镁热还原三氧化二钒和氮气氮化制备高纯度氮化钒
来源期刊:中国有色金属学报(英文版)2019年第8期
论文作者:徐瑞 吴跃东 张国华
文章页码:1776 - 1783
关键词:镁热还原反应;氮化反应;氮化钒粉体;制备
Key words:magnesiothermic reduction reaction; nitridation reaction; vanadium nitride powder; preparation
摘 要:以三氧化钒(V2O3)为原料,通过两步法制备高纯度氮化钒(VN)粉末。首先,在873 K、Ar气氛中通过镁热还原反应将V2O3还原,得到V和MgO的混合物;然后,在1473 K、N2气氛中对样品进行氮化;最后,通过酸浸获得高纯度的VN粉末。采用X射线衍射仪和场发射扫描电子显微镜对样品的相变和形貌演变进行分析。实验结果表明,所制备的VN粉末的总体形貌保持初始V2O3粉末的形貌。通过酸浸除去MgO后,可以得到多孔VN颗粒,其氧含量为0.178%(质量分数)。与传统方法相比,这种方法可获得含有少量氧且不含碳的高纯度VN粉末。
Abstract: High purity vanadium nitride (VN) powders were prepared via a two-step process using vanadium trioxide (V2O3) as the raw material. The V2O3 was firstly reduced at 873 K in Ar atmosphere via magnesiothermic reduction reaction to get the mixture of V and MgO, and then the products were further nitrided at 1473 K in N2 atmosphere. Finally, the as-prepared samples were acid-leached to obtain pure VN powders. X-ray diffractometry and field-emission scanning electron microscopy were used to analyze the phase transition and morphological evolution of the samples. The results reveal that the overall morphology of the obtained VN powder retains the morphology of the initial V2O3 powders. After removing MgO by acidic leaching, the porous VN particles can be obtained, with the oxygen content of 0.178 wt.%. Compared with the traditional methods, high purity VN powders with a small amount of oxygen and no carbon can be obtained.
Trans. Nonferrous Met. Soc. China 29(2019) 1776-1783
Rui XU, Yue-dong WU, Guo-hua ZHANG
State Key Laboratory of Advanced Metallurgy, University of Science and Technology Beijing, Beijing 100083, China
Received 27 December 2018; accepted 9 June 2019
Abstract: High purity vanadium nitride (VN) powders were prepared via a two-step process using vanadium trioxide (V2O3) as the raw material. The V2O3 was firstly reduced at 873 K in Ar atmosphere via magnesiothermic reduction reaction to get the mixture of V and MgO, and then the products were further nitrided at 1473 K in N2 atmosphere. Finally, the as-prepared samples were acid-leached to obtain pure VN powders. X-ray diffractometry and field-emission scanning electron microscopy were used to analyze the phase transition and morphological evolution of the samples. The results reveal that the overall morphology of the obtained VN powder retains the morphology of the initial V2O3 powders. After removing MgO by acidic leaching, the porous VN particles can be obtained, with the oxygen content of 0.178 wt.%. Compared with the traditional methods, high purity VN powders with a small amount of oxygen and no carbon can be obtained.
Key words: magnesiothermic reduction reaction; nitridation reaction; vanadium nitride powder; preparation
1 Introduction
In the past few years, metal nitrides, especially transition metals nitrides, have received more and more attention owing to their excellent chemical and physical properties [1-3]. Among them, vanadium nitride (VN) with good wear resistance, extremely high hardness, high melting point and high thermal conductivity has been widely applied in cutting tools, superconducting devices, and catalysis. Furthermore, VN is an excellent alloy additive and widely used in the production of high-strength low-alloy (HSLA) [4-6].
