不同显微组织的块体双相Ag-25Cu合金在NaCl溶液中的腐蚀行为
来源期刊:中国有色金属学报(英文版)2019年第7期
论文作者:曹中秋 尹晓桐 贾中秋 田秋月 鲁捷 张轲 王艳
文章页码:1495 - 1502
关键词:纳米晶;Ag-Cu块体合金;机械合金化;液相还原;显微组织;电化学腐蚀
Key words:nanocrystalline; Ag-Cu bulk alloy; mechanical alloying; liquid phase reduction; microstructure; electrochemical corrosion
摘 要:为了更好地了解块体双相Ag-25Cu(at.%)合金的腐蚀机制,采用液相还原(LPR)、机械合金化(MA)和粉末冶金(PM)法分别制备显微组织不同的2种纳米晶和1种常规尺寸的Ag-25Cu块体合金,并采用电化学方法对比研究此3种不同显微组织Ag-25Cu合金在NaCl溶液中的腐蚀行为。结果表明:粉末冶金法制备的常规尺寸PMAg-25Cu合金的显微组织极不均匀;相反,液相还原法和机械合金化法制备的纳米晶LPRAg-25Cu和MAAg-25Cu合金的显微组织较均匀,而纳米晶LPRAg-25Cu合金的显微组织最均匀。MAAg-25Cu合金的腐蚀速率高于PMAg-25Cu合金的腐蚀速率,但低于LPRAg-25Cu合金的腐蚀速率。3种Ag-25Cu合金表面形成的钝化膜均具有n型半导体特征。LPRAg-25Cu合金的钝化电流密度低于PMAg-25Cu合金的钝化电流密度,但高于MAAg-25Cu合金的钝化电流密度。
Abstract: In order to have a better understanding on the corrosion mechanisms of bulk two-phase Ag-25Cu (at.%) alloys with different microstructures, two bulk nanocrystalline Ag-25Cu alloys and one coarse grained counterpart were prepared by liquid phase reduction (LPR), mechanical alloying (MA) and powder metallurgy (PM) methods, respectively. Their corrosion behavior was investigated comparatively using electrochemical methods in NaCl aqueous solution. Results show that the microstructure of the coarse grained PMAg-25Cu alloy is extremely inhomogeneous. On the contrary, compared with PMAg-25Cu alloy, the microstructures of the nanocrystalline LPRAg-25Cu and MAAg-25Cu alloys are more homogeneous, especially for LPRAg-25Cu alloy. The corrosion rate of MAAg-25Cu alloy is higher than that of PMAg-25Cu alloy, but lower than that of LPRAg-25Cu alloy. Furthermore, the passive films formed by three Ag-25Cu alloys exhibit n-type semiconducting properties. The passive current density of LPRAg-25Cu alloy is lower than that of PMAg-25Cu alloy, but higher that of MAAg-25Cu alloy.
Trans. Nonferrous Met. Soc. China 29(2019) 1495-1502
Zhong-qiu Cao1, Xiao-tong Yin1, Zhong-qiu Jia1, Qiu-yue Tian1, Jie Lu2, Ke ZHANG1, Yan WANG1
1. College of Chemistry and Chemical Engineering, Shenyang Normal University, Shenyang 110034, China;
2. Experimental Teaching Centre, Shenyang Normal University, Shenyang 110034, China
Received 30 October 2018; accepted 11 April 2019
Abstract: In order to have a better understanding on the corrosion mechanisms of bulk two-phase Ag-25Cu (at.%) alloys with different microstructures, two bulk nanocrystalline Ag-25Cu alloys and one coarse grained counterpart were prepared by liquid phase reduction (LPR), mechanical alloying (MA) and powder metallurgy (PM) methods, respectively. Their corrosion behavior was investigated comparatively using electrochemical methods in NaCl aqueous solution. Results show that the microstructure of the coarse grained PMAg-25Cu alloy is extremely inhomogeneous. On the contrary, compared with PMAg-25Cu alloy, the microstructures of the nanocrystalline LPRAg-25Cu and MAAg-25Cu alloys are more homogeneous, especially for LPRAg-25Cu alloy. The corrosion rate of MAAg-25Cu alloy is higher than that of PMAg-25Cu alloy, but lower than that of LPRAg-25Cu alloy. Furthermore, the passive films formed by three Ag-25Cu alloys exhibit n-type semiconducting properties. The passive current density of LPRAg-25Cu alloy is lower than that of PMAg-25Cu alloy, but higher that of MAAg-25Cu alloy.
