DOI:10.19476/j.ysxb.1004.0609.2017.09.23
从低冰镍中高效浸提Ni、Cu、Co
陈光炬,王会刚,张 梅,郭 敏
(北京科技大学 冶金与生态工程学院,北京 100083)
摘 要:以低冰镍为研究对象,采用FeCl3-HCl溶液体系高效浸提目标金属Ni、Cu、Co,系统地研究FeCl3溶液的浓度、盐酸溶液的浓度、浸出温度和时间对Ni、Cu、Co浸出率的影响,并对Ni的浸出动力学进行探讨。结果表明:在最优浸出条件下,即FeCl3溶液的浓度为1.0 mol/L、盐酸溶液的浓度为0.5 mol/L、浸出温度90 ℃、浸出时间7 h时,Ni、Cu、Co浸出率分别达到98.4%、98.9%和97.3%。当温度为60~90 ℃时,Ni的浸出反应符合未反应核收缩模型,代入动力学方程分析后发现,Ni浸出反应过程是界面化学反应控速,表观活化能为38.4 kJ/mol。
关键词:低冰镍;FeCl3-HCl溶液体系;浸出;Ni;Cu;Co;动力学
文章编号:1004-0609(2017)-09-1936-07 中图分类号:TF815 文献标志码:A
镍是国民经济、社会发展、国防工业建设以及科学技术发展必不可少的基础材料和重要的战略物资,广泛应用于冶金、化工、建筑、机械制造、电池、电镀、航天等领域[1]。世界上约70%金属镍从硫化镍矿冶炼得到,中国镍资源85%为硫化镍矿[2]。硫化镍矿中,最常见的含Ni相是镍黄铁矿((Fe,Ni)9S8))[3],而且常伴生有Cu和Co,部分伴生有铂族金属[4],因此,在硫化镍冶炼时高效综合回收各有价金属长期引起关注。我国传统硫化镍冶炼工艺[1, 5]:1) 在闪速炉或电炉中对硫化镍精矿进行熔炼,分离脉石后得到目标金属富集的低冰镍,该过程Ni、Cu、Co等目标金属损失少;2) 在转炉对低冰镍进行吹炼,降低Fe和S含量,得到目标金属更进一步富集的高冰镍,该过程会产生污染气体SO2,且70%Co及部分Ni和Cu会进入转炉渣[6];3) 对高冰镍进行精炼,如采用阳极电解法、硫酸选择性浸出法、氯化浸出法等,得到目标金属或其化合物。为了提高镍冶炼过程各有价金属的回收率,探索新的冶炼工艺流程具有非常重要的意义。
低冰镍是硫化镍冶炼过程的中间产物,即硫化物熔体。相对于硫化镍矿,低冰镍充分富集了硫化镍矿中的有价金属元素Ni、Cu、Co;相对于高冰镍,其有价金属元素损失较少。目前,国内外以低冰镍为原料进行提镍的研究较少[7-8],并且均是在一定高压条件下氧化浸出,常压氧化浸出未见报道。考虑到低冰镍的主要组成物相为一些硫化物及其硫化物固溶体,因此,传统的针对含镍硫化物的处理方法和工艺如:加压酸浸[7, 9-10]、加压氨浸[11]和常压酸浸[12-13]、常压氨浸[14]等方法可以应用于低冰镍的浸出研究。PARK等[9, 11]针对硫化物熔体(包含CuFeS2, CuS2, (Fe,Ni)9S8, Ni3S2等)进行了氧压酸浸和氧压氨浸的探讨,发现氧压酸浸时Cu、Ni、Co回收率分别达到99.2%、99.3%和99.5%;氧压氨浸时Cu、Ni、Co回收率分别为93.8%、85.3%及76.5%。虽然氧压浸出能够高效回收各有价金属,但高温高压条件对设备要求高,且操作复杂,一定程度上限制了它的应用。MUZENDA等[14]对镍铜锍(包含Ni3S2、Cu2S、Ni合金等)进行了常压氧化氨浸的研究,发现常压氨浸时Ni合金难溶,Ni和Cu的回收率小于50%,即常压氨浸难以高效回收镍铜锍中有价金属。近年来,常压酸浸方法因其操作简单、反应条件温和越来越引起人们的关注,特别是FeCl3-HCl体系常被用于浸出硫化物[12-13],在常压条件下,利用溶液中Fe3+的氧化性,实现金属硫化物熔体(包含CuFeS2、CuS2、(Fe,Ni)9S8, Ni3S2等)中Cu、Ni、Co的高效浸出及目标金属的高效回收。然而,迄今为止,采用常压下FeCl3-HCl体系氧化浸出低冰镍的研究未见报导。
