交联海藻酸钙凝胶固定化酵母醇脱氢酶研究
李桂银1, 3,杨栋梁2, 3,蒋玉仁2,丁 萍2,黄可龙2,李元建1
(1. 中南大学 药学院,湖南 长沙,410078;
2. 中南大学 化学化工学院,湖南 长沙,410083;
3. 湖南科技职业学院 轻化工程学院,湖南 长沙,410118)
摘 要:对用海藻酸钙包埋﹑戊二醛交联法固定酵母醇脱氢酶催化苯乙酮酸合成(R)-扁桃酸的过程进行研究,比较游离酶与固定化酶的酶学性质。实验结果表明:固定化酶的热稳定性显著提高,游离酶在70 ℃时酶蛋白变性失去活力,而固定化酶在65 ℃保温1 h的能保持64%的酶活力,在70 ℃时酶活力仍可保留48.6%;固定化酶的最适温度由30 ℃升至40 ℃,最适反应pH值由6.8下降为5.8;固定化酶保留了62.72%的游离酶活性, 固定化酶的表观米氏常数和最大反应速率分别为37.33 mmol/L和358.42 nmol/min。该固定化酶具有良好的储存稳定性和操作稳定性。
关键词:海藻酸钠;固定化;酵母醇脱氢酶
中图分类号:O642 文献标志码:A 文章编号:1672-7207(2009)06-1510-07
Immobilization saccharomyces cerevisiae alcohol dehydrogenase on cross-linked calcium alginate beads
LI Gui-yin1, 3, YANG Dong-liang2, 3, JIANG Yu-ren2, DING Ping2, HUANG Ke-long2, LI Yuan-jian1
(1. School of Pharmaceutical Sciences, Central South University, Changsha 410078, China;
2. College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
3. Department of Chemical Engineering, Hunan Vocational College of Science and Technology, Changsha 410118, China)
Abstract: The optimum technology of immobilized Saccharomyces cerevisiae alcohol dehydrogenase (SCAD) within a calcium alginate matrix which was cross-linked with glutaraldehyde was studied. The SCAD was compared with its immobilized enzyme in some properties. The results show that the free enzyme loss all its activity when heated at 70 ℃ for 1 h but the immobilized enzyme still keeps 48.6% of the original activity. The maximal activity of the native SCAD appears at 30 ℃, but the optimum temperature of the immobilized SCAD is as high as 40 ℃. The optimal reaction pH of the immobilized enzyme is 5.8 compared to 6.8 of the free SCAD. For the reduction of phenylglyoxylic acid by immobilized SCAD, the kinetic analysis of data indicates that the immobilized SCAD retains 62.72% activity of its original activity, the maximum specific activity and the Michaelis constants for phenylglyoxylic acid are 358.42 nmol/min and 37.33 mmol/L, respectively. Furthermore, the immobilized SCAD enhances storage stability and good durability in the repeated use.