A large number of methods have been proposed to prepare VN, such as direct reaction of metal vanadium and nitrogen, solid-state metathesis (SSM) at an elevated temperature [7], ammonolysis of precursor compounds of vanadium [8], and mechanochemical synthesis under a pressurized nitrogen atmosphere [9]. In the industrial production, VN is produced on a large scale by the carbonitrothermic reduction process. Carbon has been widely used in industrial fields with some attractive advantages such as easily-obtained and cheap. However, carbon is not a strong reducing agent at temperatures below 1873 K, which has been proved by Ellingham diagram [10]. For this reason, excessive carbon is always added to ensure the completion of the reduction reaction, which inevitably leads to the existence of residual carbon in the reduction products [11,12]. Therefore, to get a high purity vanadium nitride, a reducing agent with a stronger reducibility should be chosen.
Based on the Ellingham diagram of oxides, it can be found that Ca, Mg and Al are the possible reductants to reduce V2O3. Among them, Ca has an extremely chemical affinity to oxygen and is the strongest reducing agent. Although Al also has a strong reducing ability, it is hard to remove the generated Al2O3 from the products. The reduction ability of Mg is not as strong as Ca, but it is much cheaper than Ca, meanwhile, the byproduct MgO can be easily removed by leaching in HCl solution, as described in Eq. (1). Consequently, Mg is selected as the reductant in the current study [13,14].
MgO+2HCl=MgCl2+H2O (1)
In this work, a new method for preparing high quality VN based on magnesium reduction was proposed. V2O3, Mg powders and MgO were used as raw materials. Since magnesiothermic reduction reaction was an extremely exothermic reaction, a certain amount of MgO was added. Owing to a high melting point (3125 K), MgO would not participate in the reaction, but act as a diluent in the reaction to reduce the reaction temperature and the volatilization of Mg. This method has the following advantages: (1) VN without the contamination of C could be obtained; (2) the residual oxygen content could be controlled to a low level. Therefore, this method may give a possible way for producing high purity VN.
2 Experimental
2.1 Raw materials
The main chemicals used in this process, including Mg powder, hydrochloride acid, anhydrous ethanol, and MgO were supplied by Sinopharm Chemical Reagent Beijing Co., Ltd., China. V2O3 powders (>95 wt.%) were purchased from Alfa Aesar, England.
2.2 Experimental procedures
According to the stoichiometric proportion, high purity V2O3 (>95 wt.%, with the other component of high-valence vanadium oxide), Mg (>99.8 wt.%) powers and MgO (>98 wt.%) were weighed and mixed uniformly in an agate mortar. Considering the volatilization behavior of Mg, which had a low melting point (921 K), excessive Mg was added. The mixture was pressed into cylindrical briquettes in a stainless steel mold and then placed into an alumina crucible, which was accommodated into the constant-temperature zone of a vertical tube furnace with MoSi2 rods as the heating elements. The schematic diagram of the experimental equipment is shown in Fig. 1. The diameter of the alumina tube was 600 mm.
At the first reduction stage as shown in Eq. (2), a series of experiments were carried out to find the optimal temperature, reaction time and the amount of MgO addition. High purity argon (>99.999%) with a flowing rate of 400 mL/min was introduced into the furnace during the whole reaction process. After reaction, the sample was cooled down to the room temperature before being taken out from the furnace. And then, the nitridation stage was carried out in N2 atmosphere at 1273 K or 1473 K as described by Eq. (3). The temperature of the system was raised to the desired temperature at a heating rate of 5 K/s and held for 4 h, and then cooled to room temperature. During this process, high purity nitrogen (>99.999%) with a flowing rate of 400 mL/min was introduced into the furnace.
V2O3+3Mg+xMgO=(3+x)MgO+2V (2)
2V+N2(g)=2VN (3)
To separate MgO from the as-product, the sample was milled and leached in 1 mol/L HCl at a room temperature under magnetic stirring for 2 h. Then, the sample was filtered and washed several times with deionized water and anhydrous ethanol (> 99.7 wt.%). Then, the wet powders were placed in the dry oven for 2 h to obtain the grey-brown VN powders.