本文作者以低冰镍为研究对象,首先对其组成物相及有价元素的赋存状态进行了详细表征;然后利用FeCl3-HCl溶液体系浸提目标金属Ni、Cu、Co,系统研究FeCl3溶液的浓度、盐酸溶液的浓度、浸出温度和时间对Ni、Cu、Co浸出率的影响,并对Ni的浸出动力学进行了探讨。
1 实验
1.1 实验原料及表征
本研究中所用低冰镍由吉林某镍冶炼厂提供,经90 ℃干燥24 h后,研磨至75 μm以下。实验所用试剂FeCl3·6H2O和盐酸溶液(36%~38%)均为分析纯。
经分析可知,低冰镍的化学成分如表1所列,主要元素成分为Fe、Ni、Cu、Co和S。其中Ni、Cu、Co、Mg、Mn和Al的含量通过电感耦合等离子体发射光谱仪(ICP-OES)分析得到,Fet和Fem含量通过滴定法分析得到,S含量通过BaSO4重量法分析得到。低冰镍的X射线衍射(XRD)分析见图1,其主要物相为镍黄铁矿((Fe,Ni)9S8)、磁铁矿(Fe3O4)、FeNi合金(FeNi3)及斑铜矿(Cu5FeS4)。其中以FeNi合金形式存在的金属Fe含量为2.97%。根据FeNi3中Ni与Fe的质量比,可以估算出以FeNi合金形式存在的金属Ni含量约为9.35%,即约30%的Ni以FeNi合金形式存在,约70%的Ni以镍黄铁矿形式存在。
为了进一步了解各元素和物相分布状况,采用矿相解离分析仪(MLA)分析低冰镍,电子显微镜照片及相应元素的面扫描图片如图2所示,表2所列为图2(a)中不同区域的能谱分析结果。通过对低冰镍表征的综合分析可知,低冰镍中(Fe,Ni)9S8是最主要物相,Fe3O4晶粒较大且分布集中,FeNi3合金主要以条状嵌布在(Fe,Ni)9S8中,Cu5FeS4较少,呈不规则形状均匀分布其中。低冰镍中Ni、Cu、Co元素赋存状态为:Ni以(Fe,Ni)9S8和FeNi3形式存在,Cu以Cu5FeS4形式存在,Co与Fe、Ni性质相似,主要以类质同象形式分布在(Fe,Ni)9S8与Fe3O4中。
表1 低冰镍主要化学成分
Table 1 Main chemical composition of low nickel matte (mass fraction, %)


图1 低冰镍的XRD谱
Fig. 1 XRD pattern of low nickel matte
1.2 实验仪器及分析
实验仪器:TTR3型X射线衍射仪用于分析低冰镍物相组成,工作电压为40 kV,工作电流为200 mA,扫速为10 (°)/min;MLA250型矿相解离分析仪用于分析低冰镍中各物相分布状态;ARL ADVANT XP+型X射线荧光光谱仪(XRF)用于分析浸出渣的化学成分,工作电压为50 kV,工作电流为50 mA,分析范围0.001%~100%;OPTIMA 7000DV型电感耦合等离子体发射光谱仪用于分析浸出液中金属元素的浓度。
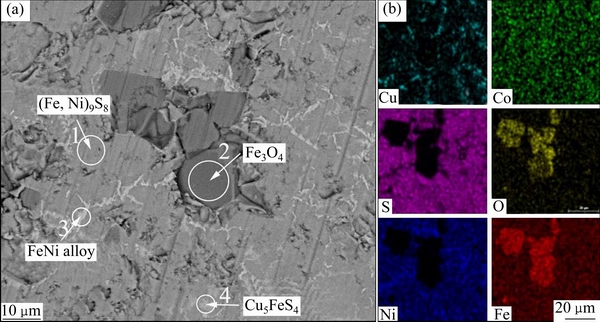
图2 低冰镍的SEM像和面扫描
Fig. 2 SEM image (a) and surface scanning images (b) of low nickel matte
表2 图2(a)中低冰镍不同区域的能谱分析结果
Table 2 EDS analysis results of low nickel matte at different areas in Fig. 2(a)