Key words: sodium alginate; immobilization; saccharomyces cerevisiae alcohol dehydrogenase
α-羟基苯乙酸俗称扁桃酸,是合成环扁桃酯、羟苄唑、匹莫林等手性药物的重要中间体。它有一个手性碳原子,有(R)-扁桃酸和(S)-扁桃酸2种光学构型。单一构型的扁桃酸被广泛应用于光学纯的氨基酸、血管紧张肽转化酶抑制剂、辅酶A等药品的不对称合成。如(R)-扁桃酸是半合成抗生素(青霉素类或头孢菌素类)的起始原料和神经安慰药物麻黄碱的中间体[1-3]。制备光学活性的扁桃酸有不对称合成法[4-5],外消旋体的拆分[6-8]和生物催化不对称还原法等方法[9-10]。而生物催化不对称还原法具有高效率、高立体选择性、反应条件温和以及经济效益和社会效益好等优点。因此,利用微生物细胞或酶作为催化剂来催化不对称还原苯乙酮酸制备具有光学活性的扁桃酸的方法受到研究者的关注。
面包酵母(Saccharomyces cerevisiae)是被广泛利用的廉价微生物之一,其细胞内存在着丰富的氧化还原酶(如醇脱氢酶)系,可催化各种羰基化合物的不对称还原反应,并对该类反应具有高度的立体选择 性[11]。Xiao等[10-11]以苯乙酮酸为原料,利用诱导后的游离酵母细胞(Saccharomyces cerevisiae FDllb)中的酵母醇脱氢酶(Saccharomyces cerevisiae alcohol dehydrogenase (SCAD) (EC1.1.1.1),在NADH参与下,合成了(R)-扁桃酸。
与游离酶相比,固定化酶具有高效、专一、温和的酶催化特性以及稳定性高、可分离回收、重复使用和生产成本低等一系列优点。国内外关于固定化SCAD酶的方法有磁性粒子吸附法、包埋法、共价结合法、交联法等[12-14]。本文作者采用海藻酸钠包埋、戊二醛交联的方法固定化酵母醇脱氢酶SCAD,优化了固定化工艺条件;以苯乙酮酸为酶促反应底物,研究了固定化酶的部分酶学性质,并对固定化酶的动力学进行了探讨。
1 实 验
1.1 仪器及试剂
仪器:SPX-100生化培养箱(上海民仪电子有限公司制造);UV2100紫外分光光度计(北京莱伯泰科仪器有限公司制造);KS-250F超声波破碎仪(宁波新芝股份有限公司制造)。
试剂:啤酒酵母(Saccharomyces cerevisiae, strain No.3,中南大学化学化工学院制药工程系菌种保藏室保藏);扁桃酸外消旋体(国药集团化学试剂有限公司生产,分析纯);R-扁桃酸(Sigma公司生产);苯乙酮酸(Sigma公司生产);尼克酰胺核苷酸(NADH) (Sigma公司生产);牛血清蛋白(BSA)(上海赛达生物药业有限公司生产);其他试剂都为分析纯。
1.2 酵母菌的培养及酵母醇脱氢酶的制备
种子培养基(g/L):蛋白胨,3;葡萄糖,50;酵母粉,2.5;MgSO4,0.5;KH2PO4,1.0;NaCl,0.5。pH值为6.0~6.5。
从斜面挑取一环接种到内有50 mL种子培养基的250 mL锥形瓶中,在温度为28 ℃、转速为120 r/min的摇床中培养28 h后,将培养液离心(在6 000 r/min转速下离心15 min),收集菌体。称量质量。将菌体悬浮在质量为其10倍的生理盐水中,混匀,用超声波破碎仪对菌体进行破碎(功率为400 W,探头振幅为25%),破壁次数为120次(操作时每工作8 s停8 s,间歇操作)。将破壁后的溶液离心(在6 000 r/min转速下离心10 min)后获得酵母醇脱氢酶的粗酶液。而后在粗酶液中缓慢加入硫酸铵至饱和度30%,在4 ℃静置,于4 000 r/min转速下离心20 min,弃沉淀,取上清液,加硫酸铵至饱和度70%;在4 ℃静置,于4 000 r/min转速下离心20 min,弃清液,沉淀用磷酸盐缓冲液(pH值为6.2)溶解后透析除盐初步纯化后,得到实验用酶液(蛋白质质量浓度为0.14 g/L,酶活力为471.0 U)。