Key words: nanocrystalline; Ag-Cu bulk alloy; mechanical alloying; liquid phase reduction; microstructure; electrochemical corrosion
1 Introduction
Nanocrystalline materials have attracted more attention due to their unique physical, chemical and mechanical properties [1-3]. Usually, the grain sizes are less than 100 nm, and the volume fractions of grain boundaries may exceed 50% in the nanocrystalline materials. These characteristics result in the corrosion behavior of nanocrystalline alloys rather different from that of their coarse grained counterparts. Thus, the researches on the corrosion behavior of nanocrystalline materials have become a hot topic in the corrosion field [4-10]. So far, the corrosion behavior of nanocrystalline materials has been reported by some researchers [11-13]. Wang et al [11] investigated the effect of grain size reduction on the electrochemical corrosion behavior of the nanocrystalline Ni produced by pulse electrodeposition. It is be found that the corrosion resistances of Ni coatings in alkaline aqueous solutions considerably increase as the grain sizes decrease from microcrystalline to nanocrystalline due to more rapid formation of continuous Ni(OH)2 passive films as compared with the corresponding coarse grained Ni coatings. Oguzie et al [12] observed the effect of surface nanocrystallization on the corrosion behavior of low carbon steels prepared by the magnetron sputtering technique. They pointed out that the surface nano- crystallization is able to increase the corrosion susceptibility of low carbon steels, leading to a decrease in interfacial impedance and an increase in the kinetics of the anodic reaction. According to this acceleration of dissolution, the change belongs to an increase of the active ability of metals on the nanocrystalline coatings, which accelerates the kinetics of the anodic reaction. Pinto et al [13] revealed the corrosion behavior of the nanocrystalline copper thin films deposited by the reactive magnetron sputtering method in 0.5 mol/L NaCl aqueous solution. They indicated that different surface morphologies and grain sizes in the corrosion processes may give rise to the formation of different oxide and chloride films. The copper thin film formed by the smaller grain size has a faster corrosion rate mainly because the number of grains per unit area increases, and thus the electroactive surface area also increases. However, so for, the systematic researches on this topic have been rare and the broad agreement on the corrosion mechanism has not occurred yet. Moreover, these studies were mainly focused on the corrosion resistances of coatings, thin films and surface nanocrystallizations. On the contrary, the corrosion behavior of bulk nanocrystalline materials, especially binary bulk nanocrystalline two-phase alloys, was very rarely reported. Thus, it is important to understand the corrosion mechanisms of bulk nanocrystalline alloys.
Based on the previous reports [14-17], it can be found that mechanical alloying (MA) and liquid phase reduction (LPR) are two kinds of extremely effective methods to prepare the nanocrystalline alloyed powders. Hot pressing the nanocrystalline alloyed powders has widely been used to obtain the bulk nanocrystalline materials with very high densities. For example, a bulk Ag-50Ni alloy with very high density was able to be prepared by hot pressing the nanocrystalline Ag-50Ni alloyed powders obtained through mechanically alloying. The continuous Ag nets are formed because the nanocrystalline Ag particles have very low melting point, and they were able to separate out from the supersaturated powders produced in the course of mechanically alloying [18]. Furthermore, another bulk nanocrystalline Ag-50Ni alloy was able to be prepared by hot pressing the alloyed powders obtained through liquid phase reduction, but the continuous Ag nets were not formed because the reactions are able to achieve a mixing on the molecular level, and at the same time the solid solubility of Ag in Ni for Ag-50Ni powders prepared by liquid phase reduction is lower than that by mechanical alloying. The microstructure of the nanocrystalline LPRAg-50Ni alloy is more homogeneous than that of the nanocrystalline MAAg-50Ni alloy, which may result in different corrosion behavior [19].
Ag-Cu alloys are similar to Ag-Ni alloys because silver and copper are also slightly soluble into each other within almost the whole composition range [20]. In the present work, two bulk nanocrystalline two-phase Ag-25Cu alloys were prepared by hot pressing the alloyed powders obtained through the liquid phase reduction and mechanical alloying method, and one coarse grained Ag-25Cu counterpart was prepared by the powder metallurgy (PM) method. The corrosion behavior of three Ag-25Cu alloys with different microstructures was also investigated in 0.6 mol/L NaCl aqueous solution in order to understand the corrosion behavior of bulk two-phase Ag-Cu alloys comprehensively.
2 Experimental
2.1 Sample preparation
A bulk nanocrystalline Ag-25Cu (at.%) alloy was prepared by hot pressing the alloyed powders obtained through the liquid phase reduction method, and here denoted as LPRAg-25Cu. The preparation processes were described below. The alloyed powders were synthesized after AgNO3 and CuSO4 aqueous solutions were added to N2H4 aqueous solution (pH=11) at 70 ℃, and afterwards they were filtered, washed with water, and dried. Figure 1 shows the X-ray diffraction (XRD) pattern of LPRAg-25Cu alloyed powders. Figure 2 shows the transmission electron microscopy (TEM) image of LPRAg-25Cu alloyed powders. It can be seen that these alloyed powders have no impurities with the average particle size of about 10 nm. Hot pressing was finished in a vacuum hot pressing furnace made by Shenyang Weitai Science & Technology Development Company of China. The alloyed powders were put in a graphite die with 20 mm in inner diameter, and afterwards pressed for 10 min under the conditions of pressure of 86 MPa, temperature of 730 ℃, and vacuum of 0.06 Pa. The density of the bulk LPRAg-25Cu alloy calculated by the drainage method exceeded 99%. The average grain size was measured by the X-ray diffraction method and its value is about 13 nm after liquid phase reduction and about 27 nm after hot pressing.