1.3 实验步骤
研究FeCl3溶液的浓度、盐酸溶液的浓度、温度对Ni、Cu、Co浸出率影响时:浸出实验在500 mL三口烧瓶中进行,三口烧瓶连接冷凝管、机械电动搅拌器和橡皮塞,采用水浴控温。按照液固比20 mL:1 g,取12.5 g低冰镍及250 mL一定浓度的FeCl3和HCl溶液,待水浴温度达到设定值,同时加入低冰镍和FeCl3-HCl溶液,并调节搅拌速度至900 r/min充分搅拌,反应开始计时。达到预定浸出时间后,用真空泵抽滤实现固液分离,用去离子水清洗浸出渣3次。浸出渣在95 ℃干燥12 h,然后称取质量并检测分析,按式(1)计算Ni、Cu、Co浸出率(η):
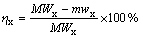 (1)
(1)
式中:x为金属元素,如Ni、Cu或Co;M和m分别为加入低冰镍的质量和浸出渣干燥后的质量,g;Wx和wx分别为x在低冰镍和浸出渣中的质量分数,%。
研究浸出时间对Ni、Cu、Co浸出率影响时:浸出实验在500 mL三口烧瓶中进行,水浴加热并机械搅拌,温度达到设定值后加入12.5 g低冰镍和250 mL一定浓度的FeCl3-HCl溶液,开始搅拌并计时。在浸出时间分别为1、2、3、5和7 h时分别用移液管取5 mL浸出液,并加入5 mL初始浓度FeCl3-HCl溶液,保持浸出液体积不变。然后将不同浸出时间的浸出液定容稀释,检测溶液中Ni、Cu、Co的浓度,按式(2)计算Ni、Cu、Co浸出率(ζ):
 (2)
(2)
式中: 为x的浓度,g/L;V为浸出液体积,L; M为低冰镍的质量,g;Wx为低冰镍中x的质量分数,%。
为x的浓度,g/L;V为浸出液体积,L; M为低冰镍的质量,g;Wx为低冰镍中x的质量分数,%。
1.4 实验原理
根据对低冰镍的表征分析,为了综合浸提低冰镍中的Ni、Cu、Co,金属硫化物与FeNi合金需被氧化溶解。酸性溶液中Fe3+具有较强的氧化性,常被用于氧化浸出金属硫化物。另外,考虑到氯化物体系浸出金属硫化物时生成的金属氯化物溶解度大,并且反应生成的单质S0为多孔疏松状[3],对反应剂扩散传质的阻碍较小,有利于金属元素的浸出。综上所述分析,本论文选取FeCl3作为氧化剂,添加盐酸溶液抑制Fe3+的水解,在FeCl3-HCl溶液综合浸提低冰镍中的有价金属元素,主要反应如式(3)~(7)所示:
(Fex,Ni(9-x))S8(s)+18FeCl3(aq)=(18+x)FeCl2(aq)+(9-x)NiCl2(aq)+8S0(s) (3)
Cu5FeS4(s)+12FeCl3(aq)=5CuCl2(aq)+13FeCl2(aq)+4S0(s) (4)
FeNi3(s)+8FeCl3(aq)=9FeCl2(aq)+3NiCl2(aq) (5)
FeNi3(s)+8HCl(aq)=FeCl2(aq)+3NiCl2(aq)+4H2(g) (6)
Fe3O4(s)+8HCl(aq)=FeCl2(aq)+2FeCl3(aq)+4H2O(aq) (7)
由上述反应式可知,目标金属Ni、Cu随着(Fex,Ni(9-x))S8、Cu5FeS4和FeNi3的溶解以氯化物形式进入溶液,伴生在(Fex,Ni(9-x))S8和Fe3O4的Co随其溶解以CoCl2形式进入溶液,实现Ni、Cu和Co的高效浸出。
2 结果与讨论
2.1 浸出条件对Ni、Cu、Co浸出率的影响
2.1.1 FeCl3溶液的浓度对Ni、Cu、Co浸出率的影响
