
Synthesis and physico-chemical properties of new green electrolyte 1-butyl-3-methylimidazolium perchlorate
WANG Xiao-dan(王晓丹)1, 2, WU Wen-yuan(吴文远)1, TU Gan-feng(徐赣峰)1, JIANG Kai-xi(将开喜)1, 3
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
2. College of Applied Chemistry, Shenyang University of Chemical Technology, Shenyang 110142, China;
3. Beijing General Research Institute of Mining and Metallurgy, Beijing 100044, China
Received 13 November 2009; accepted 22 March 2010
Abstract: 1-butyl-3-methylimidazolium perchlorate ([BMIM]ClO4) was synthesized by two steps with N-methylimidazolium. Some physico-chemical properties, such as density, surface tension, viscosity, electrical conductivity as well as electrochemical window, were investigated and solvent performance was also studied. The results show that this kind of ionic liquid is an excellent electrolyte with low viscosity, high electrical conductivity and wide electrochemical window. In addition, [BMIM]ClO4 is soluble in most conventional solvents and some metal oxides have high solubility in it, which lays the foundation of direct electrolysis of metal oxides in this ionic liquid.
1 Introduction
Room temperature ionic liquids are salts with low melting points composed of an organic cation and either an organic or an inorganic anion[1]. In contrast to classical solvents, ionic liquids possess a series of outstanding advantages as electrolytes such as no vapor pressure, high thermal stability, wide range of solubility and wide electrochemical window[2]. So, researches on ionic liquids have been paid more and more attention in recent years and among them using ionic liquids as new-style environment-protective electrolytes is a hotspot[3-4].
Chloroaluminated ionic liquids are the focus of the investigations in earlier research work[5-6]. Although ionic liquids of this kind are cheaper, they are badly sensitive to air and water. Thus, studies on relatively stable non-chloroaluminated ionic liquids have increased recently[7-8] and those composed of dialkylimidazolium cations with high stability and electrical conductivity are the most representative[9-10]. Most of the anions are perfluoroanions such as 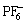 ,
,  , Tf2N- and CF3COO-, but the synthesis costs are so high that it is difficult to realize production and application of these ionic liquids. For the purpose to reduce costs and introduce new functional groups, 1-butyl- 3-methylimidazolium perchlorate based on dialkylimidazolium cation and oxygen containing inorganic anion was synthesized to discuss the effects of anion structures on properties of ionic liquids and explore the possibility of using it as electrolyte.
, Tf2N- and CF3COO-, but the synthesis costs are so high that it is difficult to realize production and application of these ionic liquids. For the purpose to reduce costs and introduce new functional groups, 1-butyl- 3-methylimidazolium perchlorate based on dialkylimidazolium cation and oxygen containing inorganic anion was synthesized to discuss the effects of anion structures on properties of ionic liquids and explore the possibility of using it as electrolyte.
2 Experimental
N-methylimidazolium (industrial product) was vacuum redistilled before being used;n-butyl bromide (CP reagent); methanol, ethanol, toluene, acetone, ethyl acetate, NaClO4 and metal oxides are all analytical reagents.
Intermediate [BMIM]Br was synthesized according to Ref.[11]. Then, 22.0 g (0.1 mol) [BMIM]Br was dissolved in 20 mL methanol with 23.9 g (0.1 mol) NaClO4 added and the mixture was stirred for 24 h at 40 °C. After filtration methanol was removed by rotary evaporation and colorless liquid could be obtained with 91% yield. The specific reaction route is shown in Fig.1.
The obtained ionic liquid was analyzed using KBr

Fig.1 Synthesis of [BMIM]ClO4 ionic liquid
tablet method by NEXUS FT-IR 470 infrared spectrometer from Thermo Nicolet Company of USA.
Before being used the ionic liquid should be dried in vacuum at 80 °C for 24 h. Then, water content was detected to be less than 0.1% by Carl Fisher method. The density was measured using bottle method and the surface tension was tested through the method of bubbling. The viscosity was measured with NDJ-TP rotary viscometer. Electrical conductivity was determined by DDSJ-308A conductivity meter with DJS-1 bright platinum electrode as conductive electrode. Electrochemical window was tested at 25 °C by ZAHNER IM (6e) electrochemical workstation using three-electrode system with a platinum wire electrode as working electrode, a platinum foil electrode as counter electrode and a silver wire electrode as reference electrode[12]. The electrodes should be polished by sand paper, washed by water and then dried before every test. The test scan rate was 100 mV/s. After two weeks exposure in air, thermal performance of the ionic liquid was analyzed at 10 °C/min by STA 449C thermal analyzer.
At room temperature, about 1 g ionic liquid was put on a glass plate and then friction between this plate and another made the ionic liquid extend as far as possible. Any one of the plates was chosen to be weighed accurately at regular intervals.
