文章编号:1004-0609(2013)12-3448-07
金在NaCN溶液中的表面产物
杨永斌,陈利娟,姜 涛,李 骞,刘晓亮
(中南大学 资源加工与生物工程学院,长沙 410083)
摘 要:结合扫描电子显微镜(SEM)和原子力显微镜(AFM)测定金氰化溶解过程表面产物的形貌。结果表明,金片表面溶蚀程度轻,少量溶解主要发生在棱和角等边界断点上。利用X射线光电子能谱(XPS)研究了金氰化溶解过程表面产物的组成元素及键合特性,得出表面产物的主要成分为AuCN、Au(OH)x和Au2(OHCN)。金在NaCN溶液中的溶解是分步进行的:第一步,Au与配位剂CN-作用形成长链结构的AuCN,AuCN进一步与CN-反应才形成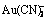 实现溶解;第二步反应只能在AuCN长链的断点处发生,因而成为总反应的控制步骤,从而使表面产物AuCN大量累积形成表面覆盖层,即表面钝化层;而棱角部位的表面产物因断点多而较易发生溶解,从而呈疏松颗粒状,未能形成紧密相连的面覆盖,即表面活性区。
实现溶解;第二步反应只能在AuCN长链的断点处发生,因而成为总反应的控制步骤,从而使表面产物AuCN大量累积形成表面覆盖层,即表面钝化层;而棱角部位的表面产物因断点多而较易发生溶解,从而呈疏松颗粒状,未能形成紧密相连的面覆盖,即表面活性区。
关键词:金;氰化物;氰化浸金;表面产物;钝化
中图分类号:TG171 文献标志码:A
Surface products of gold dissolution in cyanide solutions
YANG Yong-bin, CHEN Li-juan, JIANG Tao, LI Qian, LIU Xiao-liang
(School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The micro-morphology of gold surface products in cyanide solutions was studied by scanning electron microscopy (SEM) and atomic force microscopy (AFM). It is found that, little corrosion occurs on the gold surface except on the surface edges and horns. The element composition and binding feature of gold surface products in cyanide solutions were studied by X-ray photoelectron spectroscopy (XPS). It is indicated that the composition of surface product include AuCN, Au(OH)x and Au2(OHCN). Gold dissolution process takes place by two steps, in which, AuCN first forms into a long-chain structure AuCN that further reacts with CN- to form the soluble complex 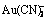 in the second step. The second step that can only take place on the end of AuCN chain essentially acts as the rate-controlling step. This mechanism inevitably causes surface covering of accumulated AuCN, i.e., the surface passivated layer. On the other side surface products on the edges and horns dissolve faster because of more breakpoints in the AuCN chain and remain in separate particles which doesn’t form compact spread covering, i.e., surface activated area.
in the second step. The second step that can only take place on the end of AuCN chain essentially acts as the rate-controlling step. This mechanism inevitably causes surface covering of accumulated AuCN, i.e., the surface passivated layer. On the other side surface products on the edges and horns dissolve faster because of more breakpoints in the AuCN chain and remain in separate particles which doesn’t form compact spread covering, i.e., surface activated area.
