Trans. Nonferrous Met. Soc. China 23(2013) 1184-1190
Hydrothermal synthesis of titanium-supported nanoporous palladium-copper electrocatalysts for formic acid oxidation and oxygen reduction reaction
Qing-feng YI 1, Xing-zhong XIAO1, Yun-qing LIU2
1. School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 27 February 2012; accepted 27 June 2012
Abstract: Nanoporous Pd and binary Pd-Cu particles were prepared by a hydrothermal method using ethylene glycol as a reduction agent and they were directly immobilized on Ti substrates named as Ti-supported Pd-based catalysts. Their electrocatalytic activity for formic acid oxidation and oxygen reduction reaction (ORR) in alkaline media was examined by voltammetric techniques. Among the as-prepared catalysts, nanoPd81Cu19/Ti catalyst presents the highest current density of 39.8 mA/cm2 at -0.5 V or 66.4 mA/cm2 at -0.3 V for formic acid oxidation. The onset potential of ORR on the nanoPd81Cu19/Ti catalyst presents an about 70 mV positive shift compared to that on the nanoPd/Ti, and the current density of ORR at -0.3 V is 2.12 mA/cm2, which is 3.7 times larger than that on the nanoPd/Ti.
Key words: Pd electrode; Pd-Cu electrode; formic acid oxidation; oxygen reduction reaction; nanoparticle; electrocatalysis
1 Introduction
Electrocatalytic oxidation of formic acid has been extensively investigated because of the important application of formic acid to fuel cells. Pd and some Pd alloys have been proved to display efficient electroactivity for formic acid oxidation [1,2]. Various Pd-containing catalysts have been developed to enhance the electroactivity in comparsion with pure and bulk Pd catalysts for formic acid oxidation. Pd nanoparticles as effective electrocatalysts for formic acid oxidation have been thoroughly studied [3-11]. In addition, Pd-based alloy catalysts, such as binary PdCo [1,12] and Pd-Au [13] catalysts, have been also paid much attention, considering their higher electroactivity for formic acid oxidation as compared with pure Pd catalysts. PARK et al [14] have prepared Pd clusters on highly dispersed Au nanoparticles and found that these Pd clusters exhibit enhanced electrocatalytic activity for formic acid oxidation relative to Pd/C. Pd/Au catalysts with hollow cone-like microstructures also exhibit significantly higher electrocatalytic activity and stability for formic acid oxidation than Pd/Au solid microhemispheres [15]. Recently, DAI and ZOU [16] have prepared Cu-Pd nanoparticles through a galvanic replacement process, and these particles exhibit much higher formic acid oxidation activities than pure Pd nanoparticles, and they are much more resistant to the surface poisoning.
Binary Pd-Cu catalysts also exhibit attractive electroactivity for oxygen reduction reaction (ORR). A binary Pd-Cu electrocatalyst, synthesized through reduction of PdCl2 and CuCl with NaBH4 in a THF solution, exhibits a 30 mV positive shift of the onset potential for ORR in a 0.5 mol/L H2SO4 electrolyte relative to a pure Pd catalyst [17]. FOUDA-ONANA et al [18] prepared Pd-Cu alloy catalysts using the dual sputtering of a palladium and a copper wafer as targets. Among these Pd-Cu alloys, the Pd50Cu50 exhibits the highest activity for ORR in a 0.1 mol/L HClO4 solution. Recently, WANG et al [19] have prepared carbon- supported pseudo-core@shell PdCu@Pt nanoparticles with low cost and high activity for ORR.
In this work, titanium-supported Pd and binary Pd-Cu particles with a nanoporous structure were prepared by a hydrothermal procedure (denoted as nanoPd/Ti and nanoPdCu/Ti, respectively). Scanning electron microscopy was used to characterize their morphological structures. The electrochemical activity of the prepared catalysts towards formic acid oxidation and ORR in alkaline solution was evaluated by voltammetric and chronoamperometric techniques.
2 Experimental
All chemicals used in this work were reagent grade products and used without further purifications. Pure water (18 MW·cm) was obtained from doubly distilled water that has been subjected to the treatment of ion exchange resins. Titanium pieces with a geometric area of 0.35 cm2 were of 99.2% purity.
