Removal of aqueous Ni(II) with carbonized leaf powder:Kinetics and equilibrium
来源期刊:中南大学学报(英文版)2016年第4期
论文作者:王恒宇 唐强 唐晓武 王艳
文章页码:778 - 786
Key words:carbonized leaf powder; Ni(II); physical adsorption; chemical adsorption; mechanism
Abstract: Nickel is a heavy metal which has the potential threaten to human’s health and attracts public concern recently. The carbonized leaf powder is expected as suitable adsorbent for Ni(II) removal became of the composition of some beneficial groups. In this work, carbonized leaf powder was evaluated for its adsorption performance towards Ni(II). According to the results, adsorbent component, dosage, initial solute concentration, solution pH, temperature and contact time can significantly affect the efficiency of Ni(II) removal. Sips model fits the test results best, and the adsorption capacity towards Ni(II) is determined around 37.62 mg/g. The thermodynamic behaviors reveal the endothermic and spontaneous nature of the adsorption. The free adsorption energy (fluctuate around 8 kJ/mol) predicted by D-R model indicates that the adsorption capacity originated from both physical and chemical adsorption. Room temperature (15-25 °C) is suitable for Ni(II) removal as well as low energy consumption for temperature enhancement. Further conclusions about the mechanism of chemical adsorption are obtained through analysis of the FT-IR test and XRD spectra, which indicates that the adsorption process occurs predominantly between amine, carbonate, phosphate and nickel ions.
J. Cent. South Univ. (2016) 23: 778-786
DOI: 10.1007/s11771-016-3123-z
TANG Qiang(唐强)1, WANG Heng-yu(王恒宇)2, TANG Xiao-wu(唐晓武)2, WANG Yan(王艳)3
1. School of Urban Rail Transportation, Soochow University, Suzhou 215131, China;
2. Research Center of Coastal and Urban Geotechnical Engineering, Zhejiang University, Hangzhou 310058, China;
3. Faculty of Architectural, Civil Engineering and Environment, Ningbo University, Ningbo 315211, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: Nickel is a heavy metal which has the potential threaten to human’s health and attracts public concern recently. The carbonized leaf powder is expected as suitable adsorbent for Ni(II) removal became of the composition of some beneficial groups. In this work, carbonized leaf powder was evaluated for its adsorption performance towards Ni(II). According to the results, adsorbent component, dosage, initial solute concentration, solution pH, temperature and contact time can significantly affect the efficiency of Ni(II) removal. Sips model fits the test results best, and the adsorption capacity towards Ni(II) is determined around 37.62 mg/g. The thermodynamic behaviors reveal the endothermic and spontaneous nature of the adsorption. The free adsorption energy (fluctuate around 8 kJ/mol) predicted by D-R model indicates that the adsorption capacity originated from both physical and chemical adsorption. Room temperature (15-25 °C) is suitable for Ni(II) removal as well as low energy consumption for temperature enhancement. Further conclusions about the mechanism of chemical adsorption are obtained through analysis of the FT-IR test and XRD spectra, which indicates that the adsorption process occurs predominantly between amine, carbonate, phosphate and nickel ions.
Key words: carbonized leaf powder; Ni(II); physical adsorption; chemical adsorption; mechanism
1 Introduction
Water and soil pollution by heavy metals represents a hot environmental issue, and attracts more and more attention recently. Among all heavy metals, Nickel is a specious one. Nickel is an essential element for human nutrition. However, excessive exposure to nickel-containing materials can result in detrimental chronic disorders to human skin, lungs, nose and bone, even increase the risk of lung and nasal cancers [1]. Nickel occurs naturally in the environment at low levels, and the nickel contaminant is usually released from nickel mine smelting, industrial waste disposal, electroplating, and battery manufacturing processes [2]. Unlike organic pollutants, which are susceptible to biological degradation, heavy metals such as Ni(II) are not biodegradable and tend to cause massive soil and water contamination, then accumulate in biological systems through food chain, posing health hazards if their concentrations exceed allowable limits [3]. Therefore, limiting the levels of nickel below allowable concentration seems vitally important. European Union recommends that the upper limit of nickel in drinking water is 0.02 mg/L [4]. According to USEPA and MHLW, the nickel in drinking water in United States and Japan should not exceed 0.04 and 0.01 mg/L [5-6], respectively.
Over the past few decades, many techniques have been developed for heavy metal removal, including precipitation, oxidation, adsorption, ion exchange, coagulation, evaporation, redox and extraction. Among them, adsorption has been widely applied in practice since its economically feasible alternative, versatile, effective, environmentally friendly and simple nature [7]. Commercial adsorbents are usually expensive, such as activated carbon. Thus, one intense debate focuses on finding a material which has abundant deposit or relative low cost as the adsorbent, and this material should possess excellent and stable adsorption performance. According to previous researches, various activated carbon and unburned carbon are produced from low-cost materials in attempt to reduce the cost of carbon production, such as coconut coirpith [8], rice husk [9],algae [10], papaya wood [11], loofa sponge and plant- immobilized biomass [12-13].
In this work, application of carbon made from a lost-cost agricultural waste, senescent leaf powder, for adsorption behavior towards Ni(II) was evaluated. The adsorption performance was evaluated under different external conditions, such as dosage, initial solute concentration, solution pH, temperature and duration. Furthermore, the adsorption mechanism was discussed based on adsorption isotherm model, BET-N2, FT-IR and XRD results.
