J. Cent. South Univ. Technol. (2008) 15: 463-468
DOI: 10.1007/s11771-008-0087-7
Synthesis and adsorption properties for Au(Ⅲ) of
alkoxycarbonyl thiourea resin
WANG Shuai(王 帅), ZHONG Hong(钟 宏), LIU Guang-yi(刘广义),
ZHANG Qian(张 骞), LI Ting(李 婷)
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: A novel alkoxycarbonyl thiourea resin(ATR) was synthesized by monomer polymerization of oxydiethane-2,1-diyl dicarbonisothiocyanatidate and polyethylene polyamine, and characterized by FT-IR. The adsorption properties of ATR were investigated by batch test. The adsorption capacities for Au(Ⅲ), Ag(Ⅰ), Cu(Ⅱ), Zn(Ⅱ), Fe(Ⅲ), Ca(Ⅱ) and Mg(Ⅱ) are 4.65, 4.40, 0.40, 0.90, 0.86, 0.0080 and 0.016 mmol/g, respectively, when the adsorption condition is as follows: contact time 24 h, temperature 30 ℃, initial concentration of Au(Ⅲ) 5.08 mmol/L and that of other metals 0.10 mol/L, and concentration of acid 1.0 mol/L. The adsorption capacity for Au(Ⅲ) increases with the increase of contact time, temperature and initial concentration of Au(Ⅲ). The capacity after five adsorption-desorption cycles remains 90% that of the first time, and the separation factors of ATR for binary metal ion solutions are larger than 995, indicating that ATR is of good regeneration property and selectivity. XPS results show that the functional atoms of ATR supply electrons for Au and coordinate with Au during the adsorption.
Key words: alkoxycarbonyl thiourea resin; Au(Ⅲ); separation factor; synthesis; adsorption
1 Introduction
Chelating resins differ from activated carbon and ion exchange resins in their high selectivity during adsorption process and their easy regeneration property during desorption process. Recently, many articles that cover a vast number of chelating resins with excellent adsorption performance for noble metal ions have been reported[1-3]. Chelating resins with two or more coordination atoms such as S, N or O may have a stronger coordination capability with the synergistic effect of multiple coordination atoms. Thiourea is an excellent ligand to noble metal ions, which is widely used in extraction, separation and recovery in precious metal hydrometallurgical field. Chelating resins with thiourea groups are expected to be new type functional materials for separation and recovery of precious metals. Recently, some attempts have been made to synthesize polymers containing thiourea groups. There are mainly two ways: 1) polymer grafting, which means to graft thiourea groups onto the main chains of such polymers as polystyrene[4-5], polyvinyl chloride[6], poly(ethylene glycol)[7], phenolic aldehyde resin[8], chitosan[9], silica gel[10] and their derivatives; 2) monomer polymerization, such as polymerization of thiourea or bisthiourea and formaldehyde[11-12], or polymerization of thiourea, amine thiourea or bisthiourea and chloromethylthiirane[13], etc. Thiourea groups of the resins synthesized by polymer grafting often locate in branches, and those of the resins synthesized by monomer polymerization often locate in main chains. Polymer grafting is the most common synthesis route, while researches on monomer polymerizing are less.
Therefore, in this work, a novel chelating resin (ATR) with alkoxycarbonyl thiourea groups in its main chains was synthesized by the reaction of oxydiethane-2, 1-diyl dicarbonisothiocyanatidate and polyethylene polyamine, and the adsorption performance and separation abilities of ATR for Au(Ⅲ) were investigated. Meanwhile, the coordination mechanism between ATR and Au(Ⅲ) was studied by X-ray photoelectron spectroscopy (XPS).
2 Experimental
2.1 Reagents and apparatus
Bis(trichloromethyl) carbonate (also called triphosgene) and polyethylene polyamine were of chemical pure grade; diethylene glycol, potassium thiocyanate, N, N-dimethylaniline (DMA), PEG-400 and gold wire were of analytical grade. Au(Ⅲ) solution was prepared by dissolving gold wire in chlorazotic acid, diluted with hydrochloric acid and water.