The nanoPd/Ti and nanoPdCu/Ti electrodes with different compositions were fabricated using a hydrothermal method according to our previous work [2] and related report [20]. 1) The pieces of Ti sheets with the thickness of 1 mm were washed with pure water (18.2 MW·cm), then etched in an 18% HCl solution at 85 °C for 10 min in order to remove the oxides on the Ti surface. After that, the sheets were washed with pure water again and subsequently subjected to ultrasonic treatment for 5 min to obtain the pretreated Ti sheets. 2) 1 mol/L NaOH solution was added dropwise into the solution composed of 5 mL of 5 mmol/L PdCl2, x mL of 5 mmol/L CuCl2 (x=0 for nanoPd/Ti and x=1.25, 1.7, 2.5, and 5 for nanoPd81Cu19/Ti, nanoPd75Cu25/Ti, nanoPd69Cu31/Ti and nanoPd48Cu52/Ti, respectively) and y mg L-glutamic acid (y=18 for nanoPd/Ti and 36 for the binary Pd-Cu catalysts), until pH value of the mixed solution reached to 11. 3) The pretreated Ti sheets were transferred in a Teflon container lined autoclave and the mixed solution was then carefully added to the container, and subsequently 1.5 mL EG was added to the container. After that, the container was heated at 160 °C for 10 h. After the reaction was finished, the Ti plates were removed and dried at 100 °C for 30 min. The prepared samples were examined by SEM and EDS on a JSM-6380LV scanning electron microscopy.
An AutoLab PGSTAT30/FRA electrochemical instrument (ECO CHEMIE in the Netherlands) and a conventional three- electrode test cell were used for electrochemical measurements. The prepared nanoPd/Ti and binary nanoPdCu/Ti catalysts were used as the working electrode. A large Pt foil and a Ag/AgCl electrode in saturated KCl solution (Ag/AgCl) were used as the counter and reference electrodes, respectively. All potentials reported in this work were quoted against Ag/AgCl. Prior to electrochemical measurements, the prepared electrodes were subjected to successive cycling potential scans in the potential range from -1.0 to 0.5 V (vs Ag/AgCl) in 1 mol/L NaOH solution until a stable CV profile was obtained. Cyclic voltammetric responses of the prepared samples and electrochemical measurements of formic acid oxidation were carried out in a 1 mol/L NaOH solution saturated with N2. For the electroactivity measurements of the prepared catalysts towards ORR, 1 mol/L NaOH solution was firstly bubbled by pure O2 gas for 15 min before measurements, and then O2 gas was continuously bubbled through the solution during the measurements. All experiments were carried out at room temperature ((25±2)) °C.
3 Results and discussion
3.1 Morphology of samples
Morphological structures of the prepared samples were examined by SEM. They present a similar morphological characteristic with a nanoporous network structure. Figure 1 shows the SEM images of nanoPd/Ti and nanoPd81Cu19/Ti as a typical sample of the prepared binary Pd-Cu catalysts. It is observed from Fig. 1 that the catalyst particles exhibit a good coverage on the surface of Ti substrate. The particle sizes are 130-180 nm for the nanoPd/Ti and 100-150 nm for the binary Pd81Cu19 catalyst particles. These nano-scale sized particles are connected with each other to form a three-dimensional texture. Further analysis of the samples was done by energy dispersive X-ray spectrometry (EDS), and the results are shown in Fig. 2. The peaks at 2.85 keV are ascribed to characteristic peaks of Pd, while the peaks at 0.9, 8.05 and 8.9 keV are attributed to the presence of Cu. The weak peaks at 2.6 and 1.05 keV are assigned to the presence of a small amount of NaCl.