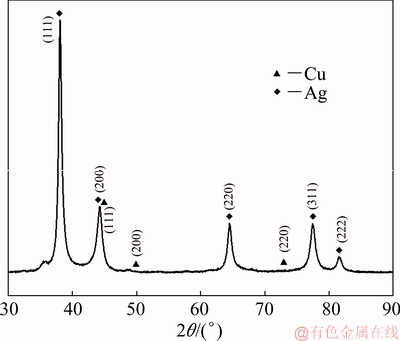
Fig. 1 XRD pattern of LPRAg-25Cu alloyed powders
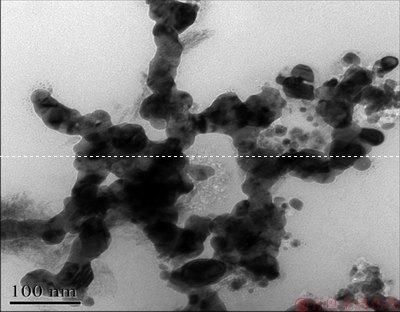
Fig. 2 TEM image of LPRAg-25Cu alloyed powders
The other bulk nanocrystalline Ag-25Cu alloy was prepared by hot pressing the alloyed powders obtained through the mechanical alloying method, and here denoted as MAAg-25Cu. The mechanical alloying processes were carried out in a QM-1SP planetary miller made by Nanjing University Instrument Company of China. Firstly, Ag and Cu powders (purity >99.9%, about 100 μm in grain size) were put in a stainless steel vial with mass ratio of powders to hardened GCr15 steel balls about 1:10. Secondly, the vial was evacuated to about 10 Pa and afterwards filled with highly pure argon in order to prevent these powders from being oxidized in the course of ball milling. Moreover, there were intermediate stops of 15 min after these powders were milled for 1 h in order to prevent the excessive heat effect. The whole powders were milled for 60 h in order to get a mixing on the nanometre level. Finally, the hot pressing processes of MAAg-25Cu alloy were similar to those of the above LPRAg-25Cu alloy. The density of the bulk alloy calculated by the drainage method also exceeded 99%. The average grain size was measured by the X-ray diffraction method and its value was about 8 nm after mechanical alloying and about 19 nm after hot pressing.
In order to compare the corrosion electrochemical properties with two bulk nanocrystalline alloys, a coarse grained Ag-25Cu counterpart was prepared by the powder metallurgy (PM) method, and here denoted as PMAg-25Cu. The preparation processes of PMAg- 25Cu alloy were similar to those of MAAg-25Cu alloy, but the time of ball milling was 1 h only. PMAg-25Cu alloy ingot was subsequently annealed in vacuum at 800 ℃ for 54 h to remove the residual mechanical stress and achieve a better phase equilibration with grain size of about 120 μm.
2.2 Electrochemical measurements
All electrochemical measurements were performed using PARM273A and M5210 potentiostat/galvanostat systems made in EG&G Company of USA. A three-electrode cell was used with saturated calomel electrode (SCE) as the reference electrode, platinum electrode as the counter electrode, and the sample as working electrode. Polarization ranges were from -1.2 to 1.5 V versus open circuit potential (OPC). The scanning rate was 0.5 mV/s. Frequency ranges were from 105 to 10-2 Hz. Electrochemical parameters were fitted by Corrview software. Electrochemical impedance spectro- scopy (EIS) analysis and fitting circuits were carried out by Zview2 software.
3 Results and discussion
3.1 Sample microstructures
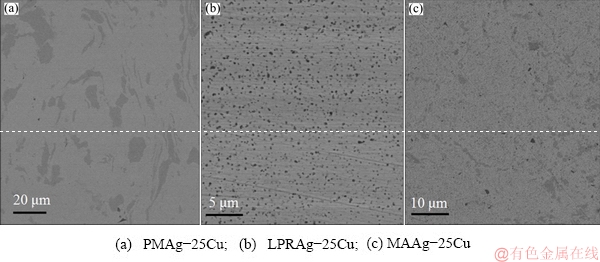
Fig. 3 Microstructures of three Ag-25Cu alloys prepared by different methods
Figure 3 shows the microstructures of three Ag-25Cu alloys prepared by different methods. The actual average compositions of three Ag-25Cu alloys are 73.22 at.% Ag and 26.78 at.% Cu for PMAg-25Cu alloy, 75.39 at.% Ag and 24.61 at.% Cu for LPRAg-25Cu alloy, 77.62 at.% Ag and 22.38 at.% Cu for MAAg-25Cu alloy according to the analysis by scanning electron microscopy and energy-dispersive X-ray microanalysis (SEM/EDX). All three Ag-25Cu alloys are composed of two phases. One is α phase rich in Ag, the other is β phase rich in Cu. In PMAg-25Cu alloy, the light a phase contains about 94.24 at.% Ag and 5.76 at.% Cu, and the medium grey b phase contains about 8.64 at.% Ag and 91.36 at.% Cu. In LPRAg-25Cu alloy, the light a phase contains about 98.80 at.% Ag and 1.20 at.% Cu, and the medium grey b phase contains about 37.30 at.% Ag and 63.70 at.% Cu. In MAAg-25Cu alloy, the light a phase contains about 85.02 at.% Ag and 14.98 at.% Cu, and the medium grey b phase contains about 24.48 at.% Ag and 75.52 at.% Cu. The microstructure of the coarse grained PMAg-25Cu alloy is extremely inhomogeneous. On the contrary, the microstructure of the nanocrystalline LPRAg-25Cu alloy or MAAg-25Cu alloy is more homogeneous than that of PMAg-25Cu alloy, while the microstructure of LPRAg-25Cu alloy is the most homogeneous.