1.3 酵母醇脱氢酶的固定化
将1 mL 酶活力为471.0 U的酶液 与1 mL 3%(质量分数)的海藻酸钠溶液混合后,用注射器(5号针头)滴到20 mL 2.0%的CaCl2溶液中,于25 ℃静置固化 6 h,过滤洗涤,加入到20 mL 0.5%(质量分数)戊二醛溶液中于25 ℃室温下交联2 h,经过滤﹑洗涤和干燥后得到颗粒状固定化酶。
1.4 酵母醇脱氢酶相对酶活力的测定
酶活的测定:酵母细胞产醇脱氢酶催化苯乙酮酸生成扁桃酸,反应过程需要辅酶NADH参与,NADH在波长340 nm处有特征吸收峰,而NAD+无吸收峰。故可通过测定340 nm处吸收值的变化来测定酶活[15]。
移取4 mL的磷酸盐缓冲液(浓度为0.02 mol/L, pH=6.8),1 mL 0.1 mol/L苯乙酮酸,1 mL 1 mmol/L NADH于30 ℃保温5 min 后,加入每次制备的固定化酶颗粒(即1 mL 游离酶制备成的固定化酶颗粒)或1 mL 游离酶(蛋白质含量为0.14 g/L),测量3 min内在340 nm处吸光度的变化。
酶活的定义:1 mL 游离酶或固定化酶(即由1 mL 游离酶制备成的固定化酶颗粒)在单位时间内(min)消耗NADH的量(nmol)定义为1个酶活单位(U)。
根据吸光度DA340,通过标准曲线换算出NADH的浓度c,再按下式计算酶活力f:
f=c×V×103/t。 (1)
其中:c为NADH浓度,mmol/L;V为酶液体积,mL;t为反应时间,min。
酶的活力回收率是指固定化酶活力与用于固定化的游离酶活力之比,%;酶的相对活力是指单因素条件下测得各组酶活力与该条件下酶的最高活力之比,%。
1.5 固定化酶包埋率的测定
构成蛋白质的某些氨基酸(如色氨酸、苯丙氨酸 等)在紫外区具有紫外吸收,通过对酵母醇脱氢酶磷酸缓冲溶液的全波长扫描发现在280 nm有最大吸收峰,故可通过测280 nm的吸光度来测定蛋白质含量。以牛血清蛋白(BSA)作为蛋白质标样,以BSA的浓度x为横坐标,A280为纵坐标y,标准曲线为y=0.086 98+ 0.630 3 x,显著性系数R=0.991 3。
将固定化酶颗粒用0.3 mol/L多聚磷酸钠溶解,剧烈搅拌破坏颗粒,离心分离,取上层清液测其在280 nm处的吸光度,而用于固定化的游离酶按相同比例稀释测其在280 nm处的吸光度,计算得到固定化酶和游离酶中的蛋白质含量,两者之比即为海藻酸钙凝胶固定化酶的包埋率。
2 结果与讨论
2.1 酵母醇脱氢酶固定化条件的优化
在海藻酸钠包埋酶的固定化方法中,固定化条件对酶活力影响很大。根据预备实验和其他的相关文献,选择对实验影响较大的5个因素,即固定化时间、固定化温度、海藻酸钠浓度、氯化钙浓度、戊二醛浓度,考察它们对固定化酶活力的影响。每个因素设4个水平,按正交设计表L16(45)安排实验,结果见表1和 表2。
表1 正交设计固定化条件因素水平
Table 1 Level of immobilization condition factor in orthogonal design
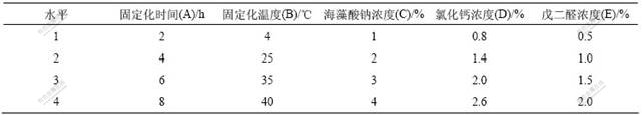
表2 正交实验直观分析
Table 2 Results of Orthogonal Experiment