At 25 °C, about 2 g oxide was added to 25 mL ionic liquid and the mixture was magnetically stirred for 24 h followed by filtration with sand-core funnel. The obtained powder was washed with acetone, dried, and weighed. The mass difference before and after dissolution was considered as the mass of soluble compound.
3 Results and discussion
3.1 Structure characterization of [BMIM]ClO4 ionic liquid
The ionic liquid was characterized by infrared spectroscopy and the results are as follows. Stretching vibration peaks of C—H from imidazole ring are located at 3 158 and 3 119 cm-1, stretching vibration peaks of C—H from—CH3— and —CH2— on lateral chain of imidazole ring are located at 2 964 and 2 938 cm-1, skeletal vibration peaks of imidazole ring are located at 1 574 and 1 467 cm-1, in-plane bending vibration peak of C—H from imidazole ring located at is located al 1 170 cm-1 and stretching vibration peak of ClO4- is located at 1 097 cm-1. This indicates that the synthesized product is the aim product with [BMIM]+ and ClO4- ions.
3.2 Physical properties of [BMIM]ClO4 ionic liquid
Density at different temperatures is given in Fig.2. According to the figure, density of [BMIM]ClO4 at 20 °C is 1.26 g/cm3. Densities of most ionic liquids at atmosphere pressure and room temperature are higher than water, generally ranging from 1.1 to 2.4 g/cm3. Densities of common ionic liquids [BMIM]PF6 and [BMIM]BF4 are 1.33 g/cm3 (20 °C) and 1.21 g/cm3 (25 °C)[2], respectively. It is known that the effect of anions on density is relatively obvious and density increases with the bigger anion. Density is linearly changed against temperature with the data fitted to get ρ=1.27-6.42×10-4(T-298.15).
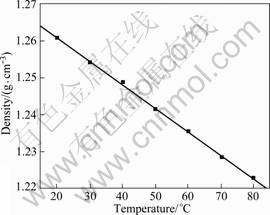
Fig.2 Density of [BMIM]ClO4 ionic liquid at different temperatures
Surface tension of [BMIM]ClO4 at different temperatures is shown in Fig.3. As can be seen that, at 20 °C the surface tension which is lower than that characterized for water (72.7 mN/m)[13] and higher than that of normal alkanes (16.0-27.0 mN/m)[13], is 43.9 mN/m. The surface tensions of [BMIM]BF4 and [BMIM]PF6 are 38.4 and 42.9 mN/m (63 °C), respectively[13], while the surface tension of [BMIM]ClO4 at 60 °C is 42.5 mN/m, which accords with the bigger anions and surface tension[13]. Otherwise, it is observed that surface tension of [BMIM]ClO4 linearly decreases with temperature increasing and fitted formula is γ=44.3-0.03(T-298.15).
Fig.4 shows the relationship between viscosity of the ionic liquid and temperature. The viscosity is 47.5 mPa?s at 20 °C and decreases with temperature increasing. Generally, when symmetry of the ionic liquid anion is higher, viscosity is higher because of larger van der Waals force and smaller electrostatic interaction. For example, viscosity with 312 mPa?s[2] of [BMIM]PF6 at 20 °C is much higher than that of [BMIM]ClO4, and one reason might be different symmetry of these two ionic liquid anions. 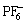 anion belongs to octahedral configuration with high symmetry, while
anion belongs to octahedral configuration with high symmetry, while 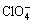 anion belongs to tetrahedral configuration with symmetry lower than
anion belongs to tetrahedral configuration with symmetry lower than 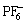 anion. [BMIM]ClO4 with low viscosity is not easy to cause adherence to reactor, so it can be used at room temperature.
anion. [BMIM]ClO4 with low viscosity is not easy to cause adherence to reactor, so it can be used at room temperature.

Fig.3 Surface tension of [BMIM]ClO4 ionic liquid at different temperatures

Fig.4 Viscosity of [BMIM]ClO4 at different temperatures
The electrical conductivity of [BMIM]ClO4 ionic liquid at different temperatures is shown in Fig.5. Commonly, the higher the viscosity and the lower the conductivity, the larger the density and the higher the conductivity[13]. Up to now, the highest conductivity of ionic liquids ever reported is 120 mS/cm[14], but rigorous conditions of synthesis limited the applications of this kind of ionic liquid. [BMIM]ClO4 possesses high conductivity of 9.44 mS/cm at 25 °C as shown in Fig.5; however the conductivity of [BMIM]PF6 is only 1.8 mS/cm (22 °C)[2]. When plotting ln σ with 1/T (Fig.6), one can get a line, illustrating that the relationship between electrical conductivity and temperature is in accordance with Arrhenius equation (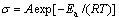 ) with the calculated activation energy Ea(σ) of 26.74 kJ/mol based on the fitted data.