2 Materials and methods
2.1 Theoretical backgrounds
2.1.1 Kinetic studies
Kinetic experiments were conducted not only to assess the uptake rates and contact times needed for completion of adsorption reactions but also can be used to optimize the residence time of contaminated waters in treatment systems. The test data were analyzed using three kinetic models as follows (pseudo-first order kinetics, pseudo-second order kinetics and intraparticle diffusion model). The pseudo-first order kinetic equation is
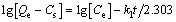 (1)
(1)
where Qe and Cs are the amount of solute adsorbed per unit adsorbent at equilibrium and any time, respectively (mg/g), and k1 is the pseudo-first order rate (min-1). The pseudo-second order kinetic equation is
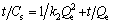 (2)
(2)
where k2 is the pseudo-second order rate constant (g·min-1·mg-1). The intraparticle diffusion model is
 (3)
(3)
where kint is the relevant rate constant  and C is the intercept.
and C is the intercept.
2.1.2 Adsorption isotherms
Three two-parameter isothermal models (Langmuir, Freundlich, and D-R models) and two three-parameter models (Redlich-Peterson and Sips models) were applied to evaluate the test results in order to poke more information on adsorption mechanisms. Langmuir model assumed that adsorption occurs uniformly on the active sites of the adsorbent, and once adsorbate occupies a site, no further adsorption can take place at the same one. The Langmuir isotherm can be written as [14]
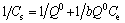 (4)
(4)
where Ce is the equilibrium solute concentration (mg/L); Q0 is the maximum adsorption capacity of the adsorbent (mg/g); and b is the Langmuir constant (L/mg). The Freundlich model stipulates that the ratio of solute adsorbed to the solute concentration is a function of the solution, which can be expressed as
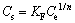 (5)
(5)
where KF (mg/g) indicates the adsorption capacity and strength of the adsorptive bond, and n is the heterogenity factor.
The D-R model assumes a uniform pore-filling adsorption and can predict the free adsorption energy change by which the adsorption type can be judged [15]. The D-R model is written as
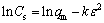 (6)
(6)
where qm is the maximum adsorption capacity (mol/g), k is a model constant related to the free sorption energy and ε is the Polanyi potential written as
 (7)
(7)
The mean free energy of adsorption (E) is
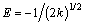 (8)
(8)
The Redlich-Peterson model can be applied either in homogeneous or heterogeneous systems due to its versatility expressed as [16]
 (9)
(9)
where KRP (L/g) and αRP (L/mg)β are Redlich-Peterson model constants and β is the exponent which lies between 0 and 1.
Sips model is a combined form of Langmuir and Freundlich expressions deduced for predicting the heterogeneous adsorption systems [17]. At low concentrations, it reduces to Freundlich isotherm; while at high concentrations, it predicts a monolayer adsorption form of the Langmuir isotherm as
 (10)
(10)
where qmS is the Sips maximum adsorption capacity (mg/g); KS is the Sips equilibrium constant 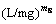 ; and mS is the Sips model exponent.
; and mS is the Sips model exponent.
2.1.3 Adsorption thermodynamics
Thermodynamic parameters such as enthalpy change (△H0), entropy change (△S0) and Gibbs free energy change (△G0) can be estimated with the following Gibbs free energy equations:
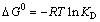 (11)
(11)
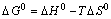 (12)
(12)
where R is the ideal gas constant (8.314 J/(mol·K)); T is the absolute temperature (K); KD is the distribution coefficient of the solute between the adsorbent and the solution in equilibrium Cs/Ce (mL/g). Equations (11) and (12) can be written in a linearized form between KD and 1/T as:
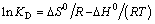 (13)
(13)
2.2 Adsorbent and solutions
The senescent leaves were collected from the Yuquan campus of Zhejiang University. Most of them were firmiana simplex leaves. The collected leaves were oven dried at 105 °C overnight and then pulverized in a mortar. The powder was placed on a ceramic plate which was then transferred into a muffle furnace (15 cm×25 cm×40 cm). The temperature was regulated at 250 °C, with an increment of 25-30 °C/min, and then pertained for 5 h. There was no gas supply into the oven during the calcination process. The carbonized product was cooled to room temperature, and passed 24 mesh screen with diameter of (355 ±13) μm. This product was designated as carbonized leaf powder (CLP), and utilized in the following test.
Stock solution was prepared by dissolved nickel chloride (ZHENXIN, China) in analytical grade into de-ionized water to 1 mol/L standard solution, then stored in a refrigerator around 4 °C. Before the test, the 1 mol/L standard solution was diluted to the target concentrations for use. Conical flasks and PVC tubes (Polyvinylchlorid Centrifuge Tubes) were immersed in 0.01 mol/L HNO3 solution overnight then rinsed three times with de-ionized water.
2.3 Characterization
The specific surface area of CLP was determined by BET N2 adsorption test (Autosorb 1-MP apparatus, Quantachrome Corporation, USA). The results were analyzed by using the BET adsorption theory to predict the specific surface area, volume of micropores and average pore size, etc. FT-IR spectra was recorded using Fourier Transform Infrared Spectroscopy (Nexus-670, Nicolet, USA) to study the functional groups with respect to investigating the mechanism of Ni(II) adsorption. The pH of samples was measured by glass electrode potentiometer (pH 213, China). The XRD spectra of CLP and Ni(II) laden CLP were obtained by D/MAX-RA diffractometer (Rigaku Corporation, Japan).