从表2可以看出:第10组实验制备的固定化酶的活力为363.81 U,明显比其他组的高。通过极差分析(R)得到影响因素显著性的顺序从大至小为:A,C,B,E,D。通过比较每一组的K1,K2,K3和K4可选出优化水平组合,即A3B2C3D3E1。而此条件恰好不包含在已做过的16次实验中,按此条件进行确认试验,测得固定化酶的酶活力为383.51 U,综合以上结果得固定化最佳条件为:3.0%海藻酸钠,2.0%氯化钙,0.5%戊二醛,固定化温度为25 ℃,固定时间为6 h。其中,固定化时间和海藻酸钠浓度的影响最显著。
在实验中发现,海藻酸钠浓度偏低(<2%)时,酶泄漏严重,清洗酶珠时,酶损失较多,酶活力下降;海藻酸钠浓度偏高时,微环境效应和扩散阻力对反应影响很大,且不易操作,很难将酶液从注射器中挤出。因此,选用3%的海藻酸钠对酶进行固定化较适宜。
钙离子对固定化酶活力有2个方面的影响:一方面,钙离子浓度对固定化酶的强度起正面影响;另一方面,钙离子会抑制酶的活力。当CaCl2浓度小于1.4%时,造粒效果不理想,颗粒状固定化酵母醇脱氢酶的机械强度偏小,抽滤时容易塌陷变形,酵母醇脱氢酶包埋不完全;当CaCl2浓度过大时,高浓度的Ca2+对酵母醇脱氢酶活性有抑制作用。
戊二醛作为一种双功能交联剂[16],可以使海藻酸钠分子发生交联,同时,也能使酶交联在载体上,能够增强海藻酸钠的机械强度和固定化酶的稳定性,但同时也影响酵母醇脱氢酶的活性。
当固定化时间为6 h时,固定化酶活力最强。固定化时间过短或过长,固定化酶活力均有不同程度的下降。究其原因,固定化酶的活性受到2个方面的综合影响:一方面,随着固定化时间的延长,酵母醇脱氢酶固化越来越充分,酶在海藻酸钙凝胶中包埋得更牢固,有利于固定化酶活力的提高;另一方面,随着固定化时间的延长,Ca2+对固定化酶的抑制作用加剧,使酶活力下降。综合考虑以上因素,通过正交实验得到的最适固定化条件比较合理。
2.2 最佳固定化条件下固定化酶的包埋率
在最佳固定化条件下进行5组平行实验,测定蛋白质包埋率并计算相对标准偏差(RSD),结果如表3所示。由表3可见,平均包埋率为45.6%,相对标准偏差为4.6%。这说明该固定化条件较好,有较好的稳定性。
表3 固定化酶的包埋率
Table 3 Entrapment rate of immobilized SCAD

2.3 固定化酶酶学性质
2.3.1 固定化酶的最适反应pH值
以苯乙酮酸为酶促底物,将固定化酶和游离酶在不同pH值(5.0,5.8,6.4,6.8,7.0,7.4和8.0)的磷酸盐缓冲液中反应后,测量其酶活力,考察酶的最适反应pH值。实验结果如图1所示。

1—游离酶;2—固定化酶
图1 固定化酶的最适反应pH
Fig.1 Optimal reaction pH of immobilization SCAD
由图1可看到,游离酶的最适反应pH值为6.8,固定化酶的最佳反应pH值为5.8。酶经过固定化后,其最适pH值会发生一些变化。影响固定化酶的最适pH值的因素主要有2个:一个是载体的带电性质,另一个是酶催化反应产物的性质。一般来说,用带负电荷的载体制备的固定化酶,其最适pH值比游离酶的最适pH值高;用带正电荷的载体制备的固定化酶的最适pH值比游离酶的最适pH值低;而用不带电荷的载体制备的固定化酶,其最适pH值一般不改变。酶催化作用的产物性质对固定化酶的最适pH值有一定的影响。一般来说,当催化反应的产物呈酸性时,固定化酶的最适pH值要比游离酶的最适pH值高一些;当产物呈碱性时,固定化酶的最适pH值要比游离酶的最适pH值低一些;当产物呈中性时,最适宜pH值一般不变[17]。海藻酸钙凝胶带正电,而固定化酶催化苯乙酮酸生产扁桃酸,产物呈酸性,2个因素作用的方向相反。在此实验中,可能前者因素起主要作用,故结果显示固定化酵母醇脱氢酶的最适反应pH值为5.8。
2.3.2 固定化酶的最适反应温度
