
Micro and sub-micro morphology of Mg65Cu25Y10 bulk amorphous alloy containing primary crystalline phases
GUAN Le-ding(关乐丁)1, 2, YAN Biao(严 彪)1, 2, YANG Sha(杨 沙)1, 2
1. Shanghai Key Laboratory of D&A for Metal-Functional Materials, Tongji University, Shanghai 200092, China;
2. School of Materials Science and Engineering, Tongji University, Shanghai 200092, China
Received 15 July 2007; accepted 10 September 2007
Abstract: Acoustic-frequency induction melting together with rapid quenching was used to prepare Mg65Cu25Y10 bulk amorphous alloy containing primary crystalline phases. Transmission electron microscopy (TEM), X-ray diffractometry (XRD), environmental scanning electron microscopy (ESEM) and atomic force microscopy (AFM) were used to study the amorphization and morphology of the prepared alloy. Under micro condition, the sample alloy is composed of amorphous structure with cauliflorate shape, primary CuMg2 dendrite phase, mixed primary CuMg2 and Cu2Y crystalline phases, as well as Mg phase with small size. Further study under sub-micro condition shows that, the planar amorphous structure of cauliflorate shape is composed of the three-dimensional sub-micro morphology of “bulge-concavity” pattern, which extends into the space in terms of certain period. It is estimated that the major factors influencing the micro and sub-micro morphologies of amorphous alloy are its super-cooling liquid structure, rapid quenching transformation, as well as the melting conditions.
Key words: Mg-based amorphous alloys; induction melting; rapid quenching; morphology
1 Introduction
Among numerous amorphous alloys, Mg-based amorphous alloys have attracted great attention because of a high specific strength and a low glass transition temperature[1]. Mg-Cu-Y alloy system exhibits higher glass forming ability, which was discovered by INOUE and MASUMOTO[2]. Thus, appropriate compound ingredients and their proportions can be identified based on such system. INOUE et al[2-3] have made great progress in preparation and study of Mg-based amorphous alloys since 1990s. The effective size of the amorphous Mg65Y10Cu15Ag5Pd5 alloy prepared reached 12 mm[3], which was the largest Mg-based amorphous alloy reported so far. At present, researchers pay much attention to adding microelements to the Mg-based alloys so as to better improve their glass forming ability and mechanical properties[4]. Mg-based amorphous alloys possess good mechanical properties and corrosion- resistant properties[2], whose outstanding hydrogen- storage property is also superior to that of Zr-based amorphous alloys[5]. Therefore they have a promising future in applications.
Micro and sub-micro morphologies of amorphous alloys have hardly been reported worldwide due to the restrictions of preparation conditions, glass forming ability of the alloys, as well as the measurement of amorphous alloy. In the present work, delicate devices were used to study the micro and sub-micro morphologies of Mg-based amorphous alloy, which has not yet been understood clearly. So it is significantly important to perceive its formation mechanism and structural characteristics.
The present work used the methods of acoustic- frequency induction melting and rapid quenching to prepare Mg65Cu25Y10 bulk amorphous alloy containing primary crystalline phases. Transmission electron microscopy (TEM), X-ray diffractometry (XRD), environmental scanning electron microscopy (ESEM) and atomic force microscopy (AFM) were used to estimate the degree of amorphization, and to study the micro and sub-micro amorphous morphologies.
2 Experimental
A Mg65Cu25Y10 master ingot was prepared by melting Mg (≥99.9%), Cu (≥99.9%) and Y (≥99.9%) according to the nominal composition under vacuum. The ingot was sealed into a quartz glass tube well vacuumized.
The ingot sealed in the tube was inductively melted by XG-30 acoustic-frequency induction equipment under the output oscillating current of 900 A for 20 s. The tube was subsequently quenched by liquid nitrogen and a sample alloy with size of 10 mm×4 mm×50 mm was prepared.
Electron diffraction pattern of the sample was studied by HITACHI H-800 TEM. XRD pattern was studied by D/max 2550VB3 X-ray diffractometry, operated at a scanning rate of 10(?)/min from 10? to 85?. Microstructure and morphology of the alloy were studied by QUANTA 200 FEG-ESEM under high-vacuum mode. Sub-micro structure and morphology were studied by SPA-300HV AFM under dynamic force mode (DFM) within the scanning scope of 500 nm, with resolution of 0.2 nm and 0.01 nm on X-Y planar surface and along Z axis, respectively.