2.4 Experiment
2.4.1 Effect of adsorbent dosage
The adsorbent dosage (CLP) in the aqueous solution was increased from 0.5 to 1, 2, 5, 10 and 20 g/L in order to determine the most appropriate one. Three various initial Ni(II) concentrations (50, 100 and 200 mg/L) were set and poured into stoppered conical flasks. After mixing with the adsorbent, the flasks were put into a thermostated agitator (25 °C) (THZ-C-1, BING, China) for 24 h at 180 r/min without regulating the solution pH. The supernatant was obtained by centrifuging the mixture at 3000 r/min for 5 min (TDZ5-WS, XIANGYI, China), then the equilibrium Ni(II) concentration was determined by atomic absorption spectrophotometer (AAS) (TAS-990, PERSEE, China).
2.4.2 Adsorption kinetics
The dosage was fixed 10 g/L while the initial solute concentrations varied from 50, 100 to 200 mg/L. The solution pH was not adjusted and the reaction temperature was maintained at 25 °C. In order to measure the adsorption rate, the samples were collected at different time durations increasing from 3 to 6, 9, 12, 15, 20, 40, 60, 120, 180, 240 and 300 min. The mixture was centrifuged at 3000 r/min for 3 min for supernatant in order to determine the Ni(II) concentration.
2.4.3 Adsorption isotherms at different temperatures
Batch tests were carried out to study the Ni(II) adsorption isotherms on CLP. The CLP (10 g/L) was blended with six sets of nickel chloride solution with increasing initial Ni(II) concentration from 25 to 600 mg/L (25, 50, 100, 200, 300, 400, 500 and 600 mg/L) at five sets of increased temperatures separately from 5 to 45 oC with an increment of 10 oC. All samples were equilibrated for 24 h in the controlled temperature agitator and the equilibrium Ni(II) concentrations were measured by AAS.
2.4.4 Effect of pH
The same amount of CLP (400 mg) and Ni(II) solution (100 mg/L and 40 mL) were mixed in nine pretreated conical flasks. Then the initial solution pH of these nine centrifuge tubes was adjusted ranging from (2.0 ± 0.2) to (10.0 ± 0.2) with an increment of 1.0 by 0.1 mol/L HCl or NaOH solution. The sample flasks were then placed into a thermostated agitator (25 °C) and rotated at 180 r/min for 24 h. The pH of solutions was measured at the end of the test. After centrifugation at 3000 r/min for 5 min, the supernatants were sampled to determine the Ni(II) concentration by AAS. Blank tests were conducted in all the above tests to evaluate the adsorption of Ni(II) on the inner surface of the flasks, injunctions with that controls and parallel tests were conducted and the results were averaged.
3 Results
3.1 Characterization of adsorbent
Table 1 shows the surface properties of the prepared adsorbent. The specific surface area (SSA) was determined at 6.26 m2/g. The micropore and average pore diameter were determined at 14.0 and 20.0  , respectively. The total pore volume reaches 3.14× 10-3 cm3/g. Compared to other reported porous materials such as activated carbons, the SSA of CLP is not encouraging. However, for CLP, the adsorption towards contaminant does not depend merely on physisorption occurred at the pore space or surface areas. The reaction between the surface active sites and the objective heavy metals could contribute much more adsorption capacity than the physisorption on pore spaces. The following investigations confirmed these speculations as well as the effectiveness of the prepared material.
, respectively. The total pore volume reaches 3.14× 10-3 cm3/g. Compared to other reported porous materials such as activated carbons, the SSA of CLP is not encouraging. However, for CLP, the adsorption towards contaminant does not depend merely on physisorption occurred at the pore space or surface areas. The reaction between the surface active sites and the objective heavy metals could contribute much more adsorption capacity than the physisorption on pore spaces. The following investigations confirmed these speculations as well as the effectiveness of the prepared material.
Table 1 BET N2 adsorption results
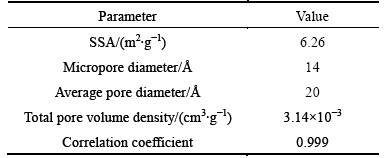
The FT-IR spectra of CLP (400-4000 cm-1) were taken to obtain information of functional groups as presented in Fig. 1. Characteristic bands related to amine are observed at wavenumbesr of 1623, 1319 and 475 cm-1 [18]. The weak band at 713 cm-1 is assigned to carbonate components [19], and the band intensities at 1431, 1119 and 875 cm-1 are also found, suggesting the existence of the carboxyl group which is a preferential adsorption site for heavy metals [20]. The weakness of band intensities of C—H group at 2920 and 2850 cm-1 shown in Fig. 1 means that the C—H group content is very low after 250 °C activation. The natural macromolecules (i.e., lignin) contained in the CLP, which remains stable even after mulched for 2 mon [21], are greatly decomposed in this respect.
Figure 2 shows the XRD spectra of CLP and Ni(II) Laden CLP. In Fig. 2(a), characteristic bands at 2θ= 15.23°, 29.4°, 30.88° and 30.1° are assigned to sodium hydrogen phosphate hydrate and calcium hydroxide phosphate, which is consistent with the observation of P—O at 501 cm-1 shown in Fig. 1 [22]. The characteristic band related to calcite is at 2θ=31.4° and 35.9°, and the intensity of the band at 2θ=28.3° indicates that CLP contains albite. Calcium phosphate also exists in CLP according to the related band at 2θ=40.53°. Quartz content is regarded to be abundant in CLP due to the band at 2θ=20.8°, 26.6°, 39.4° and 59.96° [23]. The patterns at 2θ=14.9°, 24.5°, 38.1° and 50.1° can be attributed to whewellite (calcium oxalate hydrate). The pattern at 2θ=24.5° is sharp and strong, indicating that the crystalline of this component is well conserved at 250 °C.