The temperature of synthesis was controlled by HC21005 cryostat (Chongqing Experimental Equipment Factory); infrared spectra were recorded on a G510PFTIR spectrophotometer (Nicolet Company, USA) by KBr pellet method; adsorption experiments were carried out in the SHA-C thermostatic vibrator (Jintan Medical Instrument Plant); the concentrations of Au(Ⅲ) were measured by 752S UV-Vis spectrometer (Shanghai Lengguang Technology Company), and those of other metals were measured by WYX-9003 atomic absorption spectrometer (Shenyang Analytical Instrument Factory); XPS was performed on an XSAM800 system (Kratos Company, British) to observe the changes of electron binding energies of elements.
2.2 Synthesis of ATR
Diethylene glycol bischloroformate was prepared by the reaction of 0.073 3 mol triphosgene and 0.10 mol diethylene glycol in toluene at 5 ℃ for 10 h in the presence of catalyst DMA. During the reaction the evolved hydrogen chloride was swept out of the reaction mixture by a slow stream of nitrogen, and the residual hydrogen chloride was washed out by 1% ice salt water. Then diethylene glycol bischloroformate reacted with 0.25 mol KSCN at 5 ℃ for 3.5 h in the presence of phase-transfer catalyst PEG-400, from which oxydiethane-2,1-diyl dicarbonisothiocyanatidate was synthesized. At last, oxydiethane-2,1-diyl dicarboniso- thiocyanatidate reacted with 8.75 g polyethylene polyamine (containing 0.20 mol amino groups) for 1 h at 5 ℃ to obtain ATR. ATR with the particle size of 150-250 μm was obtained after being washed, dried, crushed and size graded.
2.3 Adsorption procedure
2.3.1 Adsorption of ATR for metal ions
A batch method was used to examine the adsorption of metal ions on ATR. A certain quality of resin (0.0250 g for Au(Ⅲ) and 0.250 g for other metal ions ) was suspended in 25 mL solution in an iodometric flask, which was placed in a thermostatic vibrator. At a desired temperature, the flask was shaken at 120 r/min for a certain time period. The solution was filtrated, the concentration of Au(Ⅲ) in the filtrate was determined by crystalline-violet sepectrophotometric method[14], and the concentrations of other metal ions were determined by atomic absorption spectroscopy. Adsorption capacity was calculated as follows:
q=(ci-ce)V/m (1)
where q is the adsorption capacity; ci is the initial concentration of Au(Ⅲ) solution; ce is the concentration of Au(Ⅲ) at a certain time; V is the volume of bulk solution; m is the mass of the dry resin.
2.3.2 Regeneration of ATR
A batch method was also used to examine the regeneration of ATR. A solution containing 1.0 mol/L HCl and 10% (mass fraction) thiourea was chosen as elution in the desorption of Au(Ⅲ) adsorbed on ATR. After adsorption, ATR was washed and suspended in the elution solution in an iodometric flask. The flask was shaken for a certain time period in the thermostatic vibrator. The adsorption-desorption operation was repeated several times, and the adsorption capacity of every time was recorded.
2.3.3 Adsorption selectivity of ATR
The adsorption selectivity of ATR for metal ions was investigated in binary component systems by batch tests. 0.025 0 g resin was suspended in 25 mL mixed solution of Au(Ⅲ)-Cu(Ⅱ), Au(Ⅲ)-Zn(Ⅱ) or Au(Ⅲ)- Fe(Ⅲ) and was shaken in the thermostatic vibrator for a certain time period. Separation factor (k) was calculated according to the following equation:
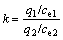 (2)
(2)
where q1 and q2 are the adsorption capacities for Au(Ⅲ) and the other metal ions, respectively; ce1 and ce2 are the concentrations at equilibrium of Au(Ⅲ) and the other metal ions, respectively.
3 Results and discussion
3.1 Preparation of ATR
The synthetic route of ATR is shown as follows.