) with the calculated activation energy Ea(σ) of 26.74 kJ/mol based on the fitted data.
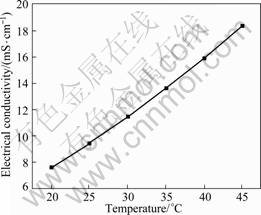
Fig.5 Electrical conductivity of [BMIM]ClO4 ionic liquid at different temperatures

Fig.6 Arrhenius curve of [BMIM]ClO4 ionic liquid
[BMIM]ClO4 ionic liquid is linearly swept to obtain cyclic voltammetric curve as shown in Fig.7. According to Fig.7, the limited cathode potential is -1.4 V and limited anode potential is 2.2 V with the electrochemical window of 3.6 V. Consisting of the same cations, the window of [BMIM]ClO4 is much larger than that of [BMIM]Br (2.2 V) but a little smaller than [BMIM]PF6 (>4 V)[1] mainly because of the larger volume and higher dispersion of  ionic liquid[15].
ionic liquid[15].
3.3 Water absorption and thermal stability of ionic liquid
Water absorption results are shown in Table 1. It can be seen from Table 1 that the mass of [BMIM]ClO4 ionic liquid increases with prolongation of exposure time but
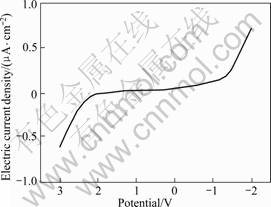
Fig.7 Linear sweep voltammetric curve of [BMIM]ClO4 ionic liquid
Table 1 Water absorption of [BMIM]ClO4 ionic liquid (g)
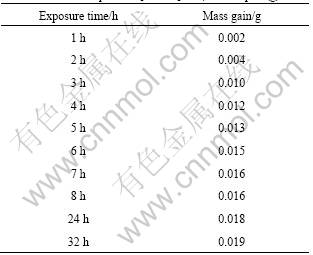
the mass gain is not very significant, and changes get slow especially after 7 h with just 3.8% (mass fraction) gain after 32 h. It can be illuminated that this kind of ionic liquid is hydrophilic, but only exhibits a certain degree of water absorption in the air.
Mass of the ionic liquid is 0.497 g before experiment, and the data in Table 1 are overall mass gains after a period of time.
DSC-TGA results of [BMIM]ClO4 are shown in Fig.8. It can be seen from Fig.8 that this ionic liquid is comparably stable before being heated to 300 °C and evaporation of some water in the ionic liquid causes the mass loss before 100 °C, which again proves the results of water absorption experiments above. The obvious mass loss of 75.3% between 300.1 and 345.6 °C should be caused by the decomposition of [BMIM]ClO4. Endothermic peak of DSC curve within this range of temperature, identical with TGA curve, is also clear. All above results indicate that [BMIM]ClO4 ionic liquid has good thermal stability with high decomposition temperature and can be used as electrolyte at wide temperature range.
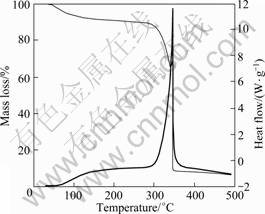
Fig. 8 DSC-TGA curve of [BMIM] ClO4 ionic liquid
3.4 Solubility of ionic liquid
Ionic liquids at room temperature show special characteristics on various aspects different from other molecular solvents and it is significant to study the solubility to organic and inorganic liquids or solids. But till now, there have been less data reported on solubility of ionic liquids. Solubility performance for routine solvents and some inorganic metal oxides were investigated, as shown in Tables 2 and 3.
Table 2 Solubility of [BMIM]ClO4 ionic liquid to routine solvents

Table 3 Solubility of some metal oxides in [BMIM]ClO4 (g/L)

It is shown in Table 2 that [BMIM]ClO4 is soluble in most conventional polar solvents and insoluble to non-polar toluene, which plays an important role in solvents choosing for ionic liquids synthesis and products separation. For example, methanol is chosen to be solvent to synthesize [BMIM]ClO4 because methanol is soluble to the intermediate [BMIM]Br and the low boiling point is helpful for its separation from the ionic liquid.
From Table 3, it can be seen that some metal oxides have high solubility in [BMIM] ClO4, while others have very low solubility. ABBOTT et al[16] have given the solubility of HOC2H4(CH3)+Cl- ionic liquid by just 10-6 order of magnitude, which indicates that sorts of anions have great influence on the solubility. The study on solubility in this work provides vital data for application of this ionic liquid in the field of mineral separation, organic synthesis and homogeneous catalysis. Furthermore, a new thought on direct electrolysis of metal oxides in ionic liquids is offered from the study.
4 Conclusions
1) A new ionic liquid [BMIM]ClO4 was synthesized with low cost by simple method.