The phase composition of sample was examined by X-ray diffraction (XRD, TTR III; Rigaku Corporation, Tokyo, Japan) using Cu Kα radiation in the range of 2θ=10°-90° with a scanning rate of 10 (°)/min. The morphology was characterized using the combination of field-emission scanning electron microscopy (FE-SEM, JSM-6701F, Japan) and energy dispersive spectroscopy (EDS). The residual oxygen content in the final products was determined by the oxygen-nitrogen-hydrogen analyzer (EMGA-830, HORIBA, Japan).
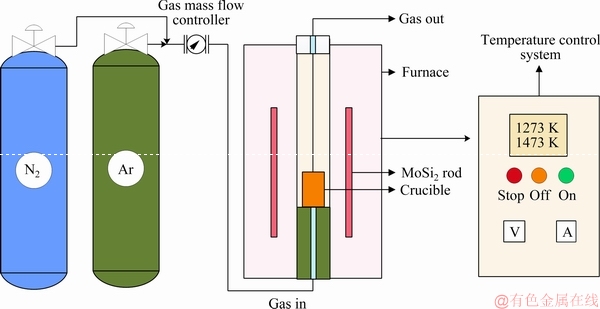
Fig. 1 Schematic diagram of experimental equipment
3 Results
3.1 Phase transition
Figure 2(a) shows the XRD patterns of products prepared after reacting at different temperatures for 2 h in a flowing Ar atmosphere with a MgO/V2O3 molar ratio of 3. It was found that all diffraction peaks were indexed as V and MgO when the temperature was higher than 873 K, indicating that V2O3 was completely reduced to V; whereas, at 823 K, the diffraction peaks of V2O3 and Mg were observed, which demonstrated that the kinetic condition was not fast enough at 823 K to reduce the V2O3 within a short time. Figure 2(b) presents the XRD patterns of samples obtained after reacting at 873 K for different time with a MgO/V2O3 molar ratio of 3. It could be seen that all diffraction peaks were indexed as V and MgO when the reaction time was longer than 45 min. However, the V2O3 and Mg were observed when the reaction time was only 30 min, and no diffraction peak of V was detected, which indicated that the reaction did not take place under this condition. Therefore, the reduction process should be carried out at a temperature higher than 873 K for about 1 h.
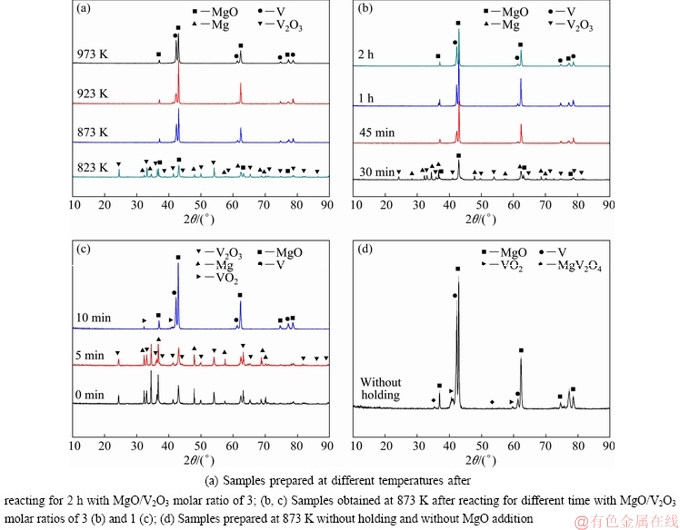
Fig. 2 XRD patterns of products obtained under different reaction conditions
To investigate the effect of MgO addition on the reaction process, the reaction temperature was set to be 873 K while the MgO/V2O3 molar ratio were 0, 1 and 3, respectively. Figure 2(c) shows the XRD patterns of the products prepared in a flowing Ar atmosphere with MgO/V2O3 molar ratio of 1, and it was found that the as-prepared sample was mainly composed of VO2, V and MgO after reacting for 10 min. Compared with the sample prepared with a MgO/V2O3 molar ratio of 3 (Fig. 2(b)), the reduction process has taken place within such a short time. The appearance of VO2 may result from the oxidation of the residual V2O3, which could be described by Eq. (4). Meantime, when there was no addition of MgO to the system, except for VO2, Mg and MgO, a small amount of MgV2O4 phase was also detected (Fig. 2(d)). The appearance of MgV2O4 may be caused by the insufficient content of Mg. The reaction temperature greatly increased without the extra addition of MgO, leading to the volatilization loss of Mg. The residual V2O3 reacted with MgO to form MgV2O4, as shown in Eq. (5). Therefore, it was necessary to add a certain amount of MgO to the system, which could decrease the actual reaction temperature and assist in decreasing the volatilization loss of Mg. The reaction condition was acceptable when the MgO/V2O3 molar ratio was 3.