3 Results and discussion
3.1 Amorphization
Fig.1 shows the selected area electron diffraction pattern of the amorphous Mg65Cu25Y10 alloy. On one hand, a defocused spot which is uniformly continuous can be seen in Fig.1, proving that amorphous structure takes a large proportion in the sample alloy. Meanwhile, several polycrystalline diffraction circles can also be seen, so that crystalline phases are generated in the sample. On the other hand, as there exist polycrystalline diffraction circles rather than spots, and the circles are fuzzy and dark, the crystalline phases are quite small, which results from rapid quenching. Fig.2 shows the X-ray diffraction pattern of amorphous Mg65Cu25Y10 alloy. A broad diffusion peak corresponds to an amorphous matrix, while two sharp peaks and another one are identified to be CuMg2 phase and Cu2Y phase, respectively. The amorphization of the alloy is incomplete for the appearance of crystalline peaks. However, the intensities of the crystalline peaks are not so large, and the number of the crystalline peaks which can be identified is quite small. It is estimated that the amorphous structure holds the major part of the alloy[6], which also contains small sized CuMg2 and Cu2Y crystalline phases.

Fig.1 Selected area electron diffraction pattern of amorphous Mg65Cu25Y10 alloy
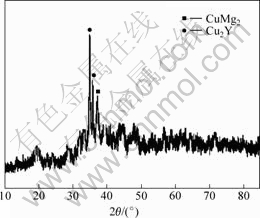
Fig.2 X-ray diffraction pattern of amorphous Mg65Cu25Y10 alloy
3.2 Micro morphology
Fig.3(a) shows the backscattered electron image of the amorphous Mg65Cu25Y10 alloy by environmental scanning electron microscopy (ESEM). As it is shown in Fig.3(a), the structure of the alloy is composed of four parts: dispersed black spots, areas with the shape of cauliflorate, areas with the shape of arborization, and the erose areas among the aforementioned areas. By combining the results of X-ray diffraction and electron diffraction, it is concluded that the cauliflorate areas which hold major parts of the alloy are amorphous phases, the arborization areas are primary crystalline phases that can generate dendrite crystals, and the erose areas among the two areas are primary crystalline phases formed in no order. Fig.3(b) shows the illustration of point element analysis of the amorphous Mg65Cu25Y10 alloy, and Table 1 lists the results of the point element analysis. Point A locates on the dendrite structure as seen in Fig.3(a), whose major component is CuMg2 as listed in Table 1. For Points B and C which locate on the erose areas, the components of them are a little complex as seen in Table 1. It is estimated that they are composed
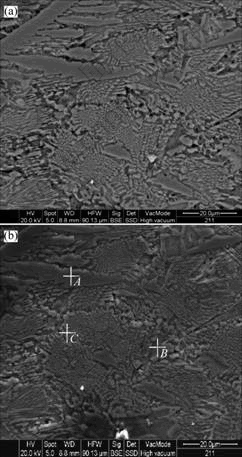
Fig.3 Backscattered electron image(a) by environmental scanning electron microscope (ESEM) and illustration of point element analysis(b) of amorphous Mg65Cu25Y10 alloy
Table 1 EDS results of amorphous Mg65Cu25Y10 alloy (mass fraction, %)
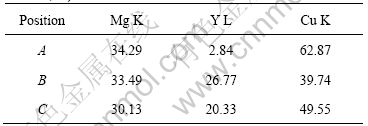
of both primary CuMg2 and Cu2Y crystalline phases. Furthermore, in terms of the principle that the lower the atomic number, the darker the backscattered electron image shown by ESEM, it is concluded that the major component of the black spots which are the darkest shown in Fig.3(a) is magnesium whose atomic number is the lowest in the master alloy. HUI et al[7] found analogous α-Mg in the study of Mg-based bulk amorphous composites, and YUAN et al [8] also found analogous hcp-Mg in the study of Mg85Cu5Zn5Y5 amorphous alloy containing nanoscale particles. However, as no legible diffraction peak of Mg-phase can be identified in X-ray diffraction pattern, the grain size of Mg-phase should be quite small, which coincides with the result shown by ESEM.