图3所示为FeCl3溶液的浓度对Ni、Cu、Co浸出率的影响。浸出条件:盐酸溶液的浓度0.3 mol/L,温度90 ℃,浸出时间7 h。由图3可知,在FeCl3溶液的浓度为0.86 mol/L时,Ni、Cu、Co浸出率分别为90.0%、87.2%和84.5%。当FeCl3溶液的浓度增加为1 mol/L时,Ni、Cu、Co浸出率明显提高,分别达到96.6%、98.8%和95.3%。继续增加FeCl3溶液的浓度至1.5、2 mol/L,Ni、Cu、Co浸出率无明显提高。由物相分析可知,低冰镍中Ni赋存在(Fe,Ni)9S8和FeNi3中,Cu赋存在Cu5FeS4中,Co主要赋存在(Fe,Ni)9S8和Fe3O4中。在浸出反应体系中,FeCl3是氧化剂,其浓度为0.86 mol/L是低冰镍中全部S从-2价被氧化为0价单质S0所需理论FeCl3浓度值。低冰镍中除硫化物需要被氧化溶解,FeNi合金溶解也会消耗氧化剂,所以FeCl3溶液的浓度为0.86 mol/L时,氧化剂不足,使得金属浸出率不高。FeCl3溶液的浓度由0.86 mol/L增大至1 mol/L能够促进反应式(3)~(5)的进行,促进(Fe,Ni)9S8、Cu5FeS4和FeNi3的充分溶解,进一步提高Ni、Cu、Co的浸出率。当FeCl3溶液的浓度为1.0 mol/L时,低冰镍中目标金属赋存相已基本反应完全,近乎达到平衡,继续增大FeCl3溶液的浓度,Ni、Cu、Co浸出基本保持不变。考虑到反应成本和后续浸出液处理,选取FeCl3溶液的浓度1 mol/L为最佳。
2.1.2 盐酸溶液的浓度对Ni、Cu、Co浸出率的影响
图4所示为盐酸溶液的浓度对Ni、Cu、Co浸出率的影响,其他浸出条件:FeCl3溶液的浓度1 mol/L,温度90 ℃,浸出时间7 h。由图4可知,盐酸溶液的浓度从0.1 mol/L增大到0.5 mol/L时,Cu浸出率基本稳定在98.8%左右,Ni、Co浸出率分别从95.5%、94.3%增大到98.4%、97.3%。在浸出反应体系中,盐酸溶液的主要作用是利用其酸性,抑制Fe3+水解,使Fe3+作为氧化剂被充分利用。盐酸溶液的浓度较低时Cu5FeS4已能完全溶解,所以增大盐酸溶液的浓度对Cu浸出率无明显提高。盐酸溶液的浓度增大能够促进反应式(6)~(7)的发生,在一定程度上促进FeNi3和Fe3O4的溶解,提高目标金属Ni、Co的浸出率。考虑到低冰镍中有价金属的充分回收,选取盐酸溶液的浓度为0.5 mol/L为最优。

图3 FeCl3溶液的浓度对Ni、Cu、Co浸出率的影响
Fig. 3 Effect of FeCl3 concentration on leaching rates of Ni, Cu and Co

图4 盐酸溶液的浓度对Ni、Cu、Co浸出率的影响
Fig. 4 Effect of HCl solution concentration on leaching rates of Ni, Cu and Co
2.1.3 浸出温度对Ni、Cu、Co浸出率的影响
图5所示为温度对Ni、Cu、Co浸出率的影响。其浸出条件:FeCl3溶液的浓度1 mol/L,盐酸溶液的浓度0.5 mol/L,浸出时间7 h。由图5可知,60 ℃时,Cu浸出率已达到93.6%;升高温度至90 ℃时,Cu浸出率达到98.9%,提高温度对Cu浸出率提高影响较小。当温度从60 ℃升高到90 ℃提升时明显,Ni、Co的浸出率分别从60 ℃时的64.4%、50.7%升高到90 ℃时的98.4%、97.3%。温度升高能降低反应发生所需的活化能,促进反应式(3)~(7)的进行,即Ni、Cu、Co赋存相的溶解,使得Ni、Cu、Co浸出率提高,所以浸出温度选取90 ℃为最优。

图5 浸出温度对Ni、Cu、Co浸出率的影响