V2O3+1/2O2=2VO2 (4)
V2O3+MgO=MgV2O4 (5)
To prepare the VN powders, the as-prepared sample after the magnesiothermic reduction was further nitrided in a nitrogen atmosphere at a high temperature. Figure 3(a) shows the XRD patterns of the VN obtained after nitriding for 4 h in a N2 atmosphere at 1273 and 1473 K, respectively. It could be found that all diffraction peaks belonged to VN and MgO phases and no V phase was detected. After the acidic leaching process, a small amount of V2N was detected from the sample obtained at 1273 K (Fig. 3(b)). This may be owing to a low gas-solid reaction rate. However, when the nitridation temperature was increased to 1473 K, the V2N phase disappeared, indicating that a high temperature was beneficial to the nitration process. The pure VN phase could be prepared after nitriding at 1473 K for 4 h in a N2 atmosphere.
3.2 Microstructure
Figure 4 shows the microstructures of the raw materials V2O3 and the prepared VN at 1473 K before and after leaching. From Fig. 4, it could be found that the overall morphology of the as-prepared VN powders retained that of the initial V2O3 powders. Before leaching process, the surface of the particles was smooth as that of the raw materials V2O3 shown in Figs. 4(b, e). However, when MgO was removed by leaching process, the prepared VN particles were more porous, as shown in Figs. 4(c, f). According to previous research [15], the reason for this phenomenon may be that during the magnesiothermic reduction process, the byproduct MgO with a high melting point was formed and distributed closely with the prepared V particles, which prevented the physical contact and sintering among different V particles. At the high temperature nitridation stage, nitrogen combined with vanadium to generate VN. After leaching in HCl solution, MgO particles were removed and the VN particles with a porous structure were formed, as shown in Figs. 4(b, c, e, f).
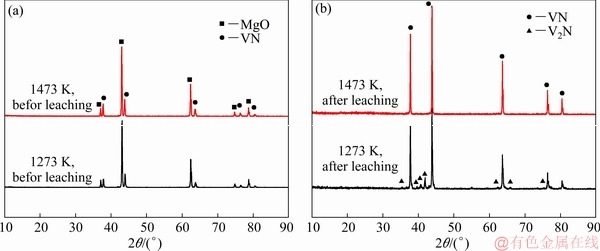
Fig. 3 XRD patterns of products prepared at 1273 and 1473 K before (a) and after (b) leaching
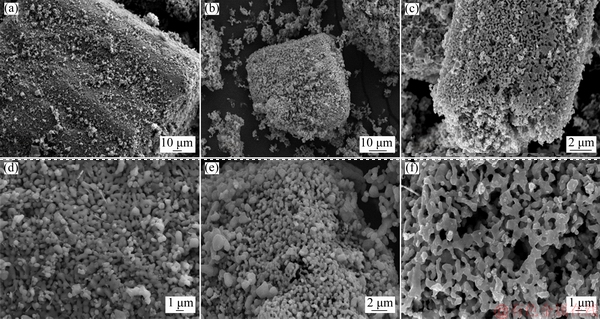
Fig. 4 SEM images of raw material V2O3 (a, d) and VN prepared at 1473 K before (b, e) and after (c, f) leaching
An area scan was conducted to further examine the elemental distributions of the sample obtained at 1473 K. It could be found from Fig. 5 that the nitrogen region was completely overlapped with the vanadium region. Moreover, a part of the oxygen was detected, which may be caused by the passivation of VN particles due to the porous structure and high specific surface [16,17].