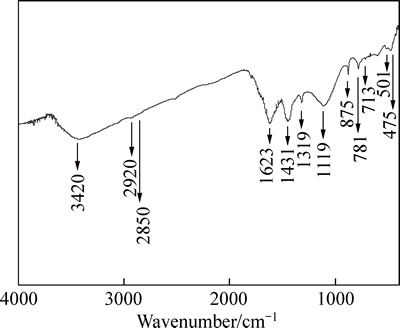
Fig. 1 FT-IR spectrum of CLP
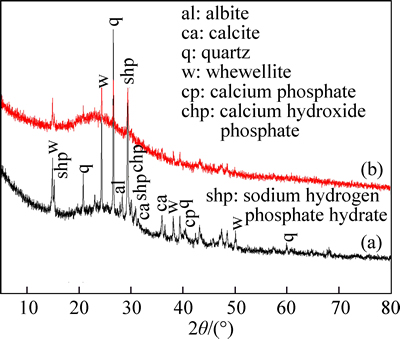
Fig. 2 XRD spectra of CLP (a) and Ni(II) Laden CLP (b)
3.2 Experiment
3.2.1 Effect of adsorbent dosage
Figure 3 shows the effect of dosage on the Ni(II) adsorption from aqueous solutions. It is apparent that the Ni(II) removal rate increases rapidly with increasing the dosage and then reaches a maximum at a dosage of 10 g/L. Thereafter, the removal amount basically remains a steady value with no obvious change when increasing the adsorbent dosage continuously.
Contrary to total removal amount, the unit adsorption amount of the CLP decreases with increasing dosage at each specific initial solute concentration, this phenomenon may be due to the less availability to adsorbent for Ni(II) when dosage increases. But thistrend is not linear, as the sharp decrease at the first stage (0.5 g/L
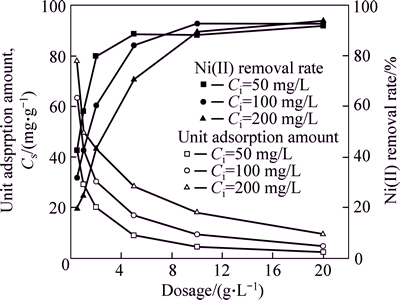
Fig. 3 Dosage effect on Ni(II) adsorption
3.2.2 Adsorption kinetics
Figure 4 shows the variation of equilibrium concentration of Ni(II) with contact time at three different initial concentrations. The plot indicates the adsorption progresses in two steps, consisting of a rapid adsorption process and a slowly adsorption step during which equilibrium is obtained. The contact time for equilibrium is found increased with increasing initial Ni(II) concentration. The contact time for equilibration is determined to be only about 15 min, when Ci=50 mg/L, but as initial Ni(II) concentration increases to 100 and 200 mg/L, 40 min and 120 min will be required for equilibrium, respectively.
Figure 5 plots Cs of Ni(II) on CLP as a function of equilibration durations. From the chart, it is obvious that the unit adsorption amount of Ni(II) reaches a relatively high value within a fairly short time (about 15 min) andthen slowly increases until it reaches a plateau after 120 min. The test data are further analyzed with the above three kinetic models (pseudo-first order kinetics, pseudo-second order kinetics and intraparticle diffusion model as shown in Eqs. (1)-(3)) to find the best suitable model.
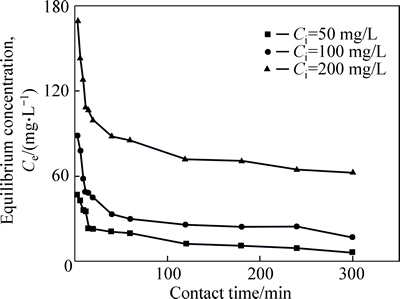
Fig. 4 Variation of equilibrium concentration of Ni(II) with contact time
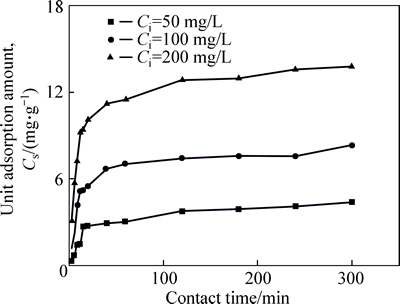
Fig. 5 Effect of contact time on unit adsorption amount
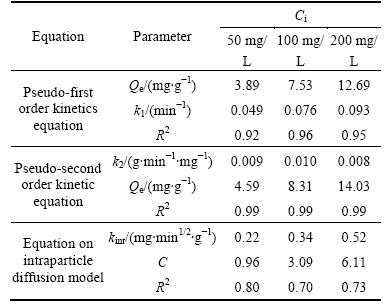
Table 2 lists the critical parameters of adsorption kinetics. From the correlation coefficient value listed, we could easily judge that the pseudo-second order kinetic equation (R2 =0.99, 0.99 and 0.99) could fit the test data best, which is similar to other previous works [25]. Besides, there is a slight decrease in the rate constant k2 from 0.009 to 0.008 g/(mg·min) when increasing the initial Ni(II) concentration from 50 to 200 mg/L. This indicates that the solution with the smallest solute concentration is likely to reach equilibrium most quickly, which is also observed in Pb(II) removal on natural Kaolin [26].
