
Thermodynamic calculation of intermetallic compounds in
AZ91 alloy containing calcium
WU Yu-feng(吴玉锋), DU Wen-bo(杜文博), NIE Zuo-ren(聂祚仁),
CAO Lin-feng(曹林锋), ZUO Tie-yong(左铁镛)
College of Materials Science and Engineering, Beijing University of Technology, Beijing 100022, China
Received 3 June 2005; accepted 5 October 2005
Abstract: Based on the Miedema model and Chou model, the activities of different solute components in Mg-Al-Zn, Mg-Ca-Zn and Mg-Al-Ca ternary systems were calculated. The results show that the variety of zinc content has little influence on the activity of Al or Ca, and the interaction of Zn and Al or Ca can be neglected when the mass fraction of Zn is lower than 2% in the AZ91 alloy containing calcium (noted as Mg-Al-Zn-Ca system). Therefore, the possible intermetallic compounds in the Mg-Al-Zn-Ca system can be predicted by directly calculating the Gibbs free energies of the reactions in Mg-Al-Ca system. The calculated Gibbs free energies in the Mg-Al-Ca system indicate that Al2Ca phase can take priority of depositing, which agrees with the experimental results in references. The consistency of calculation and experiment proves that the intermetallic compounds in the Mg-Al-Zn-Ca system can be predicted by the Miedema model and Chou model.
Key words: AZ91 alloy; Mg-Al-Zn alloy; Mg-Ca-Zn alloy; Mg-Al-Ca alloy; Al2Ca; Miedema model; Chou model; Gibbs free energy
1 Introduction
Magnesium alloy is regarded as a promising structural material in the 21 century because of its excellent properties such as low density, high specific strength, good castability and machinability[1-5]. The common magnesium alloy AZ91 is, however, unsuitable for using at the temperatures above 120 ℃ because of its poor creep resistance and strength at elevated temperatures[2]. Adding other elements, like calcium, is an important method to improve the mechanical properties of AZ91 at elevated temperatures. The studies [2, 6, 7] showed that the added calcium can modify the as-cast microstructure and improve the mechanical properties of AZ91 alloy at both room and elevated temperatures because of the existence of Ca in β-Mg17Al12 phase. However, up to now the thermodynamic calculation of the intermetallic compounds in the AZ91 alloy containing calcium (noted as Mg-Al-Zn-Ca system) has not been reported.
Miedema model has been widely used to calculate some thermal properties of binary alloys[8, 9]. Chou model is more convenient to predict excess Gibbs free energy and activity coefficients of different components in ternary system than the other thermodynamic models[10-12]. The purpose of the present paper is to calculate the activities of different solute components in Mg-Al-Zn, Mg-Ca-Zn and Mg-Al-Ca alloy systems and to predict the deposited phases in the Mg-Al-Zn-Ca system based on the Miedema model and Chou model.
2 Calculation model
2.1 Miedema model
According to Miedema model[8], the heat of formation, ΔHij, in the binary liquid i—j alloy can be calculated as
 (1a)
(1a)
in which
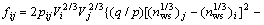
 (1b)
(1b)
where xi and xj represent the molar fractions of components i and j, respectively; V is the molar volume, 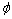 is the electron negativity, nws is the electron density; u, p, q, a and r are all empirical constants, q/p=9.4. a equals 1.0 for solid alloys and 0.73 for liquid alloys containing a transition metal and a nontransition metal, respectively, and a equals 0 for the other alloys. All the parameters above are obtained from Refs.[8, 13].
is the electron negativity, nws is the electron density; u, p, q, a and r are all empirical constants, q/p=9.4. a equals 1.0 for solid alloys and 0.73 for liquid alloys containing a transition metal and a nontransition metal, respectively, and a equals 0 for the other alloys. All the parameters above are obtained from Refs.[8, 13].
In a binary system, the relation between  and ΔHij,
and ΔHij,  is given by
is given by
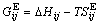 (2)
(2)
where T is the absolute temperature of system.
Considering the absolute value of  is much smaller than the absolute value of ΔHij, let
is much smaller than the absolute value of ΔHij, let  =0, then,
=0, then,  Hence,
Hence,