4 Discussion
4.1 Adiabatic temperature calculation
The magnesiothermic reduction process is a strong exothermic reaction which could result in the extreme increase of the system temperature, and leads to the volatilization of Mg. In the current study, a certain amount of MgO was added as a diluent to minimize the heat effect. If the reaction conditions are assumed to be adiabatic, the heat released from the reduction reaction will raise the system temperature and the highest temperature that a reaction system can reach is called as the adiabatic temperature (Tad) [18], which could be calculated by Eqs. (6)-(8)
 (6)
(6)
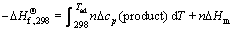 (7)
(7)

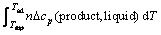 (8)
(8)
where 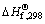 is the change of standard enthalpy of formation of the reduction reaction at 298 K; n is the mole number of each component; Δcp is the change in specific heat capacity; Tmp is the melting point of the product; and ΔHm is the change of molar enthalpy of this transformation process. Equation (6) is applicable for the case where Tad
is the change of standard enthalpy of formation of the reduction reaction at 298 K; n is the mole number of each component; Δcp is the change in specific heat capacity; Tmp is the melting point of the product; and ΔHm is the change of molar enthalpy of this transformation process. Equation (6) is applicable for the case where Tad
According to Eqs. (6)-(8), the adiabatic temperature could be calculated and the change of adiabatic temperature with the MgO addition is shown in Fig. 6. The thermodynamic data for the calculation of adiabatic temperature were obtained by HSC Chemistry 6.0 software. It could be found from Fig. 6 that the adiabatic temperature significantly decreased when the MgO/V2O3 molar ratio was less than 3. Although a much higher addition of the MgO would result in a much lower adiabatic temperature, this could also lead to a high cost and a very slow reaction rate owing to the separation effect of lots of MgO particles. Considering the factors mentioned above, the MgO/V2O3 molar ratio of 3 was chosen in the current study. Even if the adiabatic temperature is still as high as about 1900 K, the heat dissipation and the gradual release of heat within the reaction period will make the actual temperature be lower than the adiabatic temperature.
4.2 Thermodynamic analysis
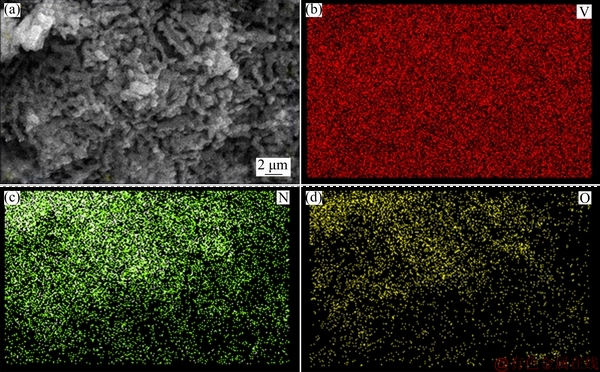
Fig. 5 SEM image (a) and element mapping distributions (b-d) of VN prepared at 1473 K
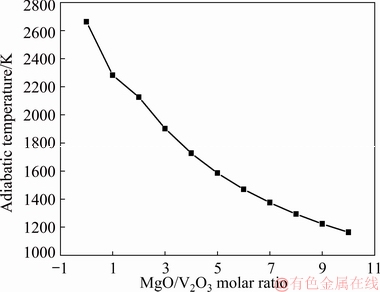
Fig. 6 Effect of MgO addition on adiabatic temperature of Reaction (2)

Fig. 7 Temperature dependence of change of Gibbs free energy for different reactions
Figure 7 shows the changes of the standard Gibbs free energy for different reactions, with the data obtained from HSC Chemistry 6.0 software. It could be found that a relative high temperature was essential for the carbothermal reduction and nitriding process to prepare VN,as described by Eq. (9). Compared with carbon, Mg was a stronger reducing agent. As indicated above, V2O3
could be easily reduced to V by Mg at a low temperature, which was further nitrided in N2 atmosphere to generate VN, as described by Eqs. (2) and (3), respectively. From Fig. 7, it can be seen that the changes of the standard Gibbs free energy are always negative for Eqs. (2) and (3), indicating that these two reactions are feasible from the viewpoint of thermodynamics.