Table 2 Predicted kinetic constants of Ni(II) adsorption on CLP
3.2.3 Adsorption isotherms
Adsorption isotherms of Ni(II) on CLP are presented in Fig. 6. The unit adsorption amount increases continually and eventually achieves a maximumadsorption amount with increasing equilibrium solute concentrations. And it can be predicted that the adsorption amount per unit keeps constant after it reaches its ultimate adsorption amount.
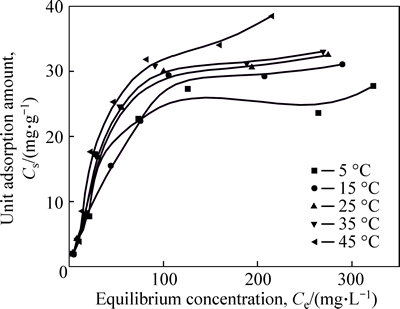
Fig. 6 Isothermal adsorption lines under different temperatures
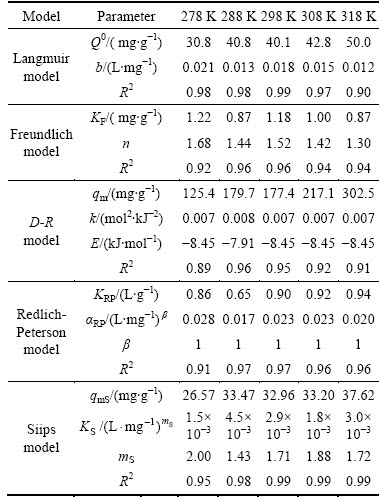
The results of predicted isothermal constants for the adsorption of Ni(II) are gathered in Table 3. It is apparent that Sips model shows the best-fit to the test data based on calculated correlation coefficients, most of which in Sips model are higher than 0.98, which means the parameters could be measured more accurately through Sips models. According to Sips model results, the predicted Ni(II) adsorption capacities of CLP are found to increase from 26.57 to 37.62 mg/g with increasing temperature from 278 to 318 K, respectively. Similarly to UNUABONAH et al [27], it is found that adsorption capacity towards Ni(II) came to the peak point at the highest temperature condition according to the above data.
The adsorption capacities estimated by D-R model are 125.4, 179.7, 177.4, 217.1 and 302.5 mg/g, respectively, which are much higher than those obtained by the Langmuir and Sips models since the inherent assumption in the D-R model that all micropores are filled with solute, which is difficult to realize in practice. The absolute values of estimated free adsorption energy by D-R moedel greatly fluctuate around 8 kJ/mol, around the boundary of physical adsorption and chemical bond[28]. Such a fluctuation of free adsorption energy indicates that no matter the temperature changes, the adsorption is predominated by the form of both physical adsorption and chemical adsorption. As the temperature is higher than 298 K (25 °C), the absolute values of free energy of adsorption |E| are a little higher than 8 kJ/mol, which indicates the adsorption capacity is mainly originated from chemical bound, or the chemical adsorption such as ion-exchange reaction is motivated, and that can also help to explain the slight increase of adsorption capacity predicted by Sips model in Table 3. In addition, it is interesting to find that even under room temperature (15-25 °C), CLP can reach an excellent performance, relative high adsorption capacity towards Ni(II). With respect to the energy saving, usage of CLP seems promising to be widely utilized in practice.
Table 3 Predicted constants of isothermal models for Ni(II) adsorption
3.2.4 Thermodynamics
The predicted constants of thermodynamics shown in Table 4 can be determined through linearization of the test data as shown in Fig. 7. The initial Ni(II) concentration has a significant effect on the thermodynamic parameters. Moreover, the Gibbs free energy is negative at various initial solute concentrations ranging from 100 to 200 mg/L, which suggests that the adsorption process is spontaneous and could be promoted by increasing temperature, closing to the results of LIN and JUANG [29]. The reason why adsorption capacity comes to peak point at highest temperature according to Langmuir isotherm could be elucidated by the above Gibbs free energy theory. In addition, with increasing initial Ni(II) concentration, almost all of the Gibbs free energy for adsorption decreases under constant temperature conditions. The change of enthalpy is 7.85, 6.93 kJ/mol and the change of entropy is 77.26 and 75.86 J/(mol·K) when the initial Ni(II) concentration increases from 100 to 200 mg/L, which implies an endothermic character to the adsorption process and increasing disorder in the system.
Table 4 Thermodynamic parameters for adsorption of Ni(II)
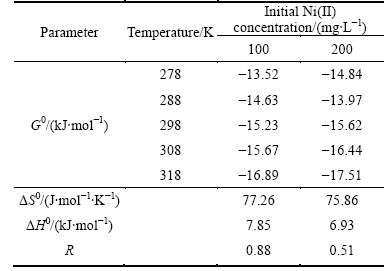
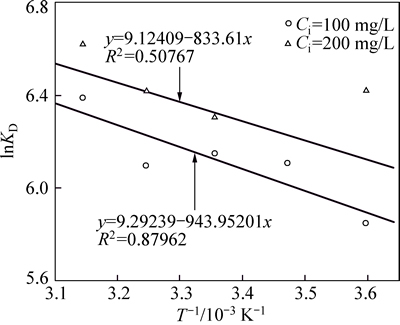
Fig. 7 Fitting test data with Gibbs free energy equations
3.2.5 Effect of pH
The effect of pH on Ni(II) removal from aqueous solutions is shown in Fig. 8. It is seen that the pH values of solutions greatly influence the adsorption amount of Ni(II) on CLP. Only 14% of Ni(II) is removed from the solution at acidic condition when pH=1.98. A sharp pH-adsorption edge could be observed between pH 1.98 and 5.91, while the removal rate of Ni(II) increases to 74.3% rapidly. Then, as can be seen from the slope in Fig. 8, the rate of Ni(II) removal becomes a bit slower when continuously increasing the pH values of the solution. The removal efficiency of Ni(II) reaches 86.1% at pH=9.00 and slightly decreases at higher pH values. Based on the general trend, nickel ions in aqueous solutions can be expected to be nearly completely removed under a strongly alkaline condition, and the decrease of adsorption capacity at lower pH can be attributed to the competition of H+ with metal ions to get adsorbed on the binding sites of the cells which are responsible for metal adsorption [30-31].