Key words: gold; cyanide; gold cyanidation; surface product; passivation
氰化浸金方法自问世以来,因具有其他浸金方法无法超越的高浸出率等优点而一直在黄金提取冶金中占据着支配地位[1]。但也存在浸金速率慢的问题,因此,一个多世纪以来国内外对氰化浸金方法及技术的研究从未间断,并出现了一系列的新技术。20世纪80年代,过氧试剂助浸技术将氰化浸金推向了高潮,实现了浸金技术的突破。此时,浸金速率似乎达到了某种极限,至今30年未能出现新的实质性提高。在此基础上,要继续提高浸金速率,需要从浸金过程的本质特性出发寻求新的思路。
众所周知,金在氰化物溶液中与配位剂CN-形成稳定的配离子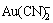 ,能有效地实现金的溶解。金在氰化物溶液中表面易生成致密的钝化产物膜,严重影响金的浸出速率[2],因此,深入地认识其钝化产物膜的组成及结构形态对于提高浸金速率是很有必要的。对于钝化膜的组成,尽管人们进行了大量的研究,但是,由于上述研究都缺乏充足的测试依据,所得结果各不相同,至今未形成一致的观点。MACARTHUR[3]认为表面沉积的是中间产物Au(CN);PAN等[4]认为是沉积了羟基金(Au(OH))或碱式氰化金(Au2(OHCN))。综合各观点,构成钝化膜的物质可能有4种:AuCNads、AuOx、Au(OH)x或Au2(OHCN)。
,能有效地实现金的溶解。金在氰化物溶液中表面易生成致密的钝化产物膜,严重影响金的浸出速率[2],因此,深入地认识其钝化产物膜的组成及结构形态对于提高浸金速率是很有必要的。对于钝化膜的组成,尽管人们进行了大量的研究,但是,由于上述研究都缺乏充足的测试依据,所得结果各不相同,至今未形成一致的观点。MACARTHUR[3]认为表面沉积的是中间产物Au(CN);PAN等[4]认为是沉积了羟基金(Au(OH))或碱式氰化金(Au2(OHCN))。综合各观点,构成钝化膜的物质可能有4种:AuCNads、AuOx、Au(OH)x或Au2(OHCN)。
总体来说,目前关于金在NaCN溶液中的表面产物报道极少,这一理论的缺乏对于金氰化溶解、钝化机理的揭示产生了严重障碍。近年来,表面分析技术发展迅速,很多新颖的研究结果是依赖于先进的表面分析技术取得的,同时,借助这一方法可以将金氰化过程表面产物的研究工作深入到分子甚至原子水平。本文作者从微观检测的界面信息出发,结合扫描电子显微镜(SEM)、原子力显微镜(AFM)和X射线光电子能谱(XPS)等多种微观测试手段[5-6],通过测定金氰化溶解过程表面产物的形貌、组成元素和键合特性等信息研究表面产物的组成及形态,为揭示金溶解过程的表面钝化与活性溶解的机理提供有力的依据,为研究浸金过程、加快浸金速率奠定基础。
1 实验
1.1 表面产物的生成
实验材料为由纯度99.99%的金粉制成的d 7.0 mm×2.0 mm圆形金片。实验前用砂纸将金片表面磨平,然后用0.06A型金相砂纸抛光,经酒精灯火焰处理,再依次用蒸馏水、质量分数为10%的稀硝酸、蒸馏水和无水乙醇分别进行超声波清洗8 min,最后用蒸馏水冲洗干净。
实验温度控制在25 ℃,pH值调节为12.0。将清洁处理后的金片置于20 mL质量分数为0.3%的NaCN溶液中浸泡,进行表面溶蚀,以生成表面产物,浸泡时间为5 d。最后取出试样,用蒸馏水冲洗,待自然晾干后进行表面产物检测。
1.2 表面产物的表征
