采用对甲苯磺酸催化研磨法合成苯甲醛缩苯胺衍生物
文瑞明,朱云辉,游沛清
(湖南城市学院 化学与环境工程系,湖南 益阳,413000)
摘要:在对甲苯磺酸催化下,将芳香醛与芳胺于室温下研磨可得较高收率的苯甲醛缩苯胺衍生物,通过单因素实验研究研磨时间、对甲苯磺酸用量及取代基类型对缩合反应的影响。研究结果表明采用对甲苯磺酸催化研磨法合成苯甲醛缩苯胺衍生物最佳工艺条件是:芳香醛、芳胺、对甲苯磺酸的摩尔比为100:100:3,反应温度为室温,研磨时间为1~3 min,在此条件下,反应收率可达86.5%~95.5%,比采用溶剂回流法和常规研磨法所得收率有显著提高,而且反应时间减少0.5 %;芳醛环上取代基对反应无影响,但芳胺环上邻、对位的强吸电子基抑制反应的进行;与传统方法相比,对甲苯磺酸催化研磨法具有反应时间短、条件温和、操作简单、收率高等优点,为同类化合物的合成提供了一个简便而有效的方法。
关键词:苯甲醛缩苯胺;Schiff碱;研磨;对甲苯磺酸;缩合反应
中图分类号:O625.6 文献标志码:A 文章编号:1672-7207(2012)04-1249-05
Synthesis of benzalanilines by p-toluene sulfonic acid using grinding method
WEN Rui-ming, ZHU Yun-hui, YOU Pei-qing
(Department of Chemistry and Environmental Engineering, Hunan City University, Yiyang 413000, China)
Abstract: Condensation of aromatic aldehydes with arylamines catalyzed by p-toluene sulfonic acid (PTSA) was carried out at room temperature to obtain benzalaniline by grinding. The factors that affect the condensation including grinding time, the amount of PTSA and the type of substituents were studied by single factor test. The results show that the optimal conditions of condensation catalyzed p-toluene sulfonic acid using grinding method are as follows. The mole ratio of aromatic aldehyde, arylamine and PTSA is 100:100:3, the reaction temperature is room temperature, and the grinding time is 1-3 min. In the optimal conditions, the yield reaches 86.5%-95.5% and the condensation time is shortened by 0.5% compared with that using traditional refluxing and grinding method. And this novel method has several advantages such as short reaction time, mild conditions, simple operation and high yields, and can provide a simple and efficient protocol for the synthesis of analogous compounds.
Key words: benzalaniline; Schiff base; grinding; p-toluene sulfonic acid; condensation