Fig. 5 Effect of leaching temperature on leaching rates of Ni, Cu and Co
2.1.4 浸出时间对Ni、Cu、Co浸出率的影响
图6所示为浸出时间对Ni、Cu、Co浸出率的影响,其他浸出条件:FeCl3溶液的浓度1 mol/L,盐酸溶液的浓度0.5 mol/L,浸出温度90 ℃。由图6可知,随着浸出时间延长,Ni、Cu、Co浸出率都随之增大,浸出率从1 h时Ni 54.7%、Cu 87.9%、Co 46.2%增大到7 h时的Ni 98.4%、Cu 98.9%、Co 97.3%。反应刚开始时,Ni、Cu、Co溶解速率比较快,随时间延长,由于氧化剂FeCl3浓度降低,以及氧化产物S0一定程度上阻碍传质,溶解速率降低。浸出反应7 h后,Ni、Cu和Co的浸出率都已超过97%,继续延长浸出时间不能有效提高Ni、Cu、Co浸出率,所以认为7 h为最佳浸出时间。

图6 浸出时间对Ni、Cu、Co浸出率的影响
Fig. 6 Effect of leaching time on leaching rates of Ni, Cu and Co
综上所述可知,通过系统研究FeCl3溶液的浓度、盐酸溶液的浓度、浸出温度和时间对Ni、Cu、Co浸出率的影响发现,FeCl3溶液的浓度、浸出温度和时间对Ni和Co浸出率影响很大,Cu5FeS4比(Fe,Ni)9S8容易氧化溶解,最优浸出条件为:FeCl3溶液的浓度1.0 mol/L、盐酸溶液的浓度0.5 mol/L、浸出温度90 ℃,时间7 h。在此条件下,Ni、Cu、Co浸出率分别达到98.4%、98.9%、97.3%,实现了高效浸提低冰镍中有价金属。
2.2 Ni的浸出反应动力学分析
2.2.1 不同控速步骤的浸出动力学方程
在FeCl3溶液的浓度1.0 mol/L,盐酸溶液的浓度0.5 mol/L,液固比20 mL:1 g,搅拌速度900 r/min的条件下,研究不同浸出温度、不同浸出时间对于Ni浸出率的影响,如图7所示。低冰镍在FeCl3-HCl溶液中浸出反应是液-固反应,可以采用未反应核收缩模型进行分析[15-16],其反应控制环节可能是扩散控速或界面化学反应控速,相应的动力学表达式如式(8)和(9)所示[17]。
1- x-(1-x)2/3~kt (8)
x-(1-x)2/3~kt (8)
1-(1-x)1/3~kt (9)
式中:k为表观速率常数,min-1;x为Ni浸出率,%;t为反应时间,min。
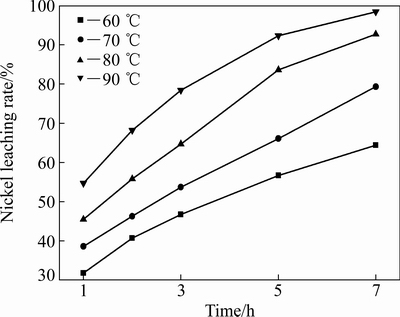
图7 不同温度下Ni浸出率随时间变化
Fig. 7 Plots of leaching rate of Ni with time at different temperatures
为了确定浸出过程的控制步骤,将图7所示的Ni浸出率分别代入式(8)和(9)并作图,以时间t为横坐标,1-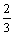 x-(1-x)2/3和1-(1-x)1/3分别为纵坐标,结果如图8所示。由于低冰镍中Ni赋存在FeNi合金和(Fe,Ni)9S8中,酸性条件下,FeNi合金比(Fe,Ni)9S8易溶且反应速度很快,所以图8中动力学曲线没有经过原点。由图8可知,1-(1-x)1/3-t(见图8(b))的线性相关性(R2>0.990)比1-