 (3)
(3)
where fij can be got from Eqn.(1b).
2.2 Calculation of activities of different solute components in ternary alloy systems
According to Chou model[10, 11], the excess Gibbs free energy in the ternary liquid i—j—k alloy can be calculated as
 (4)
(4)
where xi, xj and xk are the molar fractions of components i, j and k, respectively; ζ is an analogical coefficient, which is a special parameter introduced by Chou model, and its physical meaning and calculative method have been given in Ref.[10].  ,
,  and
and  are calculated according to Eqn.(3).
are calculated according to Eqn.(3).
The activity coefficient of any component m(m=i, j, k) can be calculated as follows[10, 11]:
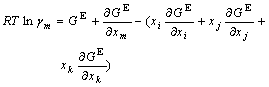 (5)
(5)
 (6)
(6)
where

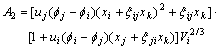



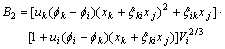
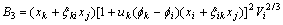
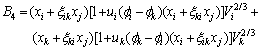

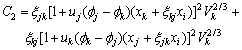

 (m=j, k) can be calculated by Eqn.(6).
(m=j, k) can be calculated by Eqn.(6).
According to the γm(m=i, j, k) calculated by Eqn.(5), the activities of component m(m=i, j, k) can be obtained:
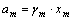 (m=i, j, k) (7)
(m=i, j, k) (7)
3 Results and discussion
3.1 Mg-Al-Zn and Mg-Ca-Zn ternary system
Fig.1 shows the activities of Al and Zn as a function of mass fraction of Zn in AZ31, AZ44 and AZ412 at 1 000 K. Fig.2 shows the activities of Ca and Zn as a function of mass fraction of Zn in Mg-Ca-xZn(x=1, 2, 5) at 1 000 K. These two figures indicate that the increase in mass fraction of Zn increases the activity of Al in Mg-Al-Zn ternary system; while the increase in mass fraction of Zn decreases the activity of Ca in Mg-Ca-Zn ternary system. The change of activities of Al and Ca component with mass fraction of Zn is, however, extremely small, when the mass fraction of Zn is lower than 2%. Moreover, it is suggested that a small content of Zn is difficult to form intermetallic compounds with other components like Al and Ca based on the consideration that the solubility of Zn in Mg matrix is comparatively high[14]. Therefore the possible inter- metallic compounds in Mg-Al-Zn-Ca system are predicted by calculating simply the Gibbs free energies of the reactions between Mg-Ca and Al-Ca in Mg-9Al-xCa systems, ignoring the purpose of Zn component.
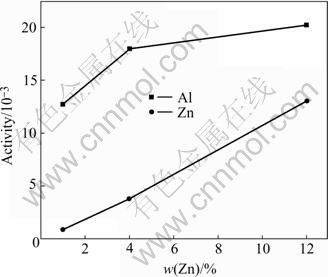
Fig.1 Activities of Al and Zn as function of mass fraction of Zn at 1 000 K

Fig.2 Activities of Ca and Zn as function of mass fraction of Zn at 1 000 K
3.2 Mg-Al-Ca ternary system
The calculated activities of Al and Ca components at 900 K in Mg-9Al-xCa(x=0.5, 1, 3) systems are listed in Table 1.
Table 1 Activities of Al and Ca component in Mg-9Al-xCa(x= 0.5, 1, 3) at 900 K

According to Al-Ca and Mg-Ca binary phase diagrams, the intermetallic compounds of which the melting point is higher than 900 K include Al2Ca, Al4Ca and Mg2Ca. Hence the possible reactions in Mg-9Al-xCa(x=0.5, 1, 3) systems at 900 K can be given as follows:
2[Al]+[Ca] = Al2Ca(s) (a)
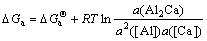 (8)
(8)
4[Al]+[Ca] = Al4Ca(s) (a′)
 (9)
(9)
2[Mg]+[Ca] = Mg2Ca(s) (a″)
 (10)
(10)
Reactions(a), (a′) and (a″) can be obtained by simplifying these reactions as follows:
2Al(s)+Ca(s)=Al2Ca(s) (b)
4Al(s)+Ca(s)=Al4Ca(s) (b′)
2Mg(s)+Ca(s)=Mg2Ca(s) (b″)
Al(s)=[Al] (c)
Ca(s)=[Ca] (d)
Mg(s)=[Mg] (e)
Since (a)=(b)-2(c)-(d), (a′)=(b′)-4(c)-(d), (a″)=(b″)-2(e)-(d),  ,
,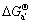 and
and 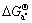 can be denoted as
can be denoted as