V2O3+3C+N2(g)=2VN+3CO(g) (9)
The Ellingham diagrams of MgO, V2O3, VO, and V-O solutions were calculated and could be found in previous work [20]. As known to all, the change of the standard Gibbs free energy for oxidation reaction of different elements (oxygen potential) as a function of the temperature, and the order of stability of various mental oxides could be intuitively shown in the Ellingham diagram. The lower the line position in the diagram is, the more stable the oxides are. It was found that the line position of MgO was lower than that of V2O3, VO and V-0.6wt.%O solid solution which was formed by the dissolution of oxygen in the lattice of V metal. This indicated that Mg was stronger enough to reduce the oxygen content in V metal to an extremely low value in the temperature range of 873-1273 K. The oxygen content of VN powder prepared in current study was 0.178 wt.%.
4.3 Reaction mechanism
As mentioned above, the addition of MgO was beneficial to slowing the reaction process. The main reason for this was that a part of the released heat was absorbed by MgO. As calculated above, the adiabatic temperature was sharply decreased when MgO was added. The added MgO particles were uniformly distributed between Mg and V2O3, and separated them from each other. The distribution of raw materials with different MgO additions is shown in Fig. 8. It can be seen from Fig. 8(a) that when no MgO was added to the raw materials, V2O3 was surrounded by Mg. With the increase of MgO addition, as shown in Figs. 8(b) and (c), the distance between Mg and V2O3 particles increased accordingly. When the temperature of the system was raised to 873 K, the reaction began to take place in a very small region. If there is no MgO distributed between Mg and V2O3 particles, the reaction region will be easily connected together and the whole reduction reaction will be carried out quickly. When MgO is added, due to the presence of MgO, Mg cannot contact with V2O3 easily, and the reduction reaction will only happen in a small region. As the reaction proceeded, the heat released from the reaction accumulated and the temperature of the system rose continuously. The whole reaction region was finally linked together to make the reaction complete. Therefore, with the increase of the MgO addition, more heat was absorbed and it became more difficult to connect the small reaction regions, which led to a longer reaction time. Furthermore, it should be noted that the MgO addition decreased the temperature of the system, which is beneficial to the decrease of the volatilization of Mg.
According to the experimental results and the above thermodynamic calculation, possible mechanism for the preparation of the porous VN is shown in Fig. 9. In order to simplify the analysis, it was assumed that the shapes of all raw materials were spherical. Firstly, the raw materials Mg, V2O3 and MgO were mixed uniformly. At the magnesiothermic reduction stage, V2O3 was reduced to V and MgO by Mg. Then, at the nitridation stage, with the continuously introducing of N2 to the system, vanadium started to combine with nitrogen to form vanadium nitride. Based on the experimental results, a higher temperature could accelerate gas-solid reaction rate that was beneficial to the nitridation process. The MgO particles, which were distributed around VN particles, prevented the sintering of VN particles. When MgO was removed by acidic leaching, the porous VN particles were obtained.