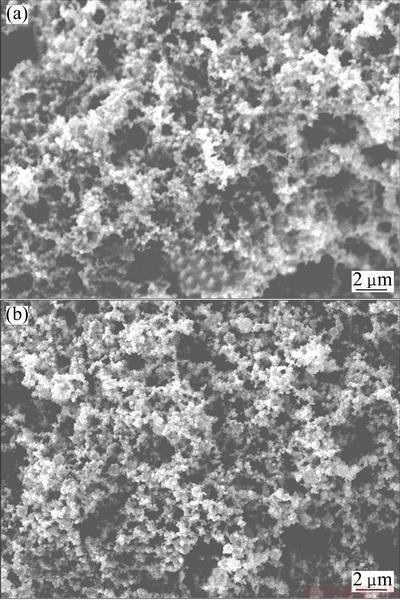
Fig. 1 SEM images of nanoPd/Ti (a) and nanoPd81Cu19/Ti (b)
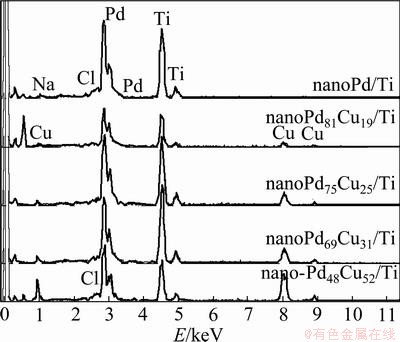
Fig. 2 EDS patterns of samples
3.2 Electroactivity of samples for formic acid oxidation
Figure 3 shows the CV profiles of nanoPd/Ti and nanoPd81Cu19/Ti as a typical sample of the binary Pd-Cu catalysts. The nanoPd/Ti catalyst presents a typical voltammetric profile of Pd electrode in alkaline solution (Fig. 3(a)), where the small anodic peaks at -0.72 and -0.54 V, and cathodic peak at -0.85 V are ascribed to the H-adsorption/desorption processes. The large cathodic peak (r) at -0.41 V is caused by the reduction of Pd oxides formed during the forward-going potential sweep. For the nanoPd81Cu19/Ti catalyst, it presents similar CV profile (Fig. 3(b)) to that of nanoPd/Ti catalyst except that on the binary Pd-Cu catalysts, only an anodic peak for the H-desorption is observed and a new anodic peak from -0.14 to -0.12 V develops, which is related to the addition of Cu to Pd particles. It is also found from Fig. 3 that the potential of the large cathodic reduction peak (r) on the binary Pd-Cu catalyst shifts to more positive direction compared to that on the nanoPd/Ti electrode. In addition, the reduction peak r on the nanoPd81Cu19/Ti catalyst displays larger current density than that on the nanoPd/Ti catalyst, showing that more oxides are formed during the forward-going scan due to the addition of Cu into Pd particles.
Electrochemical activity of the prepared catalysts towards formic acid oxidation in 1 mol/L NaOH solution was investigated by cyclic voltammetry. It is found from Fig. 4 that both nanoPd/Ti and binary Pd-Cu catalysts display high electroactivity for formic acid oxidation. For the CV response of the nanoPd/Ti electrode shown in Fig. 4(a), the anodic current density on the positive- going scan presents a rapid increase until the anodic potential is larger than 0.36 V. That is to say, the anodic peak A1 arises at a more positive potential, showing that the significantly large surface area of the nanoPd/Ti electrode favors the adsorption of formic acid and restrains the formation of Pd oxides on the nanoPd/Ti surface. The anodic peak A2 on the return-going scan presents a smaller current density than the peak A1, further indicating that only a small amount of Pd oxides form during the forward-going scan. However, the binary Pd-Cu catalysts present a little bit different cyclic voltammetric responses for formic acid oxidation, as shown in Fig. 4(b)-(e), where a wider anodic peak (A1) on the forward-going scan and a well-defined anodic peak (A2) on the negative-going scan arise. The decrease of the formic acid oxidation current after the anodic peak A1 is ascribed to the deactivation of the catalyst surfaces caused by the formation of Pd oxides at high anodic potentials. During the negative-going scan, the formed Pd oxides start to be reduced in the potential range from -0.2 to -0.24 V, resulting in the restoration of the catalyst electroactive surfaces. This enables the electrooxidation of formic acid to proceed with the potential scan to negative direction.