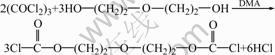 (3)
(3)
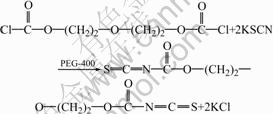 (4)
(4)
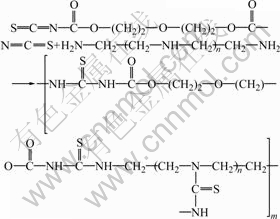 (5)
(5)
Infrared spectrum of ATR is shown in Fig.1. Characteristic peaks appear as follows: N—H stretch vibration, 3 374.9 cm-1; N—H bend vibration, 765.6 cm-1; C—N stretch vibration, 1 369.2 cm-1; C=O stretch vibration, 1 697.1 cm-1; C=S stretch vibration, 1 074.2 cm-1; N—C—N stretch vibration and N—H bend vibration, 1 537.0 cm-1; S=C—N, 457.1 cm-1; C—O stretch vibration, 1 243.8 cm-1; and C—O stretch vibration of ether groups, 1 126.2 cm-1, which justify that ATR contains the functional groups of amidocyanogen, alkoxycarbonyl and thioureido.
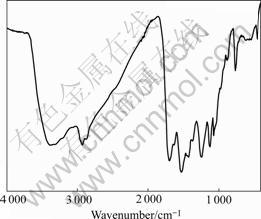
Fig.1 FT-IR spectrum of ATR
3.2 Adsorption capacities of ATR for metal ions
The adsorption condition was as follows: contact time 24 h, temperature 30 ℃, initial concentration 5.08 mmol/L for Au(Ⅲ) or 0.10 mol/L for other metal ions, and concentration of HNO3 1.0 mol/L for Ag(Ⅰ) or HCl 1.0 mol/L for other metal ions. The adsorption capacities for Au(Ⅲ), Ag(Ⅰ), Cu(Ⅱ), Zn(Ⅱ), Fe(Ⅲ), Ca(Ⅱ) and Mg(Ⅱ) are 4.65, 4.40, 0.40, 0.90, 0.86, 0.008 0 and 0.016 mmol/g, respectively. According to the hard and soft acids and bases theory(HSAB)[15], functional groups containing S or N donor atoms are soft bases. So they interact strongly with soft acids such as precious metal ions, but have weak chelating capabilities to borderline acids such as Cu(Ⅱ), Zn(Ⅱ) and Fe(Ⅲ) and no chelating capabilities to hard bases such as Ca(Ⅱ) and Mg(Ⅱ). Single functional groups containing O donor atoms are generally hard bases, and don’t interact with precious metal ions. However, alkoxycarbonyl groups may reduce the hardness of thiourea groups and make ATR become a softer base. With the synergistic action of three coordinating atoms, ATR keeps high adsorption capabilities for soft acids but low adsorption capabilities for borderline and hard acids.
3.3 Effect of adsorption conditions on adsorption of ATR
3.3.1 Effect of contact time on adsorption
The effect of contact time on the adsorption of ATR for Au(Ⅲ) is shown in Fig.2. The adsorption capacity increases with the increase of contact time until an equilibrium is established in 10 h, and the equilibrium adsorption capacity is 4.65 mmol/g.
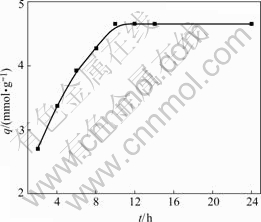
Fig.2 Effect of contact time on adsorption of ATR for Au(Ⅲ) (Initial concentration of Au(Ⅲ) 5.08 mmol/L, concentration of HCl 1.0 mol/L, temperature 30 ℃)
Generally, the adsorption of resins for metals can be described as follows: 1) diffusion of the metal ions from the solution to the liquid films surrounding the particles; 2) diffusion from the films to the particle surfaces; 3) diffusion from the surfaces to the internal sites of particles; and 4) chemical reaction of metal ions and function groups of resins. The diffusion rate of metal ions in the solution is generally quicker than other steps, so the adsorption may be controlled by one or more of the last three steps, namely liquid film diffusion, particle diffusion or chemical reaction. The rate limiting step can be judged by the following equations[16].
Liquid film diffusion:
-ln(1-F)=kt (6)
Particle diffusion:
1-3(1-F)2/3+2(1-F)=kt (7)
Chemical reaction:
1-(1-F)1/3=kt (8)
where F is the fractional attainment of equilibrium at time t and is obtained by the expression F=(ci-ct)/(ci-ce); k is the adsorption rate constant. The results fitted by Eqns.(6)-(8) are shown in Fig.3.
