction, which is an irreversible reaction of adsorbed HCHO with hydrogen evolution. The second reaction which occurs at -0.40 V is the secondary oxidation reaction, which is a quasi-reversible reaction of adsorbed HCHO without hydrogen evolution. The third reaction which occurs at -0.17 V is an irreversible oxidation of anode copper.
2) With the increase of HCHO concentration, the current density of Peak 1 (-0.62 V) increases gradually and the one of Peak 2 (-0.40 V) remains almost constant. The current density of Peak 3 (-0.17 V) increases with the increase of THPED concentration or the decrease of HCHO concentration. The complexation of THPED promotes the dissolving of anode copper.
References
[1] FARID H, ZABDEL H, AAL A A. Controlling factors affecting the stability and rate of electroless copper plating [J]. Materials Letters,2004, 58(1/2): 104-109.
[2] ZHENG Ya-jie, ZOU Wei-hong, YI Dan-qing, GONG Zhu-qing, LI Xin-hai. Electroless copper plating system of potassium sodium tartrate and EDTA·2Na [J]. Journal of Central South University of Technology, 2005, 36(6): 971-976.
[3] RAMASUBRAMANIAN M, POPOV B N. A mathematical model for electroless copper plating deposition on planar substrates [J]. Electrochemical Society, 1999, 146(1): 111-116.
[4] HOLGER G, LIENHARD P. Development of a micro-manipulator based on piezoelectric-technology [J]. Micro-electronic Engineering,2007, 84(5/8): 1333-1336.
[5] JENGQ S T, SHEU H S, YE Chang-lin. High-G drop impact response and failure analysis of a chip packaged printed circuit board [J]. International Journal of Impact Engineering,2007, 34(10): 1655-1667.
[6] VASKELIS A, NORKUS E, STALNIONIENE I, STALNIONIS G. Effect of the Cu electrode formation conditions and surface nano-scale roughness on formaldehyde anodic oxidation [J]. Electrochimica Acta,2004, 49(9/10): 1613-1621.
[7] LI Ning. Electroless practice technology [M]. Beijing: Chemistry Industry Press, 2004: 203-210. (in Chinese)
[8] ZHENG Ya-jie, ZOU Wei-hong, YI Dan-qing, GONG Zhu-qing, LI Xin-hai. Electroless copper plating in the presence of THPED and EDTA·2Na as the dual-chelating agent [J]. Materials Protection, 2006, 39(2): 20-24. (in Chinese)
[9] GU Xin, WANG Zhou-cheng, LIN Chang-jian. An electrochemical study of the effects of chelating agents and additives on electroless copper plating [J]. Electrochemistry, 2004, 10(1): 14-18.(in Chinese)
[10] TIAN Zhao-wu. Electrochemical investigation technology [M]. Beijing: Science Press, 1984: 302-320. (in Chinese)
[11] GU Lin-ying. Principle and application of electrochemical measure [M]. Beijing: Chemistry Industry Press, 1986: 101-132. (in Chinese)
[12] PAUNOVIC M. Ligand effects in electroless copper deposition [J]. Journal of Electrochemical Society, 2004, 124(3): 349-354.
[13] JUSYS Z, VASKELIS A. The kinetic H/D Isotope effect in electroless copper plating [J]. Electrochimica Acta, 2002, 42(3): 449-454.
[14] NUZZI F J. Accelerating the rate of electroless copper plating [J]. Plating and Surface Finishing, 2003, 70(1): 51-54.
(Edited by ZHAO Jun)
Foundation item: Project(200501045) supported by Innovation Fund of Guangdong Province of China
Received date: 2007-12-15; Accepted date: 2008-05-10
Corresponding author: ZHENG Ya-jie, Professor, PhD; Tel: +86-731-8836285; E-mail: zzyyjj01@yahoo.com.cn