采用JSM-6360LV型扫描电镜(SEM)对比金片腐蚀前后的形貌变化,采用di NanoMan Vs型原子力显微镜(AFM)测试表面产物的形貌特征,采用Kα 1063型X射线光电子能谱仪(XPS)分析表面产物的元素组成和键合特性。其中,当进行X射线光电子能谱仪分析时样品室内的本底真空度为1×10-7 Pa,X射线源为Al Kα,通过能量为50 eV。
2 结果与讨论
2.1 表面产物的形貌分析
通过SEM对溶蚀前后金片的表面微观形貌进行对比。由图1可知,金片于氰化物溶液中溶蚀5 d后,表面形貌的整体结构没有受到明显破坏,溶解前后表面沟壑和坑凹的分布状况基本相同,表明金片表面溶蚀程度不大。但是从细微的变化上仍能获得表面溶解现象的信息。在表面平滑的区域,可以观察到粗糙度略有增加,表面沟壑和坑凹出现了较明显的边缘溶蚀及沟壑变宽和蔓延的现象(见图1中的A,B及A′,B′处),而对于表面的显微凸起,则更是出现了明显缩小甚至消失的现象(见图1中的C及C′处)。因此,无论是平滑表面,还是凹、凸部位,都发生了不同程度的溶解。相比之下,凹、凸部位的溶解程度明显大于平滑表面的溶解程度。说明金片表面溶解主要发生在棱、角等边界断点上,形成点蚀甚至缝隙腐蚀[7]。
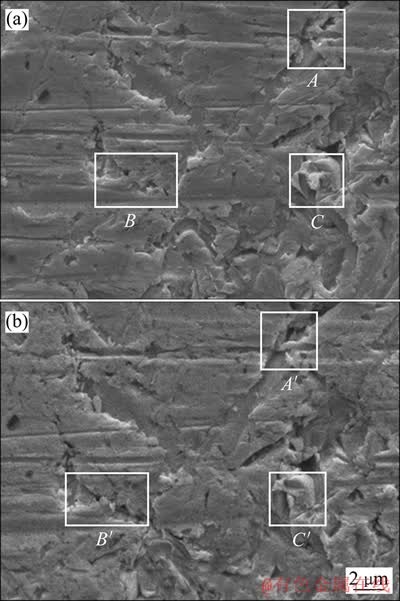
图1 金片溶蚀前后的SEM像
Fig. 1 SEM images of gold surface before(a) and after(b) cyanidation
为进一步深入研究表面溶蚀状态,利用AFM轻敲模式测定溶蚀前后的金片表面形貌,扫描面积为5 μm×5 μm,通过多次扫描,发现溶蚀前的金片表面平整,无明显颗粒状(见图2)。而溶蚀后的金片,在表面平滑处(形貌1)和棱角部位(形貌2)出现两种不同的形貌特征。平滑区域(见图3)的表面光滑平整,无缝隙或裂痕,但与图2相比,其表面明显被一层微细颗粒覆盖,且结构致密。棱角部位(见图4)结构较为疏松,有明显的裂纹,表面呈现明显的颗粒状,且颗粒大小不一。这一结果表明,金溶解过程中存在产物的积累,其积累程度在平滑表面和棱角部位表现出明显的差异性。平滑处表面产物积累成致密覆盖层,而棱角部位的表面产物积累成松散的颗粒状。这种差异性说明表面产物溶解速率在平滑表面和棱角部位存在差异,即平滑部位表面产物因溶解速率慢而积累成面,而棱角部位的表面产物因溶解速率快而未能连接成面,仅以松散颗粒状存在。这种表面产物溶解速率上的差异导致了图1所示金片溶蚀程度在平滑表面和棱角部位的差异。
2.2 表面产物的成分分析
为了研究表面产物的元素组成和结构形态,对溶蚀金片表面进行XPS全程扫描,结果如图5所示。由图5可知,溶蚀金片表面主要含有Au、C、N和O这4种元素。各元素的含量(摩尔分数)为68.10%Au、19.26%C、6.25%N和6.39%O。C、N和O的存在表明金片溶解过程中溶液的成分(CN-和OH-等)在金片表面发生了沉积,形成了某种表面产物。
为了考察表面产物的化学形态,对各元素的键合特性进行研究。为此,对表面产物中的Au、C、N和O元素进行能量窄扫描。采用Thermo Avantage曲线拟合程序分别对所得Au、C、N和O谱线进行拟合,根据拟合的波峰面积计算出同一元素不同键合形态的原子数占总原子数的百分含量,以此推断表面产物的化学形态(结果见表1)。
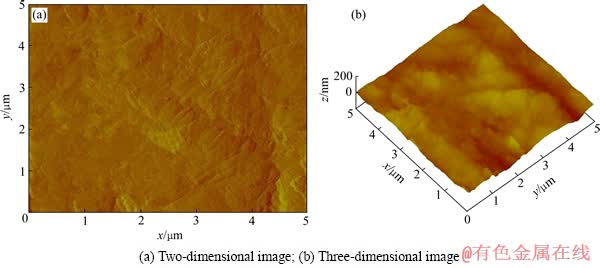
图2 溶蚀前金片表面的AFM图
Fig. 2 AFM images of gold surface before cyanidation

图3 溶蚀后金片表面(形貌1)的AFM像