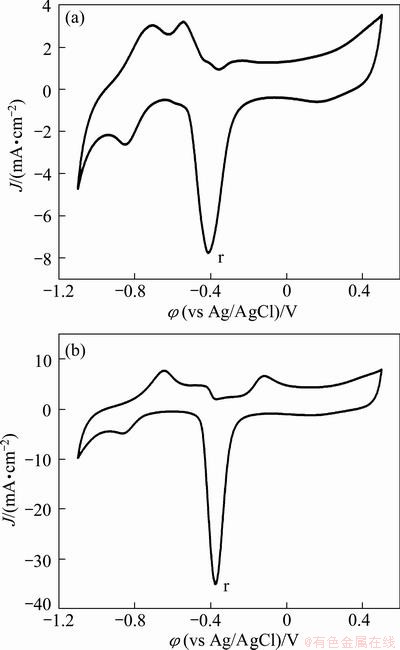
Fig. 3 Cyclic voltammograms of nanoPd/Ti (a) and nanoPd81Cu19/Ti (b) in 1 mol/L NaOH solution at scan rate of 50 mV/s
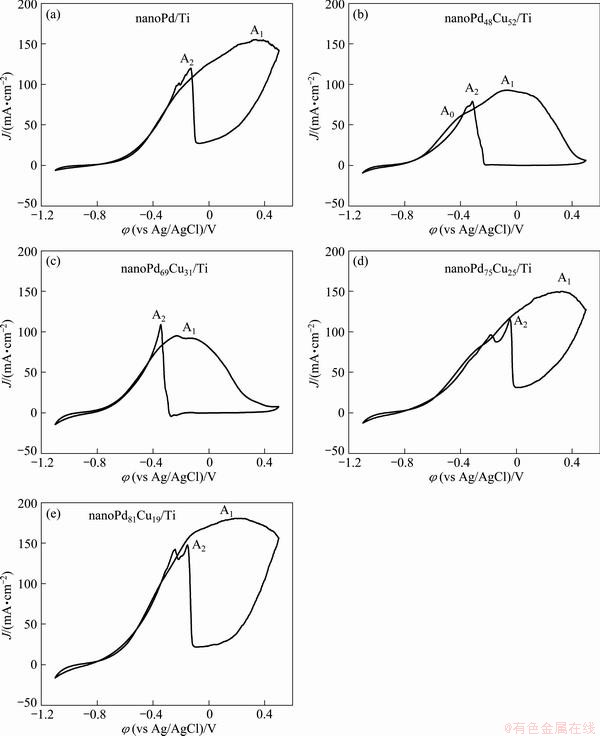
Fig. 4 Cyclic voltammograms of prepared samples in 1 mol/L NaOH+0.5 mol/L HCOOH solution at scan rate of 50 mV/s
It is generally accepted that formic acid oxidation on the Pd electrode follows a direct oxidation mechanism [1,2,5]. That is to say, on the prepared Pd-based catalysts, formic acid is likely to be directly oxidized to CO2 without the formation of poisoning CO as the reactive intermediate,
Pd+HCOOH+OH-→Pd-HCOOad+H2O+e (1)
Pd-HCOOad+OH-→Pd+CO2+H2O+e (2)
It is found from reactions (1)-(2) that alkaline media facilitate both the adsorption of formic acid and the oxidation of adsorbed formate. Compared to the oxidation of formic acid in acidic solutions [2], the oxidation of formic acid in alkaline media commences at a much negative potential. A small anodic peak A0 at more negative potential develops on the nanoPd48Cu52/Ti catalyst while this peak (A0) on other Pd-Cu/Ti catalysts is not prominent. In the range of low anodic potentials corresponding to the initial oxidation stage of formic acid, the prepared binary Pd-Cu catalysts present larger formic acid oxidation current than the nanoPd/Ti electrode. For example, during the forward-going scan, the anodic current density at -0.5 V on the nanoPd81Cu19/Ti catalyst is 48 mA/cm2 while it is 22 mA/cm2 on the nanoPd/Ti.