3.2 Corrosion properties
Figure 4 exhibits the curves of open circuit potentials versus time for three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution. It can be found that the free corrosion potentials become stable rapidly with time. The free corrosion potential is equal to -60 mV for the coarse grained PMAg-25Cu alloy, -11 mV for the nanocrystalline LPRAg-25Cu alloy, and 5 mV for the nanocrystalline MAAg-25Cu alloy. The free corrosion potential of the nanocrystalline LPRAg-25Cu alloy is higher than that of the coarse grained PMAg-25Cu alloy, but lower than that of the nanocrystalline MAAg-25Cu alloy. It can be seen that the free corrosion potentials of two nanocrystalline Ag-25Cu alloys move toward positive values in different degrees, which indicates that their corrosion tendencies decrease with the grain size reduction.
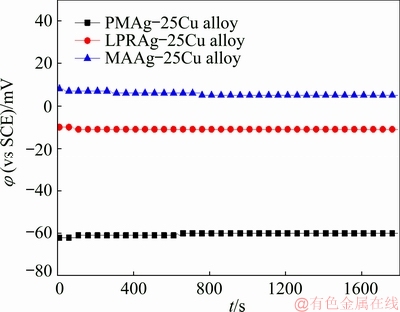
Fig. 4 Curves of open circuit potentials versus time for three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution
Figure 5 exhibits the potentiodynamic polarization curves of three Ag-25Cu alloys with different micro-structures in 0.6 mol/LNaCl aqueous solution. Table 1 lists the electrochemical parameters fitted by Corroview software such as corrosion potentials (φcorr), corrosion current densities (Jcorr), passivation potentials (φp), passivation current densities (Jmax), and passive current densities (Jp). It can been seen that the corrosion current density of MAAg-25Cu alloy is higher than that of PMAg-25Cu alloy, but is lower than that of LPRAg-25Cu alloy. Thus, the corrosion rates become faster after the grain sizes are decreased, which also indicates that corrosion properties decrease with the grain size reduction.
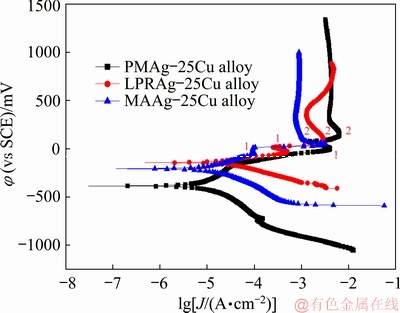
Fig. 5 Potentiodynamic polarization curves of three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution
Table 1 Electrochemical parameters of three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution
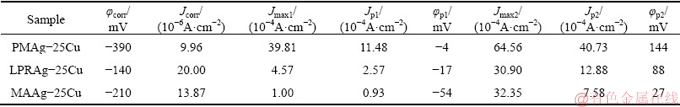
Fig. 6 EIS plots of three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution
Figure 6 exhibits the EIS plots of Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution. The EIS plot of the coarse grained PMAg- 25Cu alloy is composed of a single capacitive loop without the diffusion-induced Warburg impedance tail. This also indicates that the corrosion process of the alloy is controlled by the electrochemical reactions. On the contrary, the EIS plot of the nanocrystalline MAAg-25Cu or LPRAg-25Cu alloy is composed of a single capacitive loop with the diffusion-induced Warburg impedance tail. This also indicates that the corrosion processes of these two nanocrystalline Ag-25Cu alloys are controlled by the diffusion. Moreover, there is a contraction phenomenon of the real part in low frequency range. This shows the absorption and desorption of passive films in the course of the corrosion [21]. Figure 7 exhibits the equivalent circuits of three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution, where Rs is denoted as the solution resistance, Wo as Warburg resistance, Rt as the charge transfer resistance, and CPE as the constant phase angle element by dispersion effect. The impedance of CPE could be calculated by Formula (1) [22]
Z=T-1ω-P[cos(Pπ/2)-jsin(Pπ/2)] (1)
where Z is denoted as the impedance of CPE, j as the imaginary part, ω as the angular frequency, T as a constant, and P as the exponent (0≤P≤1). Moreover, Warburg resistance, Wo, consists of the resistance R and the impedance of CPE. And Wo may be described by Wo-R, Wo-T and Wo-P.