Under micro condition, the amorphous structure of the alloy takes on a cauliflorate shape and is separated into several areas by crystalline phases, which may be caused by the joint efforts of rapid quenching and induction melting, with the latter playing an important role. Induction melting possesses significant characteristic of skin effort, i.e. eddy current density decreases from surface to inside in the workpiece, which means melting conditions are different if the distances to the axis of induction loop differ from each other. As melting time is a little short in the present study, the components of the master alloy may not be uniformly fused during the process of induction melting. Thus, the differences of melting conditions act apparently among master alloys, whose relative positions are different from one another. During the brief process of induction melt and the course for melting liquid to fill in the cavity of the glass tube, melting liquid of the master alloy with similar melting conditions will unite together, and then form amorphous structure after rapid quenching. However, there will obviously exist componential concentration gradient and temperature gradient among liquid areas, whose melting conditions vary greatly. Crystalline phases are likely to form in such areas that have the two kinds of the gradient aforementioned, therefore further separating the amorphous structure modally under micro condition.
3.2 Sub-micro morphology
The amorphous structure of the sample takes on a cauliflorate shape under micro condition in planar surface, as shown by ESEM, and the average size of such amorphous areas divided by crystalline phases is around 20 μm×15 μm. More details about their morphology in three-dimensional space under sub-micro condition were studied. Fig.4 shows the three-dimensional sub-micro morphology of the amorphous structure in the Mg65Cu25Y10 alloy by atomic force microscopy. It is clearly identified that amorphous structure possesses three-dimensional morphology of “bulge-concavity” pattern in the space size of 500 nm×500 nm×50 nm under sub-micro condition. The characteristic of such morphology is that four structural bulges are formed based on the four corners of the planar coordinate as datum marks in the space above the coordinate. The four bulges surround their umbilication, which constitutes the structure of “bulge-concavity” pattern configurationally. By extending three-dimensionally in terms of certain period, such sub-micro morphology therefore forms amorphous cauliflorate micro structure aforementioned.
Fig.5 shows the three-dimensional morphology of amorphous structure in the alloy simulated by ESEM. The amorphous structure is composed of bulges as well as concavities among them, which is coincident with the results obtained by AFM. YANG et al[9] also observed similar structure in amorphous and amorphous/ nanocrystalline Fe85.2Zr3.3Nb3.5B3Si3Al1Cu1 alloy under contact mode by AFM.
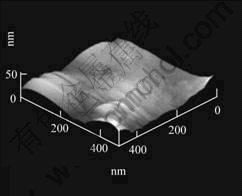
Fig.4 Three-dimensional morphology image of amorphous structure in amorphous Mg65Cu25Y10 alloy under sub-micro condition by atomic force microscopy
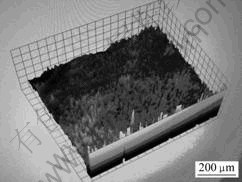
Fig.5 Three-dimensional morphology of amorphous structure in amorphous Mg65Cu25Y10 alloy simulated by ESEM
As research on amorphous morphology is not much, and conclusions resulting from different amorphous alloys vary from each other, general principles about micro and sub-micro morphologies of amorphous alloys have not yet been summarized. In the present study, it is estimated that there are three major factors that influence amorphous morphology: super-cooling liquid structure of amorphous alloy, rapid quenching transformation from liquid to solid, and melting conditions. Firstly, when alloys are melted, a lot of componential fluctuation and structural fluctuation will occur in the melting liquid because of higher temperature, which makes the structure of melting liquid uncertain to some degree. So the structure of super-cooling liquid which originates from melting liquid is also unsure extraordinarily. Secondly, cooling rate plays a crucial role in the forming of micro structure of amorphous alloy when alloy system is definite[10]. According to the classical theories of amorphous alloys, when cooling rate is higher than the critical cooling rate of amorphous alloy which is needed for rapid quenching, liquid atoms don’t have enough time to form nucleus or diffuse so as to be frozen up and generate amorphous structure. However, when cooling rate is lower than the critical cooling rate, crystalline phases or mixture of amorphous structure and crystalline phases are expected to form. Thirdly, when all components of the alloy are evenly mixed, more compact random packing configuration is likely to form[11]. Melting conditions affect mixed degree of components, thus further affect super-cooling structure and glass forming ability of amorphous alloy. Furthermore, melting liquid of amorphous alloy contains several kinds of multi-component chemical short ranger order (MCSRO)[12], which is also one of the factors that influence the forming, structure and morphology of amorphous alloy. With joint efforts of such influencing factors aforementioned, diversified micro and sub-micro amorphous morphologies are achieved under different conditions. For amorphous Mg65Cu25Y10 alloy containing primary crystalline phases studied in the present work, the morphology of cauliflorate under micro condition and “bulge-concavity” pattern under sub-micro condition are likely to be related with the above influencing factors, especially the super-cooling liquid structure of the master alloy under preparation condition.