Chronoamperometry is a powerful method to investigate the electroactivity of catalysts. The chronoamperometric responses recorded at -0.5 and -0.3 V on various prepared catalysts are presented in Fig. 5. It is found from Fig. 5(a) that the current density related to the electrooxidation of formic acid is clearly increased by the addition of Cu to Pd nanoparticles in comparison with the current density obtained on nanoPd/Ti catalyst. Figure 5(a) also shows that on the nanoPd81Cu19/Ti catalyst, the oxidation current of formic acid presents a rapid decline at the initial stage of electrolysis and subsequently displays a slow increment with the electrolysis time. This would be attributed to the presence of Cu in Pd nanoparticles. Namely, at lower potentials, a weaker binding of formate with nanoPd81Cu19/Ti surface would be propitious to the oxidation of the adsorbed formate, as shown in reaction (2). According to the CV response of nanoPd81Cu19/Ti catalyst in NaOH solution in the absence of HCOOH (Fig. 3(b)), the anodic peak at about -0.13 V shows the oxidation of Cu and subsequent formation of Cu oxides, resulting in the enhancement on further oxidation of the adsorbed formate. Figure 5(a) also shows that even at low potential of -0.5 V, nanoPd81Cu19/Ti catalyst exhibits large formic acid oxidation current density. The stable current density of formic acid oxidation on nanoPd81Cu19/Ti at 600 s is 39.8 mA/cm2, which is about 1.4, 1.3, 1.5 and 1.4 times larger than that on nanoPd/Ti, nanoPd48Cu52/Ti, nanoPd69Cu31/Ti and nanoPd75Cu25/Ti catalysts, respectively. At a higher potential step of -0.3 V (shown in Fig. 5(b)), the binary nanoPd81Cu19/Ti catalyst still displays the best catalytic activity for formic acid oxidation among the prepared catalysts. At the potential step of -0.3 V, the stable current density of formic acid oxidation on nanoPd81Cu19/Ti at 600 s is 66.1 mA/cm2, which is about 1.4, 1.6, 1.9 and 1.4 times larger than that on nanoPd/Ti, nanoPd48Cu52/Ti, nanoPd69Cu31/ Ti and nanoPd75Cu25/Ti catalysts, respectively. It is further observed from Fig. 5 that the Pd-based catalysts of the present investigation present relatively stable currents for formic acid catalytic-oxidation without obvious current decay, showing the stable electrocatalytic activity of the prepared catalysts for formic acid oxidation in alkaline media.
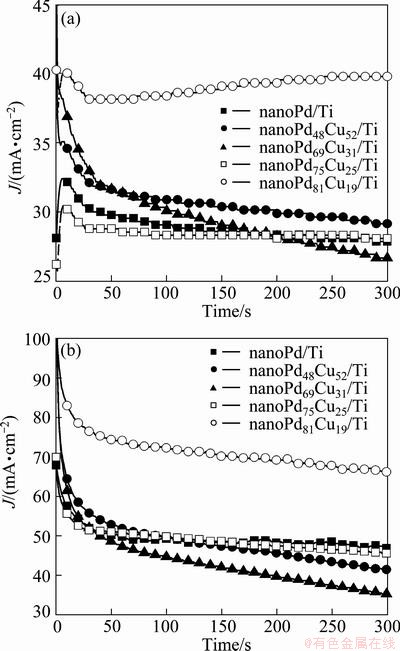
Fig. 5 Chronoamperometric responses of prepared catalysts at potential steps of -0.5 V (a) and -0.3 V (b) in 1 mol/L NaOH+0.5 mol/L HCOOH solution
The electrocatalytic stability of the Pd-based catalysts towards the formic acid oxidation was examined according to the repeatedly sweeping cyclic voltammograms in 1 mol/L NaOH+0.5 mol/L HCOOH. For example, after 50 cycles, the drop was about 1.2% of the anodic peak current density in the positive-going scan on nanoPd81Cu19/Ti. This proves that these Pd-based electrodes are stable catalysts for formic acid oxidation in alkaline media.