Fig. 3 AFM images of gold surface with morphology 1 after cyanidation
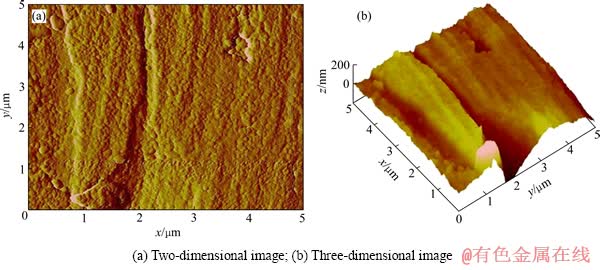
图4 溶蚀后金片表面(形貌2)的AFM像
Fig. 4 AFM images of gold surface with morphology 2 after cyanidation
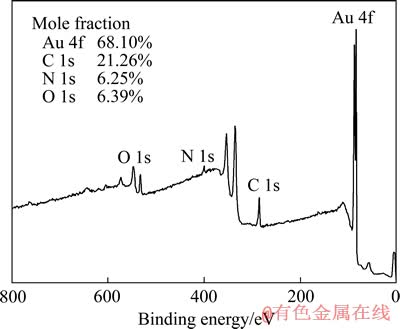
图5 金氰化溶解过程表面产物的XPS全谱
Fig. 5 XPS survey scan of gold surface products in cyanide solutions
2.2.1 Au元素的键合形态
图6所示为原始表面与溶蚀表面中Au的窄扫描曲线。从图6可知,两个峰对应的结合能均在83.90 eV左右,与XPS手册[8]及文献报道的单质Au的4f7峰结合能基本一致,可确认金片表面存在大量单质Au。但是,相对于原始表面,溶蚀表面的特征峰向高能量方向发生了明显的位移,说明溶蚀金片表面发生了部分Au原子周围电荷密度下降的现象,即出现正价态的金,因而表面产物含有化合态金,即金片表面存在金的氧化产物。
表1 表面产物各元素化学状态及其含量
Table 1 Chemical state and relative concentration of elements on surface products
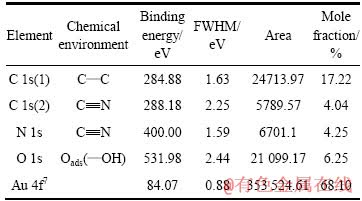
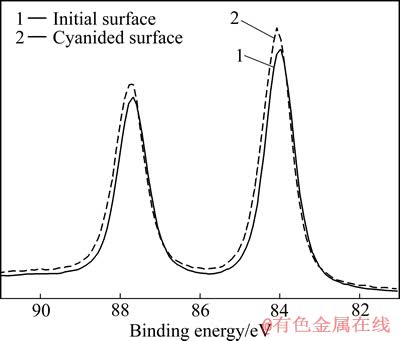
图6 原始表面与溶蚀表面Au的4f XPS谱
Fig. 6 Contrast of Au 4f XPS spectra of cyanided surface to initial surface
2.2.2 C元素的键合形态
原始金片表面与溶蚀表面C元素窄扫描曲线如图7所示。由图7可知,C 1s峰的主峰位于284.80 eV左右[9],表明表面C接近非束缚态C的特征峰,这主要是来自于环境中非束缚态C的污染。但溶蚀金片表面的C峰表现出明显不对称特性,且向高结合能方向偏移0.4 eV。这表明表面溶蚀产物中出现其他形式的含碳化合物,需要对其进行分峰拟合处理,以分析各化学状态的相对含量。
对比图7中原始金片与溶蚀金片的C 1s峰,遵循XPS图谱解叠原则,可将溶蚀金片表面C 1s峰解叠,如图8所示。由图8可知,表面产物中C 1s谱由2个峰组成。位于284.88 eV的峰C 1s(1),对应C—C键,为非束缚态的C(或称石墨碳)峰,此峰为真空系统中残留气氛如CO和CO2等的峰,广泛存在于各样品的XPS检测中[10]。在实际的光电离扫描过程中,一般会在高能量方向出现携上峰[11],如图8所示,284.88 eV高结合能方向的峰稍大于C—C键的标准峰谱。位于288.18 eV的峰C 1s(2),对应为—C≡N键中的C[12],其峰面积比为4.04%。表明表面产物中存在氰化物,结合前述人们对金的溶解、钝化现象的认识,推测其为AuCN,即AuCN作为中间产物在金表面发生沉积。
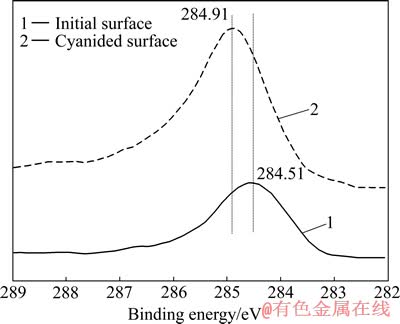
图7 原始表面与溶蚀表面C 1s的XPS谱
Fig. 7 Contrast of C 1s XPS spectra of cyanided surface to initial surface

图8 溶蚀表面的C 1s XPS谱解叠图