4 Conclusions
The method of acoustic-frequency melting together with rapid quenching was used to prepare Mg65Cu25Y10 bulk amorphous alloy containing primary crystalline phases with effective size of 4 mm. Under micro condition, the sample alloy is mainly composed of amorphous structure of cauliflorate, as well as primary CuMg2 dendrite phase, mixed structure of primary CuMg2 and Cu2Y crystalline phases, and Mg phase of small grain size, which separate amorphous areas out. The planar amorphous structure of cauliflorate under micro condition is formed by the morphology of “bulge-concavity” pattern under three-dimensional sub-micro condition, which extends into the surrounding space in terms of certain period. Such micro and sub-micro morphologies are likely to be related with the super-cooling liquid structure of amorphous alloy, rapid quenching transformation, as well as melting conditions.
References
[1] PRYDS N H. Bulk amorphous Mg-based alloys[J]. Mater Sci Eng A, 2004, 375/377: 186-193.
[2] INOUE A, MASUMOTO T. Mg-based amorphous alloys[J]. Mater Sci Eng A, 1993, 173: 1-8.
[3] AMIYA K, INOUE A. Preparation of bulk glassy Mg65Y10Cu15Ag5Pd5 alloy of 12 mm in diameter by water quenching[J]. Mater Trans JIM, 2001, 42(3): 543-545.
[4] LI Guo-qiang, ZhENG Li-jing, LI Li, LI Huan-xi, CHEN Chang-qi. Development of Mg-based bulk amorphous alloys[J]. Materials Review, 2006, 20(2): 54-57.
[5] SPASSOV T, KOSTER U. Hydrogenation of amorphous and nanocrystalline Mg-based alloys[J]. Journal of Alloys and Compounds, 1999, 287: 243-250.
[6] YANG Ying-jun, XING Da-wei, SUN Jian-fei, WEI Si-dong, SHEN Jun. Critical cooling rate and microstructure evolution of Cu-based bulk amorphous alloy[J]. Rare Metal Materials and Engineering, 2007, 36(2): 236-240. (in Chinese)
[7] HUI X, DONG W, CHEN G L, YAO K F. Formation, microstructure and properties of long-period order structure reinforced Mg-based bulk metallic glass composites[J]. Acta Materialia, 2007, 55: 907-920.
[8] YUAN G Y, ZHANG T, INOUE A. Structure and mechanical properties of Mg85Cu5Zn5Y5 amorphous alloy containing nanoscale particles[J]. Materials Letters, 2004, 58: 3012-3016.
[9] YANG Lei, YAN Biao, HE Guo-qiu, CHEN Cheng-shu. Research on surface of nanocrystalline multi-component iron-based alloy[J]. Shanghai Nonferrous Metals, 2005, 26(1): 7-10. (in Chinese)
[10] HU Yong, KOU Sheng-zhong, XU Guang-ji, DING Yu-tian, YUE Wu. Characteristics and evolution of microstructure in Cu-based bulk amorphous alloys[J]. Journal of Lanzhou University of Technology, 2006, 32(6): 1-4. (in Chinese)
[11] SUN Guo-yuan, CHEN Guang. Forming ability and mechanism of Mg-based bulk metallic glasses[J]. Nonferrous Metals, 2004, 56(2): 22-26. (in Chinese)
[12] CHEN Guo-liang, HUI Xi-dong, HE Guo. Multicomponent chemical short ranger order undercooling and the formation of bulk metallic glasses[J]. Mater Trans JIM, 2001, 42(6): 1095-1102.
(Edited by YANG Bing)
Foundation item: Projects(0552nm028; 04DZ05616) supported by Shanghai Science and Technology Committee, China
Corresponding author: YAN Biao; Tel: +86-21-65982463; E-mail: yanbiao@vip.sina.com