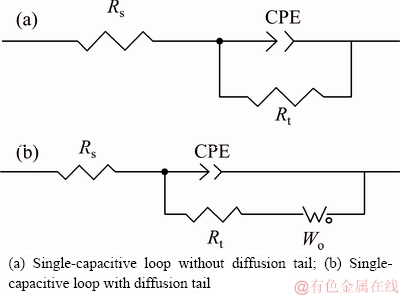
Fig. 7 Equivalent circuits of three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution
The fitting results have been listed in Table 2. The charge transfer resistance of MAAg-25Cu alloy is lower than that of PMAg-25Cu alloy, but is higher than that of LPRAg-25Cu alloy. Thus, the corrosion rate of LPRAg-25Cu or MAAg-25Cu alloy becomes faster than that of PMAg-25Cu alloy. They are consistent with the results of polarization curves.
The corrosion rates of alloys depend on the activation energy (Ea) of corrosion processes according to the Arrhenius equation. Ea is able to be obtained from the following formula [23]:
lg Jcorr=lg A–Ea/(2.303RT) (2)
where A and R are denoted as the pre-exponential factor and the mole gas constant (8.314 J/(mol·K)), respectively. It can be illustrated that the high value of Ea shows the slow corrosion rate of the alloy. Figure 8 displays the Arrhenius plots of three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution. The values of Ea were calculated and they are 22.92 kJ/mol for PMAg-25Cu alloy, 18.15 kJ/mol for LPRAg-25Cu alloy, and 19.67 kJ/mol for MAAg-25Cu alloy. The lower Ea values of two nanocrystalline Ag-25Cu alloys indicate that the corrosion rate of the nanocrystalline alloy is faster than that of the corresponding coarse grained alloy. The faster corrosion rates are attributed to different microstructures of three Ag-25Cu alloys prepared by different methods. In fact, after the grain sizes are reduced to the nanometer range by the mechanically alloying or liquid phase reduction method, these alloys are able to produce large densities of grain boundaries. A large number of atoms and defects near the grain boundaries are arranged irregularly, and their lattice distortional energies increase. The grain boundaries have a trend to decrease their energy automatically. When the alloys were immersed in NaCl aqueous solution, these large concentrations of grain boundaries and defects are much easier to be dissolved than the others. Thus, the corrosion rate of the nanocrystalline LPRAg-25Cu or MAAg-25Cu alloy is higher than that of the corresponding coarse grained PMAg-25Cu alloy. Moreover, the grain size is about 19 nm for the bulk MAAg-25Cu alloy, about 26 nm for the bulk LPRAg-25Cu alloy. Even though the grain size of MAAg-25Cu alloy is a little lower than that of LPRAg-25Cu alloy, the phase distribution of LPRAg-25Cu alloy is more homogeneous than that of MAAg-25Cu alloy and there are more electroactive points in LPRAg-25Cu alloy. It can be concluded that the corrosion rate of the nanocrystalline LPRAg-25Cu alloy is higher than that of the nanocrystalline MAAg- 25Cu alloy finally.
Table 2 Equivalent circuit parameters of three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution
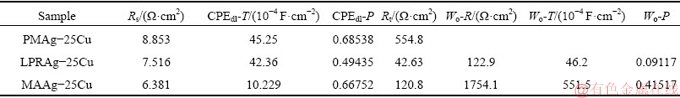
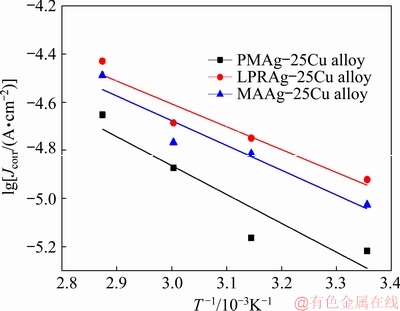
Fig. 8 Arrhenius plots of three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution
From Fig. 5 and Table 1, it can be seen that all the three Ag-25Cu alloys produce the passive phenomena with the increment of polarization potentials in 0.6 mol/L NaCl aqueous solution. When the polarization potentials keep at low values, the first passive phenomena produce and their passivation ranges are very narrow. The passivation potential φp1 and passive current density Jp1 of LPRAg-25Cu alloy are lower than those of PMAg- 25Cu alloy, but higher than those of MAAg-25Cu alloy. Moreover, when the polarization potentials keep at high values, the second passive phenomena occur in all three Ag-25Cu alloys and their passive ranges are relatively wide. The passivation potential φp2 and passive current density Jp2 of LPRAg-25Cu alloy are also lower than those of PMAg-25Cu alloy, but higher than those of MAAg-25Cu alloy. Thus, it can be concluded that MAAg-25Cu alloy can produce the passivation easily, and its passive properties are also higher than those of LPRAg-25Cu or PMAg-25Cu alloys.