分别在4,20,30,40和50 ℃用固定化酵母醇脱氢酶和游离酶催化苯乙酮酸,其结果见图2。游离酶的最适反应温度为30 ℃,而固定化酶的最适反应温度上升至40 ℃。固定化酶的最适温度有明显提高,可能是固定化载体提高了酶的空间结构对热的稳定性;但是当温度超过40 ℃后,相对酶活力大大降低,可能是因为高温使酶蛋白变性。所以,固定化酶的最佳催化温度为40 ℃。酵母醇脱氢酶经固定化后,最适反应温度的提高不仅能提高反应速度,同时可以防止外界微生物的污染,有利于酶的工业化应用。
1—游离酶;2—固定化酶
图2 固定化酶的最适反应温度
Fig.2 Optimal reaction temperature of immobilization SCAD
2.3.3 固定化酶的热稳定性
将固定化酶和游离酶在40,45,50,55,60,65和70 ℃保温1 h,测其酶活力,考察固定化酶的热稳定性,其结果见图3。SCAD经固定化后,热稳定性显著提高,游离酶在65 ℃保温1 h,酶活力仅剩22%;

1—游离酶;2—固定化酶
图3 固定化酶的热稳定性
Fig.3 Thermal stability of immobilized SCAD
在70 ℃时酶蛋白变性失去活力,而固定化酶在65 ℃保温1 h保持64%的酶活力,在70 ℃仍可保留48.6%。这可能是SCAD经海藻酸钠包埋和戊二醛交联后,酶蛋白被固定在凝胶中,分子整体运动受到限制,阻挡了不利因素对酶的作用,增加了酶构型的牢固程度,从而增加了酶的热稳定性。
2.3.4 固定化酶的pH值稳定性
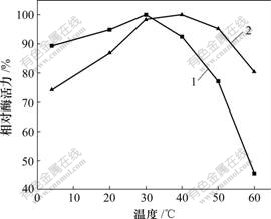
将固定化酶和游离酶置于不同pH值(4.0,5.0,5.8,6.4,7.0,7.4和8.0)的缓冲液中室温静置1 h后,测定酶活力,考察固定化酶的pH值稳定性(图4)。由图4可见,固定化酶和游离酶在酸性环境下,酶活力强;而在碱性环境下,酶活力急剧下降。并且当pH>7.0时,固定化酶颗粒开始溶解,机械强度变小,酶活力迅速降低,因此,海藻酸钠作为酵母醇脱氢酶的包埋载体,其耐碱性能有待提高。
1—游离酶;2—固定化酶
图4 固定化酶的pH稳定性
Fig.4 pH stability of immobilized SCAD
2.3.5 固定化酶的操作稳定性和贮存稳定性
将固定化酶在最适反应条件下(pH=5.8,温度为40 ℃)反应,测其酶活力。而后取反应后的固定化酶,用蒸馏水洗涤、抽滤3次,在最适条件下进行第2 次反应,如此反复。以第1次酶活力作为100%,测得前5次的相对酶活力分别为100%,67.78%,62.86%,42.65%和39.63%。随着反应次数增加,粒子逐渐胀大,酶活力降低。经过5次循环后,相对酶活力保持在39.63%。
在不加任何保护剂的条件下,以1 mL游离酶固定化所得的固体颗粒与1 mL游离酶比较,将固定化酶和游离酶于4 ℃放置20 d后游离酶活力下降近70%,而固定化酶活力下降不超过30%。可见,固定化酶的贮存稳定性比游离酶的贮存稳定性强。
2.4 酶促反应动力学
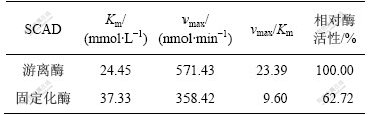
分别配制浓度为0.02,0.04,0.06,0.08和1.00 mol/L的苯乙酮酸溶液,按照SCAD酶活测定方法,测定在不同底物浓度([S])下游离酶和固定化酶的反应初速度(v)。依据米氏方程,利用Lineweaver-Burk双倒数法以1/ v对1/[S]作图(图5),并对数据进行线性回归,得到游离酶和固定化酶的动力学参数表观米氏常数Km和最大反应速率vmax,如表4所示。
表4 游离SCAD和固定化SCAD的动力学参数
Table 4 Kinetic parameters for free and immobilized SCAD
 .