Fig. 8 Fine XPS spectra of C 1s of cyanided surface
2.2.3 N元素的键合形态
原始表面的XPS全扫基本无N 1s峰出现,因此,N元素的检测一般不受环境的影响。而溶蚀表面XPS结果显示有4.25%N(摩尔分数),可知其来源于CN-中的N元素。将溶蚀表面的N 1s窄扫图进行拟合对比(见图9),N的特征峰值为399.98 eV,与C≡N键的结合能相吻合[12],结合C元素化学状态分析,从表1中C2(1s)即—C≡N的C原子与N 1s的摩尔比基本一致,可推断N元素全部以—CN的形式存在。由此可知,CN-参加了钝化膜的生成反应而进入中间产物AuCN,最后以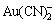 实现金的溶解。
实现金的溶解。

图9 溶蚀表面N 1s峰的XPS窄扫及拟合谱
Fig. 9 Fine spectrum and fitting curve of N 1s of cyanided surface
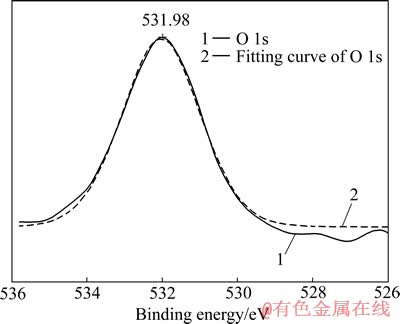
图10 溶蚀表面O 1s XPS谱及拟合曲线
Fig. 10 Fine XPS spectrum and fitting curve of O 1s of cyanided surface
2.2.4 O元素的键合形态
如图10所示,O 1s对应的结合能为531.98 eV,与吸附氧的标准峰位相重合,所以推测氧存在的形式为吸附氧Oads[13]。吸附氧Oads为电极表面吸附的含氧物种,随环境不同而变化。Oads的结合能在532.00 eV左右,一般认为是吸附的氧单质O2或羟基基团—OH[14]。在水溶液电解体系中,电极表面吸附的含氧物种以羟基基团为主,因此推测表面产物中存在羟基基团—OH,其存在形式为羟基金Au(OH)x或碱式氰化金Au2(OHCN),它们与中间产物AuCN共同存在。因为金在碱性溶液中可形成Au(OH)3和Au(OH),同时,氰化物表面具有较高的能量,在水溶液中易发生“表面羟基化”过程,形成Au2(OHCN),反应如式(1)所示:
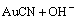 +Au=Au2(OHCN)+e (1)
+Au=Au2(OHCN)+e (1)
2.3 金氰化溶解机理探讨
以上研究表明,金的溶解是分两步进行的,第一步反应生成的不溶性中间产物在金表面积累,形成了连续的产物层覆盖面,阻碍了金的进一步反应,而金的第二步反应只能在产物层覆盖面的边界发生。图3所示为表面光滑平整处的AFM形貌,由于其表面被一层微细颗粒覆盖,表面均匀致密,没有合适的溶解边界点和边界线,第二步的溶解难以找到突破口。图4所示为表面棱角部位的AFM形貌,晶粒状形态为明显的腐蚀形态,产物表面有重叠状蚀坑,晶粒生长长程无序,有晶粒间相互连接而成的孪晶,还有沿晶粒位向扩展方向的条晶,表现出明显的腐蚀特征[15]。这是因为金溶解的第二步反应在此表面找到了溶解的基点或基线。XPS谱表明,中间产物AuCN在金表面发生沉积,更证实了该结论,即金在氰化溶液中的溶解是分两步进行的,先后发生了如式(2)和(3)的反应,首先Au与配位剂CN-作用形成长链结构的AuCN[16],其次AuCN进一步与CN-反应才形成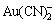 ,实现溶解。由于第二步反应只能在AuCN长链的断点处发生,因而成为总反应的控制步骤,从而使表面产物AuCN大量累积形成覆盖面。棱角部位的表面产物因断点多而较易发生溶解,呈疏松颗粒状,未能形成紧密相连的面覆盖。