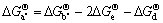
Let pure Al(s), Ca(s), Mg (s), Al2Ca(s) and Al4Ca(s) be standard state, respectively, then



thus,

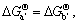
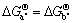
According to the thermodynamic data in Ref.[15], the values of  ,
, and
and  at 900 K are calculated as follows, respectively.
at 900 K are calculated as follows, respectively.
 -204 935 J/mol,
-204 935 J/mol, 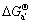 =-202 978 J/mol,
=-202 978 J/mol,
 =-35 238J/mol
=-35 238J/mol
Substituting 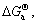
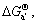
 a([Mg])=1, a(Al2Ca)=1 and a(Al4Ca)=1 into Eqns.(8), (9) and (10),
a([Mg])=1, a(Al2Ca)=1 and a(Al4Ca)=1 into Eqns.(8), (9) and (10),  ,
, and
and 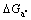 at different mass fraction of Ca are obtained, which are shown in Fig.3. It can be found that
at different mass fraction of Ca are obtained, which are shown in Fig.3. It can be found that ,
,  when the mass fraction of Ca changes from 0.5 to 3. It indicates that Al2Ca phase, not Al4Ca phase, can take priority of depositing, while Mg2Ca phase does not deposit. Moreover, the higher the mass fraction of Ca is, the more negative the
when the mass fraction of Ca changes from 0.5 to 3. It indicates that Al2Ca phase, not Al4Ca phase, can take priority of depositing, while Mg2Ca phase does not deposit. Moreover, the higher the mass fraction of Ca is, the more negative the is, and the greater the trend to formation of Al2Ca phase is.
is, and the greater the trend to formation of Al2Ca phase is.
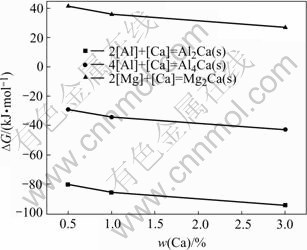
Fig.3 ΔG of different reactions as function of mass fraction of Ca at 900 K
The study in Ref.[16] indicated that only Al2Ca and α-Mg were found in Mg-9Al-0.5Zn-0.1Be-xCa alloy, while Al4Ca and Mg2Ca did not precipitate during cooling. Al2Ca precipitated prior to β-Mg17Al12 and prevented the increase of the grain size. With the mass fraction of Ca increasing from 0.5 to 3, the amount of Al2Ca increased and the effect on the refinement of grains became prominent. These experimental phenomena agree well with the results in this paper. Al2Ca precipitates at the temperature near to AZ91 alloy solidification and makes itself easy to go into β-Mg17Al12 phase and improves the thermal stability of β-Mg17Al12 phase. This just gives the reason for why Ca mostly exists in β-Mg17Al12 phase and improves the thermal stability of the AZ91 alloy introduced by Refs.[2, 17].
4 Conclusions
Based on the Miedema model and Chou model, the activities of different solute components in Mg-Al-Zn, Mg-Ca-Zn and Mg-Al-Ca systems are studied. The following conclusions are obtained.
1) In the Mg-Al-Zn and Mg-Ca-Zn alloy systems, the variety of its quantity has little influence on the activities of Al or Ca when the mass fraction of Zn is lower than 2%, therefore, the interaction of Zn and Al or Ca can be neglected. The intermetallic compounds appeared possibly in the Mg-Al-Zn-Ca system can be predicted by simply calculating the Gibbs free energies of different intermetallic compounds in Mg-9Al-xCa.
2) In Mg-9Al-xCa alloy systems, the calculated results indicate that Al2Ca phase can take priority of depositing at 900 K, which is near to the solidification temperature of AZ91 alloy. This agrees with the experimental results in Refs.[6, 16, 17]. The consistency of calculation and experiment proves that it is feasible to predict the intermetallic compounds in AZ91 alloys containing calcium by the Miedema model and Chou model.