In order to investigate the properties of passive films further, the passive films on three Ag-25Cu alloy surfaces were prepared for 30 min under 0.3 V (vs SCE). Figure 9 displays the Mott-Schottky curves for the passive films on three Ag-25Cu alloy surfaces in 0.6 mol/L NaCl aqueous solution. It can be seen that all three curves are straight lines with positive slopes. According to Mott–Schottky theory, if the slope of the straight line is a positive value, the type of the passive film should belong to the n-type semiconductor. The positive slopes of three straight lines also indicate that the types of passive films are unable to change after the grain sizes are decreased to the nanometer ranges. Moreover, the relationship between space charge capacitance (CSC) and applied potential (φ) for the n-type semiconductor can be described by the following formula [24]
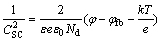 (3)
(3)
where Nd is denoted as the carrier concentration of the donor, ε as the dielectric constant of passive films (9.52 [25]), ε0 as the vacuum permittivity, e as the elementary charge, k as the Boltzman constant, T as the absolute temperature, and φfb as the flat band potential. It is well known that the smaller the carrier concentration is, the lower the conductivity becomes, which will result in a decrease in the passive current density. The carrier concentration of the passive film can be calculated from a slope in Formula (3). It is 6.00×1019 cm-3 for the coarse grained PMAg-25Cu alloy, 1.08×1019 cm-3 for the nanocrystalline LPRAg-25Cu alloy, and 6.33×1018 cm-3 for the nanocrystalline MAAg-25Cu alloys. This also indicates that the conductivity of the passive film on the nanocrystalline LPRAg-25Cu alloy is lower than that on the coarse grained PMAg-25Cu alloy, but higher than that of the nanocrystalline MAAg-25Cu alloy. Thus, the nanocrystalline MAAg-25Cu alloy with the lowest carrier concentration in the passive films has the lowest passive current density and highest stability. They are consistent with the passive current densities measured by the polarization curves.
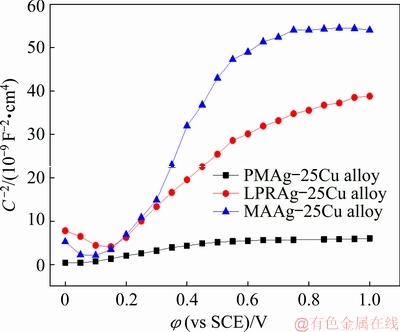
Fig. 9 Mott-Schottky curves for passive films formed on three Ag-25Cu alloy surfaces in 0.6 mol/L NaCl aqueous solution
Figure 10 shows the surface morphologies of three Ag-25Cu alloys with different microstructures after the potentiodynamic polarization in 0.6 mol/L NaCl aqueous solution. It can be seen that all three Ag-25Cu alloys show the corrosion structure of rod-like particles. The passive films of MAAg-25Cu or LPRAg-25Cu alloy are more compact than those of PMAg-25Cu alloy, while the passive films of MAAg-25Cu alloy are the most compact among three alloys. The analysis of SEM/EDX results shows that the passive films of three Ag-25Cu alloys are composed of oxides and chlorides of copper, and chlorides of silver. The lighter corrosion products are mainly composed of chlorides of silver, and the grey corrosion products are mainly composed of oxides and chlorides of copper. Figure 11 shows the X-ray diffraction (XRD) patterns of corrosion surfaces for three Ag-25Cu alloys with different microstructures after the potentiodynamic polarization in 0.6 mol/L NaCl aqueous solution. According to XRD analysis, some peaks for CuCl, CuCl2, Cu2O and AgCl are present, indicating that the corrosion products may be composed of CuCl, CuCl2, Cu2O and AgCl.