3.3 Electroactivity of samples for ORR
Besides formic acid oxidation, Pd-based catalysts are also found to possess outstanding performances in oxygen reduction reaction (ORR). Taking the advantage of relatively low cost, Pd is considered to be an appropriate alternative for Pt as a cathodic catalyst in fuel cells. Recent literatures reported that binary Pd-Cu catalysts usually show effective electroactivity for ORR in acidic electrolyte [17-19]. However, very few studies on their electrocatalytic activity for ORR in alkaline media have been carried out. Figure 6 shows the LSV curves of the prepared catalysts in order to examine their electroactivity for ORR in 1 mol/L NaOH solution. From Fig. 6, a small shoulder peak at about -0.17 V and a well-defined peak at about -0.30 V can be observed. The development of the peak at about -0.30 V is attributed to the rapid consumption of the dissolved O2 near the catalyst surface and, therefore, this peak is diffusion-controlled. It is found from Fig. 6 that the binary Pd-Cu catalysts show larger current of oxygen reduction and more positive onset potential than the nanoPd/Ti catalyst, indicating that the prepared binary Pd-Cu catalysts have higher ORR activity than the nanoPd/Ti catalyst in alkaline media. The results show that the addition of Cu to Pd particles enhances the oxygen reduction reaction on Pd. The onset potentials for ORR on nanoPd48Cu52/Ti, nanoPd69Cu31/Ti, nanoPd75Cu25/ Ti and nanoPd81Cu19/Ti catalysts are about -0.036, -0.017, -0.032 and 0.01 V, respectively, which are more positive than that of the nanoPd/Ti catalyst. The current densities of the peak at -0.30 V on nanoPd48Cu52/Ti, nanoPd69Cu31/Ti, nanoPd75Cu25/Ti and nanoPd81Cu19/Ti catalysts are about 1.66, 1.98, 1.22, and 2.12 mA/cm2, respectively, and they are 2.9, 3.5, 2.1, and 3.7 times larger than that on the nnaoPd/Ti catalyst, respectively. This implies that the prepared binary Pd-Cu catalyst can be expected as a promising ORR catalyst in alkaline media.
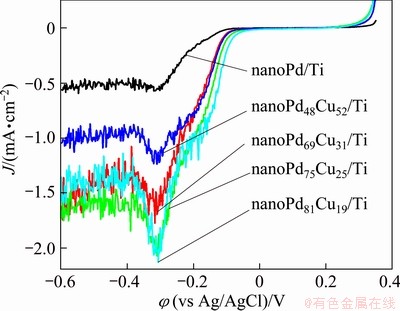
Fig. 6 Linear scan voltammetric responses of prepared catalysts at scan rate of 1 mV/s in 1 mol/L NaOH solution continuously purged with oxygen gas during measurements
Tafel plots of the prepared catalysts are also shown in Fig. 7, according to Fig. 6. The catalytic mechanism for ORR on the binary Pd-Cu catalysts is likely to be similar to that on the pure Pd catalyst. The Tafel slopes of nanoPd/Ti, nanoPd48Cu52/Ti, nanoPd69Cu31/Ti, nanoPd75Cu25/Ti and nanoPd81Cu19/Ti catalysts are 111, 129, 132, 126 and 114 mV/decade, respectively. According to Fig. 7, the exchange current density can be obtained by extrapolating the linear region to the equilibrium potential of ORR. The exchange current densities (J0) obtained from this analysis for the nanoPd48Cu52/Ti, nanoPd69Cu31/Ti, nanoPd75Cu25/Ti and nanoPd81Cu19/Ti catalysts are 8.3×10-5, 2.3×10-4, 9.8×10-5 and 1.9×10-4 mA/cm2, respectively. The results show that the J0 values for the binary Pd-Cu catalysts exhibit about an order of magnitude higher than the value of 1.2×10-5 mA/cm2 for the nanoPd/Ti electrode. The former values for the binary Pd-Cu catalysts are very close to the value of 1.09×10-7 A/cm2 reported for a commercial Pt/C catalyst [21]. These results show that the binary Pd-Cu catalysts prepared in the present work are better electrocatalysts to promote ORR.
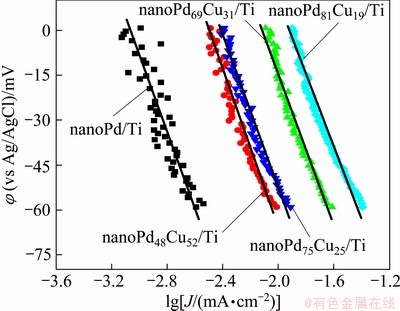
Fig. 7 Tafel plots for ORR on prepared catalysts under same experimental conditions as those in Fig. 6
4 Conclusions
1) Titanium supported nanoporous Pd (nanoPd/Ti) and binary Pd-Cu (nanoPdCu/Ti) catalysts were successfully synthesized by a hydrothermal method using ethylene glycol as a reduction agent, and their electrocatalytic activity for formic acid oxidation and ORR was evaluated in alkaline media.