.

1—游离SCAD酶;2—固定化SCAD酶
图5 由Lineweaver-Burk双曲线法得到的底物浓度和反应初速度之间的关系
Fig.5 Relationship between substrated concentration and velocity using Lineweaver-Burk double reciprocal plots
由表4可知,固定化SCAD的Km (37.33 mmol/L)是游离SCAD的Km(24.45 mmol/L)的1.53倍。Km变大,说明固定化SCAD对底物的亲和力有所降低。这可能是由于受内扩散限制,固定化凝胶粒附近底物的浓度要比溶液中的低,导致底物与酶的接触几率变小,亲和力降低。固定化SCAD的vmax为游离SCAD的1/1.59,固定化酶保留了62.72%的游离酶的活性,这可能是经过戊二醛的交联作用,引起酶空间结构发生变化,从而使酶的催化活性降低。
SCAD催化苯乙酮酸还原为(R)-扁桃酸的反应机理为:在反应过程中,苯乙酮酸的羧基接受来自NADH的氢负离子,同时又与酶结合,使羰基还原为羟基生成产物(R)-扁桃酸,辅酶NADH则转化为NAD+(如图6所示)。

图6 SCAD催化苯乙酮酸还原为(R)-扁桃酸的反应机理
Fig.6 Reaction mechanism of SCAD catalyzed resolution of phenylglyoxylic acid to (R)-mandelic acid
3 结 论
a. 采用海藻酸钙包埋﹑戊二醛交联的方法固定化酵母醇脱氢酶SCAD。利用正交实验设计法对固定化SCAD酶的条件进行了优化。固定化SCAD最佳条件如下:3.0% 海藻酸钠,2.0% 氯化钙,0.5% 戊二醛,固定化温度为25 ℃,固定时间6 h,此时,固定化酶的酶活力为383.51 U。
b. 利用固定化SCAD酶为生物催化剂,催化苯乙酮酸来合成(R)-扁桃酸。固定化SCAD酶的最适酶促反应温度为40 ℃,最适反应pH值为5.8。与游离SCAD酶相比,固定化SCAD酶的热稳定性显著提高,在 65 ℃时保持64%的酶活力,在70 ℃时酶活力仍可保留48.6%,而游离SCAD酶在70 ℃时变性失去活力。
c. 固定化SCAD的Km是游离SCAD的1.53倍,SCAD对底物的亲和力有所降低。固定化SCAD的vmax为游离SCAD的1/1.59,固定化酶保留了62.72%的游离酶的活性。重复利用固定化SCAD酶5次后仍保持39.63%酶活力,在4 ℃放置20 d后,固定化SCAD能保持70%的酶活力,显示出较强的操作稳定性和储存稳定性。
参考文献:
[1] Challener C A. Chiral intermediates[M]. London: Ashgate Publisher, 2001: 502-504.
[2] Fulenmeier A, Quitt P, Volgler K, et al. 6-Acyl derivatives of aminopenicillanic acid: US Patent 3957758[P]. 1976-05-18.
[3] LI Gui-yin, HUANG Ke-long, JIAN Yu-ren, et al. Production of (R)-mandelic acid by immobilized cells of saccharomyces cerevisiae on chitosan carrier[J]. Process Biochemistry, 2007, 42: 1465-1469.
[4] Blacker A J, Houson I N. Preparation of mandelic acid derivatives: WO 02066410[P]. 2002-08-29.
[5] 吴珊珊. 相转移催化法合成扁桃酸的工艺研究[D]. 南京: 南京理工大学化工学院, 2003.