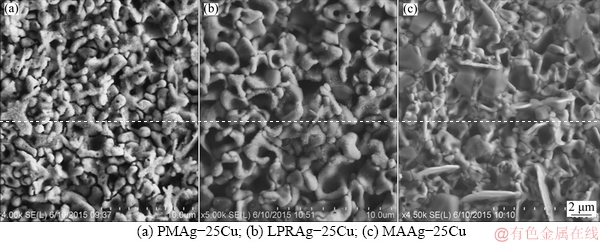
Fig. 10 Morphologies of corrosion surfaces for three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution
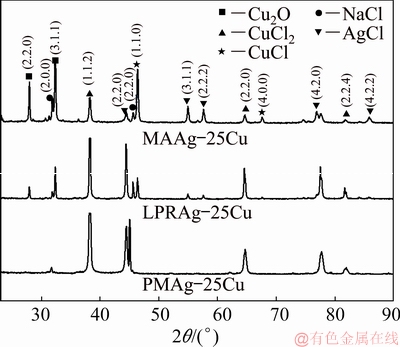
Fig. 11 XRD patterns of corrosion surfaces for three Ag-25Cu alloys with different microstructures in 0.6 mol/L NaCl aqueous solution
The cathodic reaction may be described by Formula (4):
O2+2H2O+4e=4OH- (4)
The main anodic dissolution reactions may be described as follows [26,27]:
Cu+Cl-=CuCl+e (5)
CuCl+Cl-= (6)
(6)
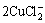 +2OH-=Cu2O+H2O+4Cl- (7)
+2OH-=Cu2O+H2O+4Cl- (7)
Cu+2Cl-=CuCl2+2e (8)
2CuCl2+2OH-+2e=Cu2O+4Cl-+2H2O (9)
Ag+Cl-=AgCl+e (10)
4 Conclusions
(1) The free corrosion potentials of two nano- crystalline Ag-25Cu alloys move toward the positive values in different degree, indicating their corrosion tendencies decrease with the grain size reduction. The corrosion rates of three Ag-25Cu alloys increase in the order of PMAg-25Cu, MAAg-25Cu and LPRAg-25Cu alloys. The corrosion rates of two nanocrystalline Ag-25Cu alloys become faster due to the large grain size reduction and more homogeneous phase distribution in nanocrystalline alloys.
(2) The EIS plot of PMAg-25Cu alloy is composed of a single capacitive loop without the diffusion-induced Warburg impedance tail. The corrosion process is controlled by electrochemical reactions. On the contrary, the EIS plot of LPRAg-25Cu or MAAg-25Cu alloy is composed of a single capacitive loop with the diffusion- induced Warburg impedance tail. Their corrosion processes are controlled by diffusion.
(3) All three Ag-25Cu alloys produce the second passive phenomena. The passive films formed on three Ag-25Cu alloy surfaces exhibit n-type semiconducting properties. Passive current densities of three Ag-25Cu alloy decrease in the order of PMAg-25Cu, LPRAg-25Cu and MAAg-25Cu. Thus, the chemical stability of the passive film on MAAg-25Cu alloy surface is higher than that on LPRAg-25Cu or PMAg-25Cu alloy surface.
References
[1] Lu K. Surface nanocrystallization (SCN) of metallic materials— Presentation of the concept behind a new approach [J]. Journal of Materials Science and Technology, 1999, 15: 193-197.
[2] PITCHAYYAPILLAI G, SEENIKANNAN P, BALASUNDAR P, NARAYANASAMY P. Effect of nano-silver on microstructure, mechanical and tribological properties of cast 6061 aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 2137-2145.
[3] HU Kun, YUAN Du, Lü Shu-lin, WU Shu-sen. Effects of nano-SiCp content on microstructure and mechanical properties of SiCp/A356 composites assisted with ultrasonic treatment [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 2173-2180.
[4] Chianpairot A, Lothongkum G, Schuh C A, Boonyongmaneerat Y. Corrosion of nanocrystalline Ni-W alloys in alkaline and acidic 3.5 wt.% NaCl solutions [J]. Corrosion Science, 2011, 53: 1066-1071.
[5] Meng G Z, Li Y, Wang F H. The corrosion behavior of Fe-10Cr nanocrystalline coating [J]. Electrochimica Acta, 2006, 51: 4277-4284.
[6] Luo W, Qian C, Wu X J, Yan M. Electrochemical corrosion behaviour of nanocrystalline copper bulk [J]. Materials Science and Engineering A, 2007, 452-453: 524-528.
[7] Pan C, Liu L, Li Y, Wang F H. Pitting corrosion of 304SS nanocrystalline thin film [J]. Corrosion Science, 2013, 73: 32-43.
[8] Bakkar A, Neubert V. Electrodeposition and corrosion characterisation of micro- and nano-crystalline aluminium from AlCl3/1-ethyl-3-methylimidazolium chloride ionic liquid [J]. Electrochimca Acta, 2013, 103: 211-218.
[9] Lu H B, Li Y, Wang F H. Dealloying behaviour of Cu-20Zr alloy in hydrochloric acid solution [J]. Corrosion Science, 2006, 48: 2106-2119.
[10] Liu L, Li Y, Wang F H. Influence of nanocrystallization on passive behaviour of Ni-based superalloy in acidic solutions [J]. Electrochimca Acta, 2007, 52: 2392-2400.
[11] Wang L P, Zhang J Y, Gao Y, Xue Q J, Hu L T, Xu T. Grain size effect in corrosion behaviour of electrodeposited nanocrystalline Ni coatings in alkaline solution [J]. Scripta Materialia, 2006, 55: 657-660.
[12] Oguzie E E, Li Y, Wang F H. Effect of surface nano- crystallization on corrosion and corrosion inhibition of low carbon steel: Synergistic effect of methionine and iodide ion [J]. Eelectrochimica Acta, 2007, 52: 6988-6996.