x-(1-x)2/3和1-(1-x)1/3分别为纵坐标,结果如图8所示。由于低冰镍中Ni赋存在FeNi合金和(Fe,Ni)9S8中,酸性条件下,FeNi合金比(Fe,Ni)9S8易溶且反应速度很快,所以图8中动力学曲线没有经过原点。由图8可知,1-(1-x)1/3-t(见图8(b))的线性相关性(R2>0.990)比1-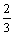 x-(1-x)2/3-t(见图8(a))的线性相关性(R2>0.969)更好,所以,认为Ni浸出反应过程可能是界面化学反应控速。
x-(1-x)2/3-t(见图8(a))的线性相关性(R2>0.969)更好,所以,认为Ni浸出反应过程可能是界面化学反应控速。
2.2.2 Ni浸出反应的表观活化能
阿伦尼乌斯(Arrhenius)方程:
k=Aexp[-Ea/(RT)] (10)
对阿伦尼乌斯方程两边同时取对数可得方程:
lnk=lnA-Ea/(RT) (11)
式中:k为速率常数,min-1;A为指前因子,min-1;Ea为浸出反应的表观活化能,J/mol;R为摩尔气体常数,R=8.314 J/(mol·K);T为热力学温度,K。
基于前面分析可知,Ni的浸出反应由界面化学反应控速,根据图8(b)中直线斜率得到温度为333、343、353、363 K时的表观速率常数k为4.66×10-4、7.09× 10-4、1.14×10-3和1.44×10-3 min-1。以lnk对T-1作图并分析,如图9所示,线性回归方程为y=6.205- 4614x,R2=0.98,得出Ni浸出反应过程由界面化学反应控速时的表观活化能Ea=4614×8.314= 38.4 kJ/mol。
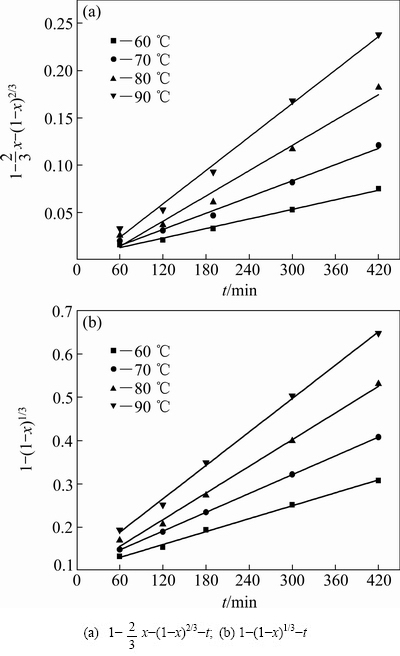
图8 不同温度下1-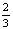 x-(1-x)2/3和1-(1-x)1/3与浸出时间的关系曲线
x-(1-x)2/3和1-(1-x)1/3与浸出时间的关系曲线
Fig. 8 Relationships between 1-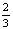 x-(1-x)2/3 and 1-(1-x)1/3 and time at different temperatures
x-(1-x)2/3 and 1-(1-x)1/3 and time at different temperatures

图9 lnk-T-1的关系图
Fig. 9 Relationship between of lnk and T-1
3 结论
1) 通过对低冰镍的XRD、SEM和EDS综合分析,低冰镍中(Fe,Ni)9S8是最主要物相,Fe3O4晶粒较大且分布集中,FeNi3主要以细条状嵌布在(Fe,Ni)9S8中,Cu5FeS4较少,呈不规则形状均匀分布其中。Ni以(Fe,Ni)9S8和FeNi3形式赋存,Cu以Cu5FeS4形式赋存,Co以取代形式赋存在(Fe,Ni)9S8和Fe3O4中。
2) 采用FeCl3-HCl体系浸出低冰镍的最优条件为:FeCl3溶液的浓度1.0 mol/L,盐酸溶液的浓度0.5 mol/L,浸出温度90 ℃和时间7 h。在此条件下,Ni、Cu和Co浸出率分别达到98.4%、98.9%和97.3%,实现了低冰镍中有价金属元素的高效浸出。
3) Ni的浸出反应动力学符合未反应核收缩模型。根据Ni在不同温度和时间时的浸出率,发现Ni在60~90 ℃之间时,浸出反应受界面化学反应控速,根据阿伦尼乌斯经验方程计算得到反应的表观活化能Ea=38.4 kJ/mol。
REFERENCES
[1] 路长远, 鲁雄刚, 邹星礼, 程红伟, 许 茜. 中国镍矿资源现状及技术进展[J]. 自然杂志, 2015, 37(4): 269-277.