Figure 9 plots the equilibrium pH on CLP versus the initial solution pH. No buffer solution is used to maintain a constant pH of the solutions. It is noted that the pH of all the solutions changes at the end of 24 h exposures:equilibrium pH values approximately lie on the diagonal line when initial solution pH<5.91 and below the diagonal initial solution pH>5.91. It is obvious that the CLP owns strong buffering capability which could resist pH changes effectively, especially for solution at pH> 5.91. The changing pattern of equilibrium pH might reflect the inherent mechanism governing the adsorption of Ni(II) on CLP, particularly regarding the effect of solution pH.
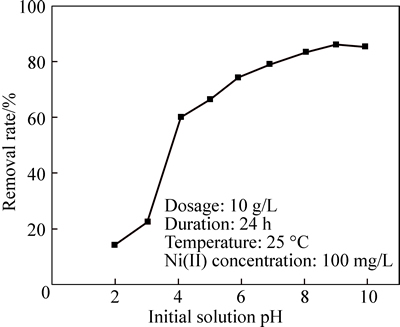
Fig. 8 Variation of Ni(II) removal percentage with varied initial solution pH
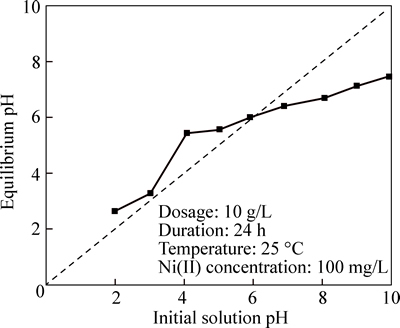
Fig. 9 Variation of equilibrium pH under different initial solution pH (pHi)
4 Discussion
The bands at 2θ=31.4 °C and 35.9 °C could be assigned to calcite content identified by the XRD spectra as shown in Fig. 2(a), consisting with the discovery of carbonate in FT-IR spectra in Fig. 1. With respect to the conclusion that calcite is proved to be a kind of effective adsorbent to remove heavy metals from solutions [32] and the fact that the calcite band intensity is weakened after adsorption, the adsorption of Ni(II) on CLP may be written as:
 →
→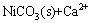 (14)
(14)
It could be linked to the results of D-R analysis (adsorption energy around -8.452 kJ/mol), indicating the presence of chemical adsorption. In addition, the existence of carboxyl groups in the surface of CLP could complex with Ni(II) in the following form:
S— →S—
→S— (15)
(15)
In Fig. 1, the bands at 475, 1319 and 1623 cm-1 could be assigned to the vibration of N—H group. Amine group is reported as a significant binding site for metal uptake in biosorbents [33]. As an alkaline functional group, amine could give rise to the solution pH, with observation in this work that the equilibrium pH of the adsorbent/water slurry (10 g/L) is higher than 8.60, which proves the presence of alkaline component in CLP. The high affinity of amine towards Ni(II) would contribute to the adsorption of Ni(II) on CLP, namely, the existence of N—H group as shown in Fig. 1 suggests that the Ni(II) adsorption process may follow the following reaction:
S—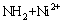 →S—
→S— (16)
(16)
S— →S—
→S— (17)
(17)
Equations (15)-(17) would be able to elucidate the observation the reason why equilibrium pH drops after Ni(II) adsorption especially when initial solution pH> 5.91. Eq. (17) is also proved by the band related to O—H (3420 cm-1) as shown in FT-IR spectra in Fig. 1 [34]. However, the deprotonated amine group would turn to possess positive charges at pH<2.0. This behavior might pronouncedly reduce the Ni(II) adsorption capacity, which could explain the minor removal rate at solution pH<2.0 and the sharp pH adsorption edge between 1.98 and 5.91, as observed in Figs. 8 and 9.
The main minerals in CLP are quartz, whewellite, albite and phosphate as determined from the characteristic bands on the XRD spectra (see Fig. 2). The patterns at 2θ=15.23°, 20.8°, 26.6°, 29.4°, 30.1°, 30.88°, 38.1°, 39.4°, 40.53° and 59.96° are weakened when Ni(II) have been adsorbed. The presence of phosphate (at 2θ= 15.23°, 29.4°, 30.1°, 30.88° and 40.53° as exhibited in Fig. 2) would contribute to the adsorption of Ni(II) in the form as follows:
 →
→ (18)
(18)
 →
→ (19)
(19)
It is noteworthy to mention that if the cation M is magnesium, Eq. (18) reaction will become insignificant with respect to its comparatively low dissolution coefficients. In addition, the weakened quartz band intensity (at 2θ=20.8°, 26.6°, 39.4° and 59.96° as shown in Fig. 2) suggests the following reaction:
 →
→ (20)
(20)
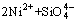 →
→ (21)
(21)
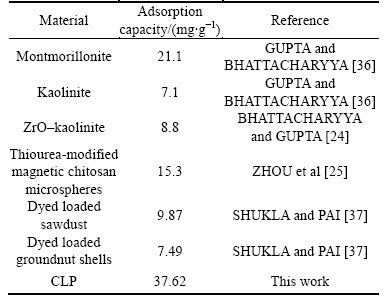
For the phenomena that the Ni(II) removal rate has a slight decrease as the pH reaches 10.0, KALBE et al [35] attributed it to amphoteric character of most heavy metals. Various adsorbents have been investigated for Ni(II) removal, and Table 5 shows the Ni(II) adsorption capacities of some recently reported adsorbents. Different and superior to other adsorbents, the CLP has relatively high Ni(II) adsorption capacity among the previous research. Besides, Firmiana Simplex, as a widely planted tree, provides a locally available cheap adsorbent resource. The activation method provided here proves effective and appears promising in the heavy metals removal from wastewaters.