2) Cyclic voltammetric and chronoamperometric data show that the Pd81Cu19/Ti catalyst exhibits large current density for formic acid oxidation and has better electrocatalytic activity than nanoPd/Ti and other binary nanoPdCu/Ti catalysts.
3) The binary Pd-Cu catalysts show significantly higher electroactivity for ORR than the nanoPd/Ti catalyst. The current density of ORR on the nanoPd81Cu19/Ti catalyst is 3.7 times larger than that on the nanoPd/Ti catalyst and the exchange current density of ORR on nanoPd81Cu19/Ti catalyst is very close to that on a commercial Pt/C catalyst.
References
[1] ZHU Y, KANG Y, ZOU Z, ZHOU Q, ZHEG J, XIA B, YAG H. Facile preparation of carbon-supported Pd nanoparticles for electrocatalytic oxidation of formic acid [J]. Fuel Cells Bulletin, 2008, 2008 (7): 12-15.
[2] YI Q F, HUAG W, LIU X P, XU G R, ZHOU Z H, CHEN A. Electroactivity of titanium-supported nanoporous Pd–Pt catalysts towards formic acid oxidation [J]. J Electroanal Chem, 2008, 619-620: 197-205.
[3] NIUF J,YIQ F. Novel titanium-supported nanoporous Pd electrodes and their electrocatalytic activity of formic acid oxidation [J]. Modern Chemical Industry, 2010, 30(12): 50-53.
[4] SHEN J Z, TANG Y W, LU T H. Catalytic ability for formic acid decomposition and electrocatalytic performance for formic acid oxidation of carbon supported Pd catalyst prepared with NH4F complexing reduction method [J]. Chinese Journal of Inorganic Chemistry, 2012, 28(2): 326-330. (in Chinese)
[5] HUANG Y, ZHOU X, LIAO J, LIU C, LU T, XING W. Preparation of Pd/C catalyst for formic acid oxidation using a novel colloid method [J]. Electrochem Commun, 2008, 10(4): 621-624.
[6] SHEN J Z, CHEN Y, YANG G X, TANG Y W, LU T H. Stability of carbon supported Pd/C catalyst in direct formic acid fuel cell [J]. Chemical Journal of Chinese Universities, 2011, 32(11): 2626-2629. (in Chinese)
[7] YANG S D, LIANG Y Y, WEN Z L, SONG Q J, ZHANG X G. Comparison of catalytic performance on different materials supported Pd catalysts for formic acid oxidation [J]. Electrochemistry, 2011, 17(2): 175-179.
[8] BAI Z, GUO Y, YANG L, LI L, LI W, XU P, HU C, WANG K. Highly dispersed Pd nanoparticles supported on 1,10-phenanthroline-functionalized multi-walled carbon nanotubes for electrooxidation of formic acid [J]. J Power Sources, 2011, 196(15): 6232-6237.
[9] WANG J, YIN G, CHEN Y, LI R, SUN X. Pd nanoparticles deposited on vertically aligned carbon nanotubes grown on carbon paper for formic acid oxidation [J]. Inter J Hydrogen Energy, 2009, 34(19): 8270-8275.
[10] HU C, BAI Z, YANG L, LV J, WANG K, GUO Y, CAO Y, ZHOU J. Preparation of high performance Pd catalysts supported on untreated multi-walled carbon nanotubes for formic acid oxidation [J]. Electrochim Acta, 2010, 55(20): 6036-6041.
[11] KIM B K, SEO D, LEE J Y, SONG H, KWAK J. Electrochemical deposition of Pd nanoparticles on indium-tin oxide electrodes and their catalytic properties for formic acid oxidation [J]. Electrochem Commun, 2010, 12(10): 1442-1445.
[12] MORALES-ACOSTA D, LEDESMA-GARCIA J, GODINEZZ L A, RODRIGUEZ H G,  L, ARRIAGA L G. Development of Pd and Pd-Co catalysts supported on multi-walled carbon nanotubes for formic acid oxidation [J]. J Power Sources, 2010, 195(2): 461-465.