LU Chang-yuan, LU Xiong-gang, ZOU Xing-li, CHENG Hong-wei, XU Qian. Current situation and utilization technology of nickel ore in China[J]. Chinese Journal of Nature, 2015, 37(4): 269-277.
[2] 周京英, 纵 凯, 付水兴. 中国镍资源全球配置研究[J]. 矿产勘查, 2015, 6(1): 86-91.
ZHOU Jing-ying, ZONG Kai, FU Shui-xing. Study on global market allocation of Chinese nickel metal resources[J]. Mineral Exploration, 2015, 6(1): 86-91.
[3] LU Z Y, JEFFREY M I, ZHU Y, LAWSON F. Studies of pentlandite leaching in mixed oxygenated acidic chloride-sulfate solutions[J]. Hydrometallurgy, 2000, 56(1): 63-74.
[4] 曾认宇, 赖健清, 毛先成, 赵 莹, 刘 嫔, 朱佳玮, 岳 斌, 艾启兴. 金川铜镍硫化物矿床铂族元素地球化学差异及其演化意义[J]. 中国有色金属学报, 2016, 26(1): 149-163.
ZENG Ren-yu, LAI Jian-qing, MAO Xian-cheng, ZHAO Yin, LIU Pin, ZHU Jia-wei, YUE Bin, AI Qi-xing. Distinction of platinum group elements geochemistry in Jinchuan Cu-Ni sulfide deposit and its implication for magmatic evolution[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(1): 149-163.
[5] WARNER A E M,  C M, DALVI A D, MACKEY P J, TARASOV A V, JONES R T. JOM world nonferrous smelter survey part IV: Nickel sulfide[J]. JOM, 2007, 59(4): 58-72.
C M, DALVI A D, MACKEY P J, TARASOV A V, JONES R T. JOM world nonferrous smelter survey part IV: Nickel sulfide[J]. JOM, 2007, 59(4): 58-72.
[6] 喻正军. 从镍转炉渣中回收钴镍铜的理论与技术研究[D]. 长沙: 中南大学, 2006: 19-20.
YU Zheng-jun. The study of theory and technology of recovery of Co, Ni and Cu from nickel converter slag[D]. Changsha: Central South University, 2006: 19-20.
[7] 尹 飞, 王振文, 王成彦, 江培海. 低冰镍加压酸浸工艺研究[J]. 矿冶, 2009, 18(4): 35-37.
YIN Fei, WANG Zhen-wen, WANG Cheng-yan, JIANG Pei-hai. Research on pressure leaching process for low nickel matte[J]. Mining and Metallurgy, 2009, 18(4): 35-37.
[8] 沈明伟, 冀成庆, 朱昌洛, 蔡 旺. 低冰镍氧压水浸试验研[J]. 云南冶金, 2012, 41(3): 32-34.
SHENG Ming-wei, JI Cheng-qing, ZHU Chang-luo, CAI Wang. Experimental study on oxygen pressure and water leaching of low nickel matte[J]. Yunnan Metallurgy, 2012, 41(3): 32-34.
[9] PARK K H, MOHAPATRA D, NAM C W, KIM H I. A comparative study of different leaching processes for the extraction of Cu, Ni and Co from a complex matte[J]. Korean Journal of Chemical Engineering, 2007, 24(5): 835-842.