,实现溶解。由于第二步反应只能在AuCN长链的断点处发生,因而成为总反应的控制步骤,从而使表面产物AuCN大量累积形成覆盖面。棱角部位的表面产物因断点多而较易发生溶解,呈疏松颗粒状,未能形成紧密相连的面覆盖。
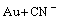 =AuCN+e (2)
=AuCN+e (2)
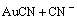 =
=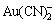 (3)
(3)
3 结论
1) 金氰化前后的表面形貌变化表明,金在氰化溶液中发生大面积钝化,很难溶解,但在表面棱角部位存在局部腐蚀。
2) 金在氰化溶液中的溶蚀表面存在两种形貌,一种表面平整而致密,且被微细颗粒覆盖,呈现钝化特征;一种表面粗糙、疏松且多蚀点和蚀坑,呈现明显的腐蚀特征。金在氰化溶液中形成的钝化膜的化学成分主要为AuCN、Au2(OHCN)和Au(OH)x。
3) 金在氰化溶液中的溶解是分两步进行的,首先Au与配位剂CN-作用形成长链结构的AuCN,然后AuCN进一步与CN-反应才形成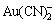 完成金的溶解。其中,第二步反应只能在AuCN长链的断点处发生,为总反应的控制步骤。
完成金的溶解。其中,第二步反应只能在AuCN长链的断点处发生,为总反应的控制步骤。
REFERENCES
[1] 胡春融, 杨 凤. 黄金选冶技术发展述评[J]. 黄金, 2000, 21(1): 29-37.
HU Chun-rong, YANG Feng. A review of the technical development of gold mineral processing and smelting[J]. Gold, 2000, 21(1): 29-37.
[2] WADSWORTH M E, ZHU X M. Kinetics of enhanced gold dissolution: Activation by dissolved silver[J]. International Journal of Mineral Processing, 2003, 72(1): 301-310.
[3] MACARTHUR D M. A study of gold reduction and oxidation in aqueous solutions[J]. Electrochem Soc, 1972, 119(6): 672-676.
[4] PAN T P, WAN C C. Anodic behavior of gold in cyanide solution[J]. Appl Electrochem, 1979, 9(5): 653-655.
[5] 朱 兵, 陈明安, 张新明. 微量Ga对高压阳极铝箔腐蚀发孔性能的影响[J]. 中南大学学报: 自然科学版, 2012, 43(11): 4211-4219.
ZHU Bing, CHEN Ming-an, ZHANG Xin-ming. Effect of trace Ga on pitting performance of high voltage anode aluminum foil[J]. Journal of Central South University: Science and Technology, 2012, 43(11): 4211-4219.