L, ARRIAGA L G. Development of Pd and Pd-Co catalysts supported on multi-walled carbon nanotubes for formic acid oxidation [J]. J Power Sources, 2010, 195(2): 461-465.
[13] NIUF J,YIQ F,LIUY Q. Electrocatalytic activity of Au modified nanoporous palladiumelectrodeforformic acid oxidation [J]. The Chinese Journal ofNonferrousMetals, 2011, 21(8): 1974-1979. (in Chinese)
[14] PARK I S, LEE K S, YOO S J, CHO Y H, SUNG Y E. Electrocatalytic properties of Pd clusters on Au nanoparticles in formic acid electro-oxidation [J]. Electrochim Acta, 2010, 55(14): 4339-4345.
[15] LI L, YIFENG E, YUAN J, LUO X, YANG Y, FAN L. Electrosynthesis of Pd/Au hollow cone-like microstructures for electrocatalytic formic acid oxidation [J]. Electrochim Acta, 2011, 56(17): 6237-6244.
[16] DAI L, ZOU S. Nanoporous PdCu alloy for formic acid electro-oxidation [J]. J Power Sources, 2011, 196: 9369-9372.
[17] MARTINEZ-CASILLAS D C, VAZQUEZ-HUERTA G, PEREZ- ROBLES J F, SOLORZA-FERIA O. Electrocatalytic reduction of dioxygen on PdCu for polymer electrolyte membrane fuel cells [J]. J Power Sources, 2011, 196(10): 4468-4474.
[18] FOUDA-ONANA F, BAH S, SAVADOGO O. Palladium–copper alloys as catalysts for the oxygen reduction reaction in an acidic media I: Correlation between the ORR kinetic parameters and intrinsic physical properties of the alloys [J]. J Electroanal Chem, 2009, 636(1-2): 1-9.
[19] WANG H, WANG R, LI H, WANG Q, KANG J, LEI Z. Facile synthesis of carbon-supported pseudo-core@shell PdCu@Pt nanoparticles for direct methanol fuel cells [J]. Inter J Hydrogen Energy, 2011, 36(1): 839-848.
[20] YANG L, HU C, WANG J, YANG Z, GUO Y, BAI Z, WANG K. Facile synthesis of hollow palladium/copper alloyed nanocubes for formic acid oxidation [J]. Chem Commun, 2011, 47: 8581-8583.
[21] BRITTO P J, SANTHANAM K S V, RUBIO A, ALONSO J A, AJAVAN P M. Improved charge transfer at carbon nanotube electrodes [J]. Adv Mater, 1999, 11: 154-157.
钛基纳米多孔钯-铜催化剂的水热法合成及对甲酸氧化和氧还原的电活性
易清风1,肖兴中1,刘云清2
1. 湖南科技大学 化学化工学院,湘潭 411201;
2. 中南大学 化学化工学院,长沙 410083
摘 要:采用水热法,以乙二醇为还原剂,直接在钛基体上沉积纳米多孔Pd和Pd-Cu颗粒, 制备钛基Pd及Pd-Cu电极材料,研究它们在碱性溶液中对甲酸氧化和氧还原反应(ORR)的电催化活性。结果表明,nanoPd81Cu19/Ti对甲酸氧化具有最大的稳定电流密度,说明Cu的加入极大地改善Pd对甲酸氧化的电活性。所制备的二元Pd-Cu催化剂对ORR的电催化活性均明显高于nanoPd/Ti。在nanoPd81Cu19/Ti电极上,ORR的起始电位相对于nanoPd/Ti电极正移了70 mV,在-0.3 V时ORR的电流密度为2.12 mA/cm2,是在nanoPd/Ti电极上的3.7倍。
关键词:钯电极;钯-铜电极;甲酸氧化;氧还原反应;纳米颗粒;电催化
(Edited by Sai-qian YUAN)
Foundation item: Project (10JJ9003) supported by Hunan Provincial Natural Science Foundation and Xiangtan Natural Science United Foundation, China; Project (11K023) supported by Scientific Research Fund of Hunan Provincial Education Department, China
Corresponding author: Qing-feng YI; Tel: +86-731-58290045; Fax: +86-731-58290509; E-mail: yqfyy2001@hnust.edu.cn
DOI: 10.1016/S1003-6326(13)62582-0