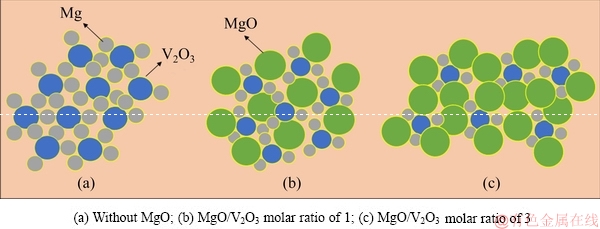
Fig. 8 Distribution of raw materials with different MgO additions
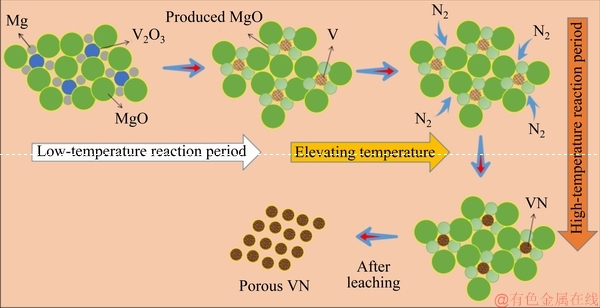
Fig. 9 Systematic diagram about preparation mechanism of VN powder
5 Conclusions
(1) High-purity VN with a porous structure was prepared by a magnesiothermic reduction of V2O3, followed by nitridation reaction in N2 gas and acidic leaching to remove MgO. During the reaction process, MgO was added to decrease the system temperature and the volatilization of Mg.
(2) Compared with the carbothermal reduction, there was no residual carbon in VN product. Furthermore, the strong chemical affinity between Mg and oxygen could reduce the residual oxygen content to be low level. The oxygen content of VN powder prepared at current study was 0.178 wt.%.
(3) The experimental results showed that the V2O3 could be reduced to V after reacting at 873 K for about 1 h with with a MgO/V2O3 molar ratio of 3. The VN phase was formed after nitriding at 1473 K for 4 h in N2 atmosphere. After removing MgO by acidic leaching, the VN with a porous structure was obtained.
References
[1] RAMANATHAN S, OYAMA S T. New catalysts for hydro- processing: Transition metal carbides and nitrides [J]. The Journal of Physical Chemistry, 1995, 99(44): 16365-16372.
[2] DISALVO F J, CLARKE S J. Ternary nitrides: A rapidly growing class of new materials [J]. Current Opinion in Solid State and Materials Science, 1996, 1(2): 241-249.
[3] GIORDANO C, ANTONIETTI M. Synthesis of crystalline metal nitride and metal carbide nanostructures by sol-gel chemistry [J]. Nano Today, 2011, 6(4): 366-380.
[4] YI Chen-qi, ZOU Jian-peng, YANG Hong-zhi, LENG Xian. Recent advances in pseudocapacitor electrode materials: Transition metal oxides and nitrides [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(10): 1980-2001.
[5] CHEN Lu-yang, GU Yun-le, SHI Liang, YANG Ze-heng, MA Jian-hua, QIAN Yi-tai. A room-temperature synthesis of nanocrystalline vanadium nitride [J]. Solid State Communications, 2004, 132(5): 343-346.
[6] WU Yue-dong, ZHANG Guo-hua, CHOU Kuo-chih. A novel process to synthesize high-quality ferrovanadium nitride [J]. Metallurgical and Materials Transactions B, 2016, 47(6): 3405-3412.
[7] PARKIN I P. Solid state metathesis reaction for metal borides, silicides, pnictides and chalcogenides: Ionic or elemental pathways [J]. Chemical Society Reviews, 1996, 25(3): 199-207.
[8] GAJBHIYE N S, NINGTHOUJAM R S. Low temperature synthesis, crystal structure and thermal stability studies of nanocrystalline VN particles [J]. Materials Research Bulletin, 2006, 41(9): 1612-1621.
[9] ROLDAN M A, LOPEZ-FLORES V, ALCALA M D, ORTEGA A, REAL C. Mechanochemical synthesis of vanadium nitride [J]. Journal of the European Ceramic Society, 2010, 30(10): 2099-2107.