[10] WANG Si-fu, WEI Chang, DENG Zhi-gan, LI Cun-xiong, LI Xin-bing, WU Jun, WANG Ming-shuang, ZHANG Fan. Extraction of molybdenum and nickel from Ni-Mo ore by pressure acid leaching[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 3083-3088.
[11] PARK K H, MOHAPATRA D, REDDY B R, NAM C W. A study on the oxidative ammonia/ammonium sulphate leaching of a complex (Cu-Ni-Co-Fe) matte[J]. Hydrometallurgy, 2007, 86(3/4): 164-171.
[12] PARK K H, MOHAPATRA D, REDDY B R. A study on the acidified ferric chloride leaching of a complex (Cu-Ni-Co-Fe) matte[J]. Separation and Purification Technology, 2006, 51(3): 332-337.
[13] 李金丽, 张明杰, 王洪宽. 三氯化铁浸出高冰镍[J]. 东北大学学报(自然科学版), 1998, 19(2): 152-154.
LI Jin-li, ZHANG Ming-jie, WANG Hong-kuan. NiS matte leached by ferric chloride[J]. Journal of Northeastern University (Natural Science), 1998, 19(2): 152-154.
[14] MUZENDA E, RAMATSA I M, NTULI F, ABDULKAREEM A S, AFOLABI A S. Parametric effects on leaching behavior of nickel-copper matte in ammonia[J]. Particulate Science and Technology, 2013, 31(4): 319-325.
[15] 陈家镛, 杨守志, 柯家骏. 湿法冶金手册[M]. 北京: 冶金工业出版社, 2005: 322.
CHEN Jia-yong, YANG Shou-zhi, KE Jia-jun. Handbook of hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 2005: 322.
[16] DRY M J, BRYSON A W. Kinetics of leaching of a low-grade Fe-Ni-Cu-Co matte in ferric sulphate solution[J]. Hydrometallurgy, 1987, 18(2): 155-181.
[17] 赵 艳, 彭 犇, 郭 敏, 张 梅. 红土镍矿微波水热法浸提镍钴[J]. 北京科技大学学报, 2012, 34(6): 632-638.
ZHAO Yan, PENG Ben, GUO Min, ZHANG Mei. Extraction of nickel and cobalt from laterite using a microwave assisted hydro-thermal leaching method[J]. Journal of University of Science and Technology Beijing, 2012, 34(6): 632-638.
High efficient leaching of Ni, Cu and Co from low nickel matte
CHEN Guang-ju, WANG Hui-gang, ZHANG Mei, GUO Min
(School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China)
Abstract: The extraction of Ni, Cu and Co with high leaching efficiencies from the low nickel matte in FeCl3-HCl solution was investigated. The effects of FeCl3 concentration, hydrochloric acid concentration, leaching temperature and time on the leaching rates of Ni, Cu and Co were systematically investigated. The results show that leaching rates of Ni, Cu and Co are 98.4%, 98.9% and 97.3%, respectively, under the optimal conditions (FeCl3 concentration: 1.0 mol/L, hydrochloric acid concentration: 0.5 mol/L, leaching temperature: 90 ℃, leaching time: 7 h). The leaching process follows the shrink-core model at the temperature of 60~90 ℃ and through kinetic analysis, the leaching efficiency of Ni is controlled by the interface chemical reaction, and the apparent activation energy is 38.4 kJ/mol.
Key words: low nickel matte; FeCl3-HCl solution; leaching; Ni; Cu; Co; kinetics
Foundation item: Project (2014CB643401, 2013AA032003) supported by National Basic Research Priorities Program of China; Project (51372019) supported by National Natural Science Foundation of China
Received date: 2016-07-21; Accepted date: 2016-12-26
Corresponding author: GUO Min; Tel: +86-10-62334926; E-mail: guomin@ustb.edu.cn
(编辑 李艳红)
基金项目:国家重点基础研究发展计划资助项目(2014CB643401, 2013AA032003);国家自然科学基金资助项目(51372019)
收稿日期:2016-07-21;修订日期:2016-12-26
通信作者:郭 敏,教授,博士;电话:010-62334926;Email:guomin@ustb.edu.cn