Table 5 Adsorption capacity towards Ni(II) in references
5 Conclusions
1) CLP studied appears prominent in Ni(II) removal from aqueous solution and exhibits promising potential of application in industry. The adsorption capacity of CLP towards Ni(II) is determined at 37.62 mg/g.
2) Kinetic studies show that the Ni(II) adsorption follows pseudo-second order kinetics, and the required time for equilibrium increases from 20 to 120 min, while initial Ni(II) concentration is raised from 50 to 200 mg/L.
3) Based on the correlation coefficients, the Sips model fits test data best, in which most correlation coefficients are higher than 0.98; Langmuir model also fits test data well, in which most correlation coefficients are higher than 0.90 .
4) The free adsorption energy (fluctuate around 8 kJ/mol) predicted by D-R model indicates that the adsorption capacity originated from both physical and chemical adsorption. Room temperature (15-25 °C) is suitable for Ni(II) removal as well as low energy consumption for temperature enhancement. The adsorption process is spontaneous and endothermic, and the system disorder increases.
5) According to the analysis to XRD and FT-IR results, the chemical adsorption occurs between nickel ions and amine, carbonate, phosphate, which are abundant in the adsorbent.
References
[1] USEPA. Nickel compounds [EB/OL]. [2014-07-24]. http://www. epa.gov/ttnatw01/hlthef/nickel.html.
[2] KADIRVELU K, NAMASIVAYAM C. Activated carbon from coconut coirpith as metal adsorbent: Adsorption of Cd(II) from aqueous solution [J]. Advances in Environmental Research, 2003, 7(2): 471-478.
[3] LIU Yao-chi, LI Xue-nong, WANG Chun-zhi, KONG Xiu, ZHONG Li-zi. Poly (styrene-co-divinylbenzene)-PAMAM-IDA chelating resin: Synthesis, characterization and application for Ni(II) removal in aqueous [J]. Journal of Central South University, 2014, 21(9): 3479-3484.
[4] EU. European drinking water directive [EB/OL]. [2014-07-13]. http: //ec.europa.eu/environment/water/water-drink/legislation_en.html.
[5] SHENG Ping-xin, TING Yen-peng, CHEN J P, HONG Liang. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms [J]. Journal of Colloid and Interface Science, 2004, 275: 131-141.
[6] MHLW. Water Supply Division, Health Service Bureau. Revision of drinking water quality standards in Japan [S]. 2004.
[7] GHAZY S E, GAD A H M. Lead separation by sorption onto powdered marble waste [J]. Arabian Journal of Chemistry, 2014, 7(3): 277-286.
[8] KADIRVELU K, THAMARAISELVI K, NAMASIVAYAM C. Adsorption of nickel(II) from aqueous solution onto activated carbon prepared from coirpith [J]. Separation and Purification Technology, 2001 24: 497–505.
[9] WONG K K, LEE C K, LOW K S, HARON M J. Removal of Cu and Pb by tartaric acid modified rice husk from aqueous solutions [J]. Chemosphere, 2003, 50: 23–28.
[10] KUMAR Y P, KING P, PRASAD V S R K. Adsorption of zinc from aqueous solution using marine green algae—Ulva fasciata sp [J]. Chemical Engineering Journal, 2007, 129: 161–166.
[11] SAEED A, AKHTAR M W, IQBAL M. Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent [J]. Separation and Purification Technology, 2005, 45: 25-31.
[12] AKHTAR N, IQBAL J, IQBAL M. Removal and recovery of nickel (II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: Characterization studies [J]. Journal of Hazardous materials, 2004, 108B: 85-94.
[13] SEKHAR K C, KAMALA C T, CHARY N S, SASTRY A R K, RAO T N, VAIRAMANI M. Removal of lead from aqueous solutions using an immobilized biomaterial derived from a plant biomass [J]. Journal of Hazardous materials, 2004, 108B: 111-117.
[14] OUADJENIA-MAROUF F, MAROUF R, SCHOTT J, YAHIAOUI A. Removal of Cu(II), Cd(II) and Cr(III) ions from aqueous solution by dam silt [J]. Arabian Journal of Chemistry, 2013, 6(4): 401-406.
[15] DENARDIS D, ROSALES-YEOMANS D, PHILIPOSSIAN L B A. Characterization of copper-hydrogen peroxide film growth kinetics [J]. Thin Solid Films, 2006, 513(1/2): 311-318.
[16] REDLICH O, PETERSON D L A. Useful adsorption isotherm [J]. Journal of Physical Chemistry, 1959, 63: 1024-1026.