WU Shan-shan. The synthesis of mandelic acid in the presence of phase transfer catalyst[D]. Nanjing: School of Chemical Engineering of Nanjing University of Science &Technology, 2003.
[6] 阮源萍, 张雪曼, 陈安齐, 等. 2, 6-二-O-β-戊基糊精涂渍Symmetry C8 柱拆分扁桃酸及其类似物对映体[J]. 色谱, 2004, 22(4): 420-423.
RUAN Yuan-ping, ZHANG Xue-man, CHEN An-qi, et al. Enantiomeric separation of mandelic acid and its analogues high performance liquid chromatography with 2,6-di-O-pentyl-β- cyclodextrin coated symmetry C8 column[J]. Chinese Journal of Chromatography, 2004, 22(4): 420-423.
[7] Ganpatid Y, Sivakumar P. Enzyme-catalysed optical resolution of mandelic acid via methyl mandelate in non-aqueous media[J]. Journal of Biochemical Engineering, 2004, 19: 101-107.
[8] Silvia R, Amaya S, Vicente U, et al. Influence of the enzyme derivative preparation and substrate structure on the enantioselectivity of penicillin G acylase[J]. Enzyme and Microbial Technology, 2002, 31: 88-93.
[9] LI Gui-yin, HUANG Ke-long, JIAN Yu-ren, et al. Preparation and characterization of Saccharomyces cerevisiae alcohol dehydrogenase immobilized on magnetic nanoparticles. International Journal of Biological Macromolecules, 2008, 42: 405-412.
[10] XIAO Mei-tian, HUANG Ya-yan, SHI Xiao-ai, et al. Bioreduction of phenylglyoxylic acid to R-(-)-mandelic acid by Saccharomyces cerevisiae FD11b[J]. Enzyme and Microbial Technology, 2005, 37: 589-596.
[11] XIAO Mei-tian, HUANG Ya-yan, MENG Chun, et al. Kinetics of asymmetric reduction of phenylglyoxylic acid to R-(-)-mandelic acid by Saccharomyces Cerevisiae FDllb[J]. Chinese Journal Chemical Engineer, 2006, 14(1): 73-80.
[12] LIAO Ming-huang, CHEN Dong-wang. Immobilization of yeast alcohol dehydrogenase on magnetic nanoparticles for improving its stability[J]. Biotechnology Letters, 2001, 23: 1723-1727.
[13] Schopp W, Grunow M. Immobilization of yeast ADH by adsorption onto polyaminomethylstyrene[J]. Application Microbial Biotechnology, 1986, 24: 271-276.
[14] Cochrane F C, Petach H H, Henderson W. Application of tris(hydroxymethyl)phosphine as a coupling agent for alcohol dehydrogenase immobilization[J]. Enzyme and Microbial Technology, 1996, 18: 373-378.
[15] Pankaj S, Himani K, Uttam C B. Purification and characterization of an enantioselective carbonyl reductase from Candida viswanathii MTCC5158[J]. Process Biochemistry, 2007, 42: 1632-1640.
[16] Murat E, Antonio R M. Production of extracellular alkaline protease by immobilization of the marine bacterium Teredinobacter turnirae[J]. Process Biochemistry, 2003, 38: 1445-1450.
[17] 刘建龙, 王瑞明, 刘建军. 酶的固定化技术研究进展[J]. 中国酿造, 2005, 9: 4-6.
LIU Jian-long, WANG Rui-ming, LIU Jian-jun. Research progress in immobilized enzyme[J]. China Brewing, 2005, 9: 4-6.
收稿日期:2009-01-25;修回日期:2009-04-09
基金项目:国家自然科学基金资助项目(B061201);湖南省教育厅资助项目(07D044);中南大学博士后科学基金资助项目(2009年)
通信作者:杨栋梁(1954-),男,湖南益阳人,教授,从事生物材料和酶催化研究;电话:0731-88830827;E-mail: dongliangyang2004@yahoo.com.cn; klhuang@mail.csu.edu.cn