[13] Pinto E M, Ramos A S, Vieira M T, Brett C M A. A corrosion study of nanocrystalline copper thin films [J]. Corrosion Science, 2010, 52: 3891-3895.
[14] Benjamin J S. Dispersion strengthened superalloys by mechanical alloying [J]. Metallurgical Transactions, 1970, 1: 2943-2951.
[15] Xu J, Herr U, Klassen T, Averback R S. Formation of supersaturated solid solutions in the immiscible Ni-Ag system by mechanical alloying [J]. Journal of Applied Physics, 1996, 79: 3935-3945.
[16] RAJABI M, SEDIGHI R M, RABIEE S M. Thermal stability of nanocrystalline Mg-based alloys prepared via mechanical alloying [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 398-405.
[17] Zhu H T, Zhang C Y, Yin Y S. Rapid synthesis of copper nanoparticles by sodium hypophosphite reduction in ethylene glycol under microwave irradiation [J]. Journal of Crystal Growth, 2004, 270: 722-728.
[18] Wang C L, Lin S Z, Niu Y, Wu W T, Zhao Z L. Microstructual properties of bulk nanocrystalline Ag-Ni alloy prepared by hot pressing of mechanically pre-alloyed powders [J]. Applied Physics A—Materials Science and Processing, 2003, 76: 157-163.
[19] Cao Z Q, Zhu X M, Li F C. Study on Nanocrystalline bulk Ag-50Ni alloy prepared by aqueous reducing method [J]. Rare Metal Materials and Engineering, 2008, 37: 1221-1224. (in Chinese)
[20] VILLARS P, PRINCE A, OKAMOTO H. Handbook of binary alloys phase diagrams [M]. Ohio: ASM International in Materials Park, 1997.
[21] Conway B E, Bockris J O, White R E. Modern aspects of electrochemical impedance spectroscopy and its applications [M]. New York: Kluwer Academic/Plenum Publishers, 1999.
[22] Cao C N. Corrosion electrochemistry [M]. Beijing: Chemical Industry Press, 1994. (in Chinese)
[23] Atkins P W. Physical chemistry [M]. 15th ed. Oxford: Oxford University Press, 1994.
[24] Morison S R. Electrochemistry at semiconductor and oxidized metal electrodes [M]. New York: Plenum Press, 1980.
[25] Young K F, Frederikse H P R. Compilation of the static dielectric constant of inorganic solids [J]. Journal of Physical & Chemical Reference Data, 1973, 2: 313-410.
[26] Kear G, Barker B D, Walsh F C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review [J]. Corrosion Science, 2004, 46: 109-135.
[27] Zhu X L, Lin L Y, Lei T Q. Process of formation of corrosion films on alloy 70Cu-30Ni in seawater [J]. Acta Metallurgica Sinica, 1997, 33: 1256-1261. (in Chinese).
曹中秋1,尹晓桐1,贾中秋1,田秋月1,鲁 捷2,张 轲1,王 艳1
1. 沈阳师范大学 化学化工学院,沈阳 110034;2. 沈阳师范大学 实验教学中心,沈阳 110034
摘 要:为了更好地了解块体双相Ag-25Cu(at.%)合金的腐蚀机制,采用液相还原(LPR)、机械合金化(MA)和粉末冶金(PM)法分别制备显微组织不同的2种纳米晶和1种常规尺寸的Ag-25Cu块体合金,并采用电化学方法对比研究此3种不同显微组织Ag-25Cu合金在NaCl溶液中的腐蚀行为。结果表明:粉末冶金法制备的常规尺寸PMAg-25Cu合金的显微组织极不均匀;相反,液相还原法和机械合金化法制备的纳米晶LPRAg-25Cu和MAAg-25Cu合金的显微组织较均匀,而纳米晶LPRAg-25Cu合金的显微组织最均匀。MAAg-25Cu合金的腐蚀速率高于PMAg-25Cu合金的腐蚀速率,但低于LPRAg-25Cu合金的腐蚀速率。3种Ag-25Cu合金表面形成的钝化膜均具有n型半导体特征。LPRAg-25Cu合金的钝化电流密度低于PMAg-25Cu合金的钝化电流密度,但高于MAAg-25Cu合金的钝化电流密度。
关键词:纳米晶;Ag-Cu块体合金;机械合金化;液相还原;显微组织;电化学腐蚀
(Edited by Wei-ping CHEN)
Foundation item: Projects (51271127, 51501118) supported by the National Natural Science Foundation of China; Project (2018304025) supported by Liaoning Provincial Key Research and Development Program, China; Project (201602679) supported by the Natural Science Foundation of Liaoning Province, China
Corresponding author: Zhong-qiu CAO; Tel: +86-24-86593313; E-mail: caozhongqiu6508@sina.com
DOI: 10.1016/S1003-6326(19)65056-9