[17] SIPS R. On the structure of a catalyst surface [J]. Journal of Chemical Physics, 1948, 16: 490-495.
[18] JONES R A. Pyrrole studies I. The infrared spectra of 2-monosubstituted pyrroles [J]. Australian Journal of Chemistry, 1963, 16: 93-100.
[19] JACKSON K D O. A guide to identifying common inorganic fillers and activators using vibrational spectroscopy [J]. Journal of Rubber Research, 1997,12(2):102-111.
[20] TANG Qiang, TANG Xiao-wu, LI Zhen-ze, WANG Yan, HU Man-man, ZHANG Xiang-jie, CHEN Yun-min. Zn(II) removal with activated firmiana simplex leaf: Kinetics and equilibrium studies [J]. Journal of Environmental Engineering, 2012, 138(2): 190-199.
[21] JIN Z, AKIYAMA T, CHUNG B Y, MATSUMOTO Y, IIYAMA K, WATANABE S. Changes in lignin content of leaf litters during mulching [J]. Phytochemisty, 2003, 64: 1023-1031.
[22] SOMYA A, RAFIQUEE M Z A, VARSHNEY K G. Synthesis, characterization and analytical applications of sodium dodecyl sulphate cerium (IV) phosphate: A new Pb (II) selective, surfactant-based intercalated fibrous ion exchanger [J]. Colloids and Surfaces A: Physicochemical Engineering Aspects, 2009, 336: 142-146.
[23] TANG Qiang, KATSUMI T, INUI T, LI Zhen-ze. Membrane behavior of bentonite-amended compacted clay [J]. Soils and Foundations, 2014, 54(3): 329-344.
[24] BHATTACHARYYA K G, GUPTA S S. Adsorption of Fe(III), Co(II) and Ni(II) on ZrO–kaolinite and ZrO–montmorillonite surfaces in aqueous medium [J]. Colloids and Surfaces A: Physicochemical Engineering Aspects, 2008, 317: 71-79.
[25] ZHOU Li-min, WANG Yi-ping, LIU Zhi-rong, HUANG Qun-wu. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres [J]. Journal of Hazardous Materials, 2009, 161: 995-1002.
[26] TANG Qiang, TANG Xiao-wu, LI Zhen-ze, CHEN Yun-min, KOU Nai-yu, SUN Zu-feng. Adsorption and desorption behaviour of Pb(II) on a natural kaolin: Equilibrium, kinetic and thermodynamic studies [J]. Journal of Chemical Technology and Biotechnology, 2009, 84: 1371-1380.
[27] UNUABONAH E I, ADEBOWALE K O, OLU-OWOLABI B I. Kinetic and thermodynamic studies of the adsorption of lead (II) ions onto phosphate-modified kaolinite clay [J]. Journal of Hazardous Materials, 2007, 144: 386-395.
[28] HELFFERICH F. Ion exchange [M]. New York: McGraw-Hill, 1962: 289.
[29] LIN S H, JUANG R S. Heavy metal removal from water by sorption using surfactant-modified montmorillonite [J]. Journal of Hazardous Materials, 2002, B92: 315-326.
[30] NASIR M H, NADEEM R, AKHTAR K, HANIF M A, KHALID A M. Efficacy of modified distillation sludge of rose (Rosa centifolia) petals for Pb(II) and Zn(II) removal from aqueous solutions [J]. Journal of Hazardous Materials, 2007, 147: 1006-1014.
[31] TANG Qiang, TAKESHI K, TORU I, LI Zhen-ze. Influence of pH on the membrane behavior of bentonite amended Fukakusa clay [J]. Separation and Purification Technology, 2015, 141: 132-142.
[32] TANG Qiang, TANG Xiao-wu, HU Man-man, LI Zhen-ze, CHEN Yun-min, LOU Peng. Removal of Cd(II) from aqueous solution with activated Firmiana Simplex Leaf: Behaviors and affecting factors [J]. Journal of Hazardous Materials, 2010, 179: 95-103.
[33] KILIC M, KESKIN M E, MAZLUM S, MAZLUM N. Hg(II) and Pb(II) adsorption on activated sludge biomass: Effective biosorption mechanism [J]. International Journal of Mineral Processing, 2008, 87: 1-8.
[34] SINITSYA A, COPIKOVA J, PRUTYANOV V, SKOBLYA S, MACHOVIC V. Amidation of highly methoxylated citrus pectin with primary amines [J]. Carbohydrate Polymers, 2000, 42: 359-368.
[35] KALBE U, BERGER W, ECKARDT J, SIMON F G. Evaluation of leaching and extraction procedures for soil and waste [J]. Waste Management, 2008, 28: 1027-1038.
[36] GUPTA S S, BHATTACHARYYA K G. Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium [J]. Journal of Environmental Management, 2008, 87: 46-58.
[37] SHUKLA S R, PAI R S. Adsorption of Cu(II), Ni(II) and Zn(II) on dye loaded groundnut shells and sawdust [J]. Separation and Purification Technology, 2005, 43: 1-8.
(Edited by FANG Jing-hua)
Foundation item: Projects(51179168, 51308310) supported by the National Natural Science Foundation of China; Project(LQ13E080007) supported by Zhejiang Provincial Natural Science Foundation, China; Project supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars of Jiangsu Province, China
Received date: 2015-01-27; Accepted date: 2015-06-11
Corresponding author: WANG Heng-yu, PhD; Tel: +86-512-67601052; E-mail: 10912019@zju.edu.cn