The correlation coefficients R2 of the three curves are very close, indicating that the adsorption process may be controlled by the three diffusion processes simultaneously.
3.3.2 Effect of concentration of HCl on adsorption
Fig.4 shows the effect of concentration of HCl on the adsorption of ATR. The adsorption capacity keeps increasing at a slow rate with the increase of concentration of HCl, which indicates that the effect of concentration of HCl is not significant. ATR remains good adsorption performance in the experimental range. Acid affects ion exchange of amidocyanogen and metal ions strongly, so the effect of concentration of acid on adsorption properties of resins containing amidocyanogen is generally significant[1]. It is not true for ATR, although there are amine groups in it. Perhaps this can be explained by the fact that ATR contains three functional groups simultaneously, and coordination between ATR and Au(Ⅲ) is dominant compared with ion exchange.
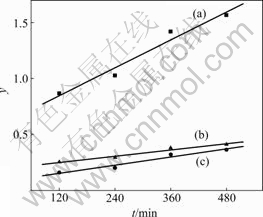
Fig.3 Linear fit results of adsorption kinetics: (a) Liquid film diffusion, y=2.070×10-3t+0.597 80, R2=0.963 9; (b) Chemical reaction, y=4.615×10-4t+0.192 70, R2=0.963 4; (c) Particle diffusion, y=6.070×10-4t+0.078 31, R2=0.962 4
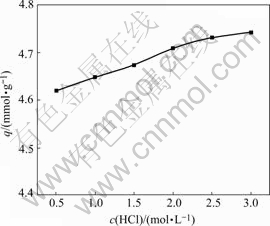
Fig.4 Effect of concentration of HCl on adsorption of ATR for Au(Ⅲ) (Initial concentration of Au(Ⅲ) 5.08 mmol/L, contact time 10 h, temperature 30 ℃)
3.3.3 Effect of initial concentration of Au(Ⅲ) on adsorption
The effect of initial concentration of Au(Ⅲ) on adsorption of ATR is presented in Fig.5. Adsorption capacity increases with the increase of initial concentration of Au(Ⅲ), but the rate changes slowly at higher concentration, being close to the maximum adsorption capacity at 30 ℃ gradually.
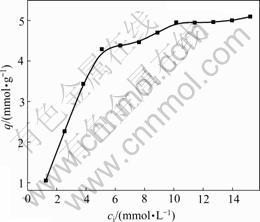
Fig.5 Effect of initial concentration of Au(Ⅲ) on adsorption of ATR for Au(Ⅲ) (Concentration of HCl 1.0 mol/L, contact time 10 h, temperature 30 ℃)
Liquid-solid adsorption behavior is generally described by Langmuir adsorption isotherm model and Freundlich adsorption isotherm model. Langmui adsorption isotherm equation[17] and Freundlich adsorption isotherm equation[18] were adopted to linearly fit the first 8 data in Fig.5. The results are shown in Fig.6.
Langmuir adsorption isotherm equation:
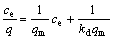 (9)
(9)
Freundlich adsorption isotherm equation:
 (10)
(10)
where kd is Langmuir constant; qm is the maximum adsorption capacity; kF and n are Freundlich constants.
The correlation coefficient of Langmuir isotherm model is larger than that of Freundlich isotherm, indicating that Langmuir isotherm is more suitable to describe the adsorption process and the adsorption of Au(Ⅲ) on ATR is a monolayer adsorption. From the intercepts and slopes of the straight lines in Fig.6, the constants are summarized as: kd=3 162 L/mol, qm=5.123 mmol/g, kF=0.013 65 and n=5.280.
3.3.4 Effect of temperature on adsorption
The effect of temperature on adsorption of ATR is shown in Fig.7. Adsorption capacity increases with the increase of temperature, which may be explained as follows: 1) the resin is swollen more completely at higher temperature, which makes Au(Ⅲ) diffuse more easily into the inside of resin; 2) the adsorption process is an endothermal one, and higher temperature is favorable for adsorption.