[6] 余 琨, 胡亚男, 谭 欣, 李少君, 陈福文. 海水激活电池用Mg-Hg-Ga合金阳极材料的腐蚀行为[J]. 中南大学学报: 自然科学版, 2012, 43(2): 466-471.
YU Kun, HU Ya-nan, TAN Xin, LI Shao-jun, CHEN Fu-wen. Corrosion behavior of Mg-Hg-Ga magnesium alloy anodes for seawater activated battery[J]. Journal of Central South University: Science and Technology, 2012, 43(2): 466-471.
[7] 韩顺昌. 金属腐蚀显微组织图谱[M]. 北京: 国防工业出版社, 2008: 1-2.
HAN Shun-chang. Atlas of microstructure on metals corrosion[M]. Beijing: National Defense Industry Press, 2008: 1-2.
[8] WAGNER C D, RIGGS W M. Handbook of X-ray photoelectron spectroscopy[M]. Minnesota: Perkin-Elmer Corporation Physical Electronics Division, 1999.
[9] 华中胜, 姚广春, 马 佳. 碳纤维表面镍镀层的XPS分析[J]. 中国有色金属学报, 2011, 21(1): 165-170.
HUA Zhong-sheng, YAO Guang-chun, MA Jia. XPS analysis of nickel layers on carbon fibers[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(1): 165-170.
[10] 周 萍, 汪小琳, 杨江荣. 铀真空热氧化膜的XPS研究[J]. 稀有金属材料与工程, 2008, 37(1): 94-97.
ZHOU Ping, WANG Xiao-lin, YANG Jiang-rong. Effect of vacuum heat treatment on oxidation of uranium surfaces[J]. Rare Metal Materials and Engineering, 2008, 37(1): 94-97.
[11] 朱永法, 郑 斌, 姚文清. 电子能谱线形分析研究碳物种的化学状态[J]. 分析化学, 1999, 27(1): 10-13.
ZHU Yong-fa, ZHENG Bin, YAO Wen-qing. The chemical states of carbon species studied using line shape analysis of X-ray photoelectron spectroscopy and auger electron spectroscopy[J]. Chinese Journal of Analytical Chemistry, 1999, 27(1): 10-13.
[12] 徐 妍, 孙宝利, 马 超. 超分散剂在莠去津颗粒表面吸附的XPS和SEM分析[J]. 光谱学与光谱分析, 2011, 31(9): 2569-2573.
XU Yan, SUN Bao-li, MA Chao. XPS and SEM spectroscopy study of hyperdispersant on atrazine surface[J]. Spectroscopy and Spectral Analysis, 2011, 31(9): 2569-2573.
[13] LIU Jun-feng, FENG Yu-jie. Investigation on the electrocatalytic characteristics of SnO2 electrodes with nanocoating prepared by electrodeposition method[J]. Science in China Series E: Technological Sciences, 2009, 52(6): 1799-1803.
[14] 冯玉杰, 刘峻峰, 崔玉虹. 环境电催化电极: 结构、性能与制备[M]. 北京: 科学出版社, 2010: 181-182.
FENG Yu-jie, LIU Jun-feng, CUI Yu-hong. Environmental electrocatalytic electrode: Structure, characteristic and preparation[M]. Beijing: Science Press, 2010: 181-182.
[15] ENGEL L, KLINGELE H. An atlas of metal damage: Surface examination by scanning electron microscope[M]. London: Carl Hanser Verlag, 1981: 232-233.
[16] NICOL M J. Anodic behavior of gold. Part Ⅱ: Oxidation in alkaline solutions[J]. Gold Bulletin, 1980, 13(3): 105-111.
(编辑 陈卫萍)
基金项目:国家自然科学基金资助项目(51074182)
收稿日期:2013-01-04;修订日期:2013-03-08
通信作者:杨永斌,副教授,博士;电话:0731-88830547;E-mail: sanyayou@126.com