[10] JEFFES J H E. Ellingham diagrams [J]. Encyclopedia of Materials: Science and Technology, 2001(2): 2751-2753.
[11] BURKER D O, MERRILL T W. Process for making vanadium carbide briquettes: US patent, 3342553 [P]. 1967-09-19.
[12] WU Yue-dong, ZHANG Guo-hua, CHOU Kuo-chih. Preparation of high quality ferrovanadium nitride by carbothermal reduction nitridation process [J]. Journal of Mining and Metallurgy B: Metallurgy, 2017, 53(3): 383-390.
[13] ZHANG Y, FANG Z Z, YANG X, HUANG Z, LEFLER H, ZHANG T Y, SUN P, FREE M L, GUO J. A novel chemical pathway for energy efficient production of Ti metal from upgraded titanium slag [J]. Chemical Engineering Journal, 2016, 286: 517-527.
[14] FANG Z Z, MIDDLEMAS S, GUO J, FAN P. A new, energy-efficient chemical pathway for extracting Ti metal from Ti minerals [J]. Journal of the American Chemical Society, 2013, 135(49): 18248-18251.
[15] ZHANG Y, FANG Z Z, YANG X, SUN P, DEVEBR B V, FREE M, LEFLER H, ZHENG S L. Hydrogen assisted magnesiothermic reduction of TiO2 [J]. Chemical Engineering Journal, 2017, 308: 299-310.
[16] YANG Ze-heng, CAI Pei-jun, CHEN Lu-yang, GU Yun-le, SHI Liang, ZHAO Ai-wu, QIAN Yi-tai. A facile route to VN and V2O3 nanocrystals from single precursor NH4VO3 [J]. Journal of Alloys and Compounds, 2006, 420(1-2): 229-232.
[17] LIAO M Y, GOTOH Y, TSUJI H, ISHIKAWA J. Crystallographic structure and composition of vanadium nitride films deposited by direct sputtering of a compound target [J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 2004, 22(1): 146-150.
[18] SHARIFITABAR M, KHAKI J V, SABZEVAR M H. Effects of Fe additions on self-propagating high temperature synthesis characteristics of TiO2-Al-C system [J]. International Journal of Refractory Metals and Hard Materials, 2014, 47: 93-101.
[19] YI H C, MOORE J J. Self-propagating high-temperature (combustion) synthesis (SHS) of powder-compacted materials [J]. Journal of Materials Science, 1990, 25(2): 1159-1168.
[20] WU Yue-dong, ZHANG Guo-hua, CHOU Kuo-chih. Preparation of vanadium nitride by magnesiothermic reduction of V2O3 in nitrogen atmosphere [J]. Metallurgical and Materials Transactions B, 2018, 49(6): 3570-3579.
徐 瑞,吴跃东,张国华
北京科技大学 钢铁冶金新技术国家重点实验室,北京 100083
摘 要:以三氧化钒(V2O3)为原料,通过两步法制备高纯度氮化钒(VN)粉末。首先,在873 K、Ar气氛中通过镁热还原反应将V2O3还原,得到V和MgO的混合物;然后,在1473 K、N2气氛中对样品进行氮化;最后,通过酸浸获得高纯度的VN粉末。采用X射线衍射仪和场发射扫描电子显微镜对样品的相变和形貌演变进行分析。实验结果表明,所制备的VN粉末的总体形貌保持初始V2O3粉末的形貌。通过酸浸除去MgO后,可以得到多孔VN颗粒,其氧含量为0.178%(质量分数)。与传统方法相比,这种方法可获得含有少量氧且不含碳的高纯度VN粉末。
关键词:镁热还原反应;氮化反应;氮化钒粉体;制备
(Edited by Wei-ping CHEN)
Foundation item: Project (51725401) supported by the National Natural Science Foundation of China
Corresponding author: Guo-hua ZHANG; Tel: +86-10-82377750; E-mail: ghzhang0914@ustb.edu.cn
DOI: 10.1016/S1003-6326(19)65085-5