2) [BMIM]ClO4 is an excellent green electrolyte with high thermal stability, low viscosity and high electrical conductivity.
3) [BMIM]ClO4 is soluble in most conventional solvents and some metal oxides have high solubility in it, which lays the important foundation for extending its application fields.
References
[1] FU Chao-peng, ZHAO Hai-hui, WU Hui-min, CHEN Jin-hua, KUANG Ya-fei. Research on electrochemical properties of nonaqueous ionic liquid microemulsions [J]. Colloid Polym Sci, 2008, 286: 1499-1504.
[2] GALIALI?SKI M, LEWANDOWSKI A, STEPNIAK I. Ionic liquids as electrolytes [J]. Electrochimica Acta, 2006, 51(26): 5567-5580.
[3] MA Jun-de, LI Bing, YAN Ling-guang, CHEN Yan. Electrodeposition of zinc from zinc chloride- 1-ethyl-3-methylimidazolium chloride molten salt [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(6): 1135-1142. (in Chinese)
[4] ZHAO C, BURRELL G, TORRIERO A A, SEPAROVIC F, DUNTOP N F, MACFARLANE D R, BOND A M. Electrochemistry of room temperature protic ionic liquids[J]. J Phys Chem B, 2008, 112(23): 6923-6936
[5] HSIU S I, HUANG J F, SUN I W, YUAN C H, SHIE A J. Lewis acidity dependency of the electrochemical window of zinc chloride–1-ethyl-3-methylimidazolium chloride ionic liquids [J]. Electrochimica Acta, 2002, 47(27): 4367-4372.
[6] WANG Xi-ran, HUA Yi-xin, ZHAO Qiu-ning, LI Yan. Electrical conductivity of AlCl3-BMIC room temperature ionic liquids [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(12): 2138-2142. (in Chinese)
[7] SUBIANTO S, MISTRY M K, CHOUDHURY N R, DUTTA N K, KNOTT R. Composite polymer electrolyte containing ionic liquid and functionalized polyhedral oligomeric silsesquioxanes for anhydrous PEM applications [J]. Applied Materials and Interfaces, 2009, 1(6): 1173 -1182.
[8] CHENG S S, YEN T F. Use of ionic liquids as phase-transfer catalysis for deep oxygenative desulfurization[J]. Energy and Fuels, 2008, 22(2): 1400-1401.
[9] PASTOR M L, VIDAL A D, CA?ADA M J A, SIMONET B M, LENDL B, VALC?RCEL M. Separation of single-walled carbon nanotubes by use of ionic liquid-aided capillary electrophoresis [J]. Anal Chem, 2008, 80(8): 2672-2679.
[10] CHANG J K, LEE M T, TSAI W T, DENG M J, CHENG H F, SUN I W. Pseudocapacitive mechanism of manganese oxide in 1-ethyl-3-methylimidazolium triocynate ionic liquid electrolyte studied using X-ray photoelectron spectroscopy [J]. Langmuir, 2009, 25(19): 11955-11960.
[11] CAI Yue-qin, PENG Yan-qing, SONG Gong-hua, HUANG Fei-fei, LU Feng. Study in the synthesis of room temperature ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate [J]. Chemical Reagents, 2005, 27(1): 1-2. (in Chinese)
[12] WANG Yi, GAO De-shu, LI Zhao-hui, JIANG Jing, SU Guang-yao. Electrochemical properties of ionic liquid on porous carbon electrode [J]. Chemical Researches, 2005, 16(2): 38-41. (in Chinese)
[13] DENG You-quang. Ionic liquid—property, preparation and application [M]. Beijing: China Petrochemical Press, 2006: 137-138. (in Chinese)
[14] HAGIWARA R, HIRASHIGE T, TSUDA T, ITO Y. Acidic 1-ethyl-3-methylimidazolium fluoride: A new room temperature ionic liquid [J]. J Fluorine Chem, 1999, 99: 1-3.
[15] MCEWEN A B, NGO H L, LECOMPTE K, GOLDMAN J L. Electrochemical properties of imidazolium salt electrolytes for electrochemical capacitor applications [J]. J Electrochem Soc, 1999, 146(5): 1687-1695.
[16] ABBOTT A P, CAPPER G, DAVIES D L, RASHEED R K, SHIKOTRA P. Selective extraction of metals from mixed oxide matrixes using choline-based ionic liquids [J]. Inorg Chem, 2005, 44: 6497-6499.
(Edited by LI Xiang-qun)
Foundation item: Project(50574031) supported by the National Natural Science Foundation of China
Corresponding author: WU Wen-Yuan; Tel: +86-13002475899; E-mail: wwy030501@yahoo.com.cn
DOI: 10.1016/S1003-6326(09)60413-1