References
[1] LU Yi-zhen, WANG Qu-dong, ZENG Xiao-qin, DING Wen-jiang, ZHUAI Chun-quan, ZHU Yan-ping. Effects of rare earths on the microstructure, properties and fracture behavior of Mg-Al alloys[J]. Materials Science and Engineering A, 2000, 278: 66-76.
[2] MIN Xue-gang, SUN Yang-shan, XUE Feng, DU Wen-wen, WU Deng-yun. Analysis of valence electron structures(VES) of intermetallic compounds containing calcium in Mg-Al-based alloys[J]. Materials Chemistry and Physics, 2002, 78: 88-93.
[3] ZHOU Hai-tao, WANG Qu-dong, WEI Yin-hong, DING Wen-jiang, ZHU Yan-ping, CHINO Y, MABUCHI M. Flow stress and microstructural evolution in as rolled AZ91 alloy during hot deformation[J]. Trans Nonferrous Met Soc China, 2003, 13(6): 1265-1269.
[4] QI Qing-ju, LIU Yong-bing, YANG Xiao-hong. Effects of rare earths on friction and wear characteristics of magnesium alloy AZ91D[J]. Trans Nonferrous Met Soc China, 2003, 13(1): 111-115.
[5] ZHANG Shi-chang, WEI Bo-kang, CAI Qi-zhou, WANG Li-shi. Effect of mischmetal and yttrium on microstructures and mechanical properties of Mg-Al alloy[J]. Trans Nonferrous Met Soc China, 2003, 13(1): 83-87.
[6] DU Wen-wen, SUN Yang-shan, MIN Xue-gang, XUE Feng, ZHU Min, WU Deng-yun. Microstructure and mechanical properties of Mg-Al based alloy with calcium and rare earth additions[J]. Materials Science and Engineering A, 2003,356: 1-7.
[7] LIU Man-ping, WANG Qu-dong, LIU Zi-li, YUAN Guang-yin, WU Guo-hua, ZHU Yan-ping, DING Wen-jiang. Behavior of Mg-Al-Ca alloy during solution heat treatment at 415℃[J]. Journal of Materials Science Letters, 2002, 21, 1281-1283.
[8] FAN Tong-xiang, YANG Guang, ZHANG Di. Thermodynamic of alloying addition on in-situ reinforced TiB2/Al composites[J]. Metallurgical and Materials Transaction A, 2005, 36A: 225-232.
[9] ZHU Yan, YANG Yan-qing, SUN Jun. Calculation of activity coefficients for components in ternary Ti alloy and intermetallics as matrix of composites[J]. Trans Nonferrous Met Soc China, 2004, 14(5): 875-879.
[10] CHOU Guo-zhi. New generation solution geometrical model and its future development[J]. Acta Metallurgica Sinica, 1997, 33(2): 126-132. (in Chinese)
[11] FAN Peng, CHOU Guo-zhi. A model for predicting thermodynamic properties of metallic solution from fundamental physical quantities of constituent elements[J]. Acta Metallurgica Sinica, 1999, 35(4): 421-426. (in Chinese)
[12] TANG Kai, JIANG Guo-chang, CHOU Guo-zhi, XU Kuang-di. The relation between the generation solution model and Self-SRem model[J]. Acta Metallurgica Sinica, 1999, 35(8): 801-804. (in Chinese)
[13] Miedema a r, de Boer p f, de Chatel p f. Cohesion in alloys fundamentals of semi-empirical model[J]. Physica, 1980, 100B: 1-28.
[14] CHEN Zhen-hua, YAN Hong-ge, CHEN Ji-hua, QUAN Ya-jie, WANG Hui-min, CHEN Ding. Magnesium Alloy[M]. Beijing: Chemical Industry Press, 2004. 30-31.(in Chinese)
[15] YE Da-lun, HU Jian-hua. Thermodynamic Data Handbook of Inorganic Materials[M]. Beijing: Metallurgical Industry Press, 2004. 30-31.(in Chinese)
[16] ZENG Xiao-qin, WANG Qu-dong, LU Yi-zhen, DING Wen-jiang, ZHU Yan-ping, ZHUAI Chun-quan, LU Chen. Microstructure and mechanical properties of Mg-9Al-0.5Zn-0.1Be-xCa alloys[J]. Materials and Mechanical Engineering, 2001, 25(5): 15-18. (in Chinese)
[17] DU Wen-wen, SUN Yang-shan, MIN Xue-gang, XUE Feng, WU Deng-yun. Influence of Ca addition on valence electron structure of Mg17Al12[J]. Trans Nonferrous Met Soc China, 2003, 13(6): 1274-1279.
Corresponding author: DU Wen-bo; Tel: +86-10-67392917; E-mail: duwb@bjut.edu.cn
(Edited by YUAN Sai-qian)