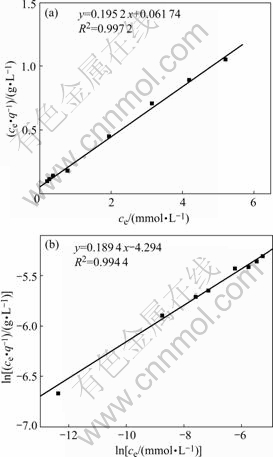
Fig.6 Langmuir isotherm and Freundlich isotherm of ATR: (a) Langmuir isotherm; (b) Freundlich isotherm
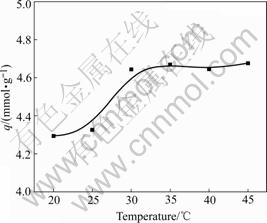
Fig.7 Effect of temperature on adsorption of ATR for Au(Ⅲ) (Initial concentration of Au(Ⅲ) 5.08 mg/mL, concentration of HCl 1.0 mol/L, contact time 10 h)
3.4 Regeneration of ATR
The adsorption-desorption operation was repeated for 5 cycles to investigate the regeneration of ATR. The results are listed in Table 1. The capacity after five regeneration cycles remains 90% that of the first time, indicating that ATR is of good regeneration property. Desorption should be operated right away after adsorption, or the resin cannot be recovered completely and the adsorption capacity will become smaller.
Table 1 Regeneration of ATR

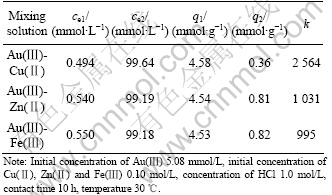
3.5 Separation property of ATR
The separation factor of the two ions is the most useful parameter in practical use. Adsorption capacities and separation factors of ATR for binary metal ions are listed in Table 2. Adsorption capacities of ATR for Au(Ⅲ), Cu(Ⅱ), Zn(Ⅱ) and Fe(Ⅲ) decrease compared with those for single ions, but adsorption capacities remain larger, so separation factors of ATR are larger, indicating that ATR is of good selectivity property.
Table 2 Separation factors of ATR for binary metal ions
3.6 Adsorption mechanism of ATR for Au(Ⅲ)
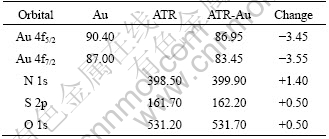
Electron binding energies of ATR and Au fore-and-aft adsorption are shown in Table 3. After adsorption, the binding energies of N 1s, S 2p and O 1s increase by 1.40, 0.50 and 0.50 eV separately, which suggests that their electron cloud density decreases. The binding energies of Au 4f5/2 and Au 4f7/2 reduce, implying that the electron cloud density increases. So a conclusion can be drawn that N, S and O provide electrons for Au in the adsorption and ATR coordinates with Au.
Table 3 Electron binding energies of ATR (eV)
4 Conclusions
1) ATR is synthesized using triphosgene, diethylene glycol, potassium thiocyanate and polyethylene polyamine as raw materials.
2) The adsorption and separation of ATR for Au(Ⅲ) are investigated by batch test. The equilibrium adsorption capacity is 4.65 mmol/g and the separation factors are larger than 995.
3) XPS results show that the functional groups of ATR provide electrons for Au during the adsorption process.
4) ATR is a favorable chelating resin for the enrichment and separation of Au(Ⅲ), so practicle application test and separation mechanism should be investigated further in the future studies.
References
[1] RAMESH A, HASEGAWA H, SUGIMOTO W, MAKI T, UEDA K. Adsorption of gold(Ⅲ), platinum(Ⅳ) and palladium(Ⅱ) onto glycine modified crosslinked chitosan resin [J]. Bioresource Technology, 2008, 99(9): 3801-3809.
[2] SHU Zeng-nian, XIONG Chun-hua, WANG Xu. Adsorption behavior and mechanism of amino methylene phosphonic acid resin for Ag(Ⅰ) [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(3): 700-704.
[3] DONIA A M, ATIA A A, ELWAKEEL K Z. Gold(Ⅲ) recovery using synthetic chelating resins with amine, thio and amine/mercaptan functionalities [J]. Separation and Purification Technology, 2005, 42(2): 111-116.
[4] ZUO G J, MUHAMMED M. Thiourea-based coordinating polymers: Synthesis and binding to noble metals [J]. Reactive Polymers, 1995, 24(3): 165-181.
[5] TROCHIMCZUK A W, KOLARZ B N. Synthesis and chelating properties of resin with methylthiourea, guanylthiourea and dithiocarbamate groups [J]. European Polymer Journal, 2000, 36(11): 2359-2363.
[6] CAO De-rong, SU Zhi-xing. Chemical conversion for macroporous spherical resins of PVC (Ⅲ): Studies on the synthesis and properties of thiourea resins [J]. Journal of Lanzhou University: Natural Science Edition, 1997, 33(2): 63-67. (in Chinese)
[7] YANG Gui-chun, CHEN Zu-xing, ZHANG Zhao-jun. Combinatorial synthesis of novel thiourea derivatives on a modified poly(ethylene glycol) [J]. Reactive and Functional Polymers, 2002, 51(1): 1-6.
[8] LIU Chun-ping, SUN Lin, LU Ju-bo, LIU Bing, QU Rong-jun. Adsorption properties and laboratory use of phenylthiourea supported phenolic aldehyde chelating resin for Ag+[J]. Ion Exchange and Adsorption, 2005, 21(4): 335-342. (in Chinese)
[9] PENG Chang-hong, CHEN Yi-feng, TANG Mo-tang. Synthesis and adsorption properties of chitosan-crown ether resins [J]. Journal of Central South University of Technology, 2003, 10(2): 103-107.
[10] LIU Peng, SU Zhi-xing, WU Xiong-zhi, PU Qiao-sheng. Application of isodiphenylthiourea immobilized silica gel to flow injection on-line microcolumn preconcentration and separation coupled with flame atomic absorption spectrometry for interferencefree determination of trace silver, gold, palladium and platinum in geological and metallurgical samples [J]. Journal of Analytical Atomic Spectrometry, 2002, 17(7): 125-130.
[11] NI Cai-hua, YI Chang-hai, FENG Zhi-yun. Studies of syntheses and adsorption properties of chelating resin from thiourea and formaldehyde [J]. Journal of Applied Polymer Science, 2001, 82(13): 3127-3132.
[12] ATIA A A. Adsorption of silver and gold on resins derived from bisthiourea and application to retrieval of silver ions from processed photo films [J]. Hydrometallurgy, 2005, 80(1/2): 98-106.
[13] XU Yu-wu, YANG Ya-he, LI Han-ping. Study on chelating resins XXX: Syntheses and adsorption properties of thiourea type resins [J]. Journal of Wuhan University: Natural Science Edition, 1999, 45(2): 129-134. (in Chinese)
[14] HU Zhang-yun. Crystalline-violet spectrophotometric method for rapid determination of gold [J]. Geological Science and Technology of Jiangxi, 1997, 24(2): 96-98. (in Chinese)
[15] HUBICKI Z, LESZCZY M. Studies of the sorption of palladium(Ⅱ) ions from model chloride systems onto an ion exchanger containing isothiourea groups and onto weakly basic anion exchangers of various types [J]. Adsorption Science and Technology, 2004, 22(8): 603-614.
[16] MA Hong-mei, ZHU Zhi-liang, ZHANG Rong-hua, LIN Jian-wei, ZHAO Jian-fu. Kinetics of adsorption of copper from water by weak base epoxy anion-exchange resin [J]. Ion Exchange and Adsorption, 2006, 22(6): 519-526. (in Chinese)
[17] CAO Zuo-ying, WEI Qi-feng, ZHANG Qi-xiu. Template synthesis and adsorption properties of chitosan salicylal Schiff bases [J]. Journal of Central South University of Technology, 2004, 11(2): 169-172.
[18] SHEN Li, XIA Jin-lan, HE Huan, NIE Zhen-yuan, QIU Guan-zhou. Biosorption mechanism of Cr(Ⅳ) onto cells of Synechococcus sp. [J]. Journal of Central South University of Technology, 2007, 14(2): 157-162.
Foundation item: Projects(20476105, 50604016) supported by the National Natural Science Foundation of China
Received date: 2007-11-20; Accepted date: 2008-03-01
Corresponding author: ZHONG Hong, Professor, PhD; Tel: +86-731-8830603; E-mail: zhongh@mail.csu.edu.cn
(Edited by CHEN Wei-ping)