芳香族Schiff碱是一类重要的有机合成中间体,可作螯合剂、稳定剂、生物活性剂、植物生长调节剂、分析试剂和催化剂等,广泛应用于化工生产和科学研究[1-4]。Schiff碱的合成一般采用酸催化下的有机溶剂回流法,由于该反应是可逆反应,所以,常用共沸方法除去生成的水[5-6]。目前,合成Schiff碱的方法有微波及超声波辐射法[1, 7]、室温研磨法[8-10],但采用这些方法反应时间较长,且反应之后还需放置一段时间才能反应完全。固相研磨反应是绿色化学的重要组成部分。研究表明:许多固相研磨反应在反应速度、反应收率以及反应选择性等方面均较液相反应有显著改善;同时,还因其可避免使用有毒的有机溶剂,减少环境污染,提高反应效率和原子经济性,降低能耗等特点而受到重视[11-13]。在对甲苯磺酸催化下,芳香醛与芳胺于室温下研磨缩合制备Schiff碱。该反应不使用有毒有害的有机溶剂,减少了环境污染,操作简便,反应时间短,是一种合成Schiff碱类化合物快捷而实用的方法。合成路线如下:

1 实验
1.1 试剂与仪器
仪器为:INOVA-400 MHz型核磁共振谱仪(TMS内标, CDCl3溶剂);XRC-1显微熔点仪(温度计未校正)。所有试剂均为分析纯,液体醛、胺在使用前经蒸馏纯化。
1.2 合成
称取10 mmol 芳香醛(1)、10 mmol 芳胺(2)和0. 3 mmol对甲苯磺酸(PTSA)加入研钵中,于室温下研磨一定时间至混合固体的颜色逐渐由浅黄色变成黄色或橙红色。反应完毕后,用95%乙醇洗涤固体,用无水乙醇重结晶、干燥,得Schiff碱固体。
2 结果与讨论
2.1 合成化合物的理化性质及波谱表征
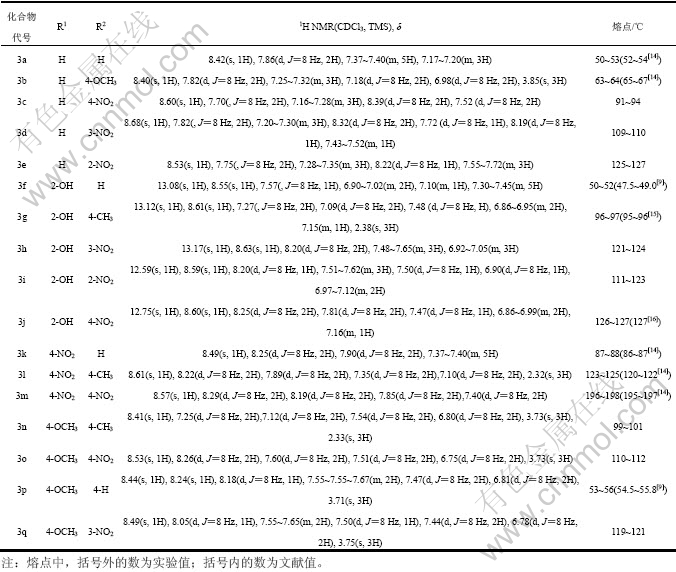
化合物3a~3q均采用相似的方法合成,其结构经熔点测定与文献报道结果比较及1H NMR证实结果见 表1。
表1 化合物的1H NMR和熔点
Table 1 1H NMR and melting point of compounds 3a~3q
2.2 对甲基苯磺酸用量对反应的影响
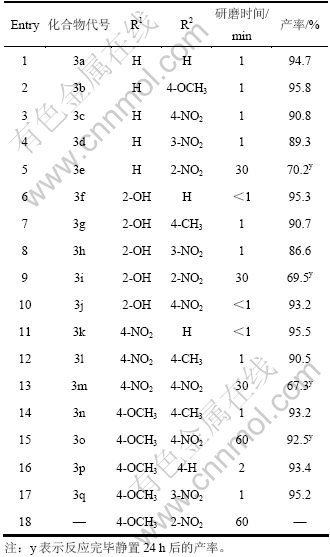
以水杨醛(10 mmol)和对硝基苯胺(10 mmol)的反应为例,考察对甲苯磺酸用量对反应的影响,结果如表2所示(其中,n表示物质的量,mol)。
表2 对甲苯磺酸用量对反应的影响
Table 2 Effect of content of PTSA on reaction

由表2可知:未添加对甲苯磺酸,即使研磨30 min,也无产物生成;随着对甲苯磺酸用量的增加,反应收率逐渐升高;当对甲苯磺酸用量增至0.3 mmol时,研磨1 min反应即完全,收率可达93.7%,继续增加对甲苯磺酸用量,收率变化不明显。经综合考虑,选择水杨醛、对硝基苯胺和对甲苯磺酸摩尔比为100:100:3为宜。
2.3 取代基对反应的影响
在n(芳醛):n(芳胺):n(对甲苯磺酸)为100:100:3的条件下,取代基类型对反应的影响如表3所示。
表3表明:芳香醛环上的取代基无论是给电子基还是吸电子基对缩合反应收率的影响都不大。可能是氨基氮上未共用电子对进攻羰基发生亲核加成反应。由于醛羰基碳的亲电能力较强,反应速率较快,故芳醛环上的取代基对反应的影响较小。苯胺环上邻、对位有强的吸电子基如硝基时,反应较难进行,即使研磨30~60 min,收率仍很低甚至不反应(见表3中Entry 5, 9, 13, 15, 18),其他反应的研磨时间均小于3 min。此外,反应残留物(3e, 3i, 3m, 3o)于60 ℃恒温干燥3 h,所获产品收率与室温静置24 h时的产率相近。
2.4 3种反应方法的比较
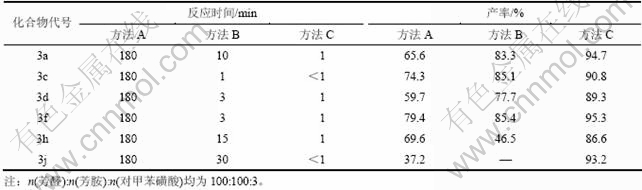
Schiff 碱的传统合成采用有机溶剂回流法,反应能耗高,操作时间长。目前,作为绿色化学合成的手段,研磨法在有机反应中得到广泛应用。在相同的反应条件下,溶剂回流法(方法A)、研磨法(方法B)和对甲苯磺酸催化研磨法(方法C)合成苯甲醛缩苯胺的反应时间和产率对比见表4。
表3 取代基对缩合反应的影响
Table 3 Effect of substituents on condensation
从表4可以看出:溶剂回流法合成目标产物需要回流3 h左右且收率不高;采用研磨法,只需在室温下研磨约10 min即可反应完全;加入对甲苯磺酸催化,反应时间可缩短至1 min,仅为常规方法的0.5 %,且产率明显提高。与常规方法相比,对甲苯磺酸催化研磨法具有反应时间短、条件温和、产率高、难以反应的化合物3j也可顺利进行等优势。因为分子在固态反应中通常被认为是运动的,研磨作用使固体颗粒减小,反应局部浓度增加,体系的总自由能增加,从而使体系活化并提高了反应速度和反应产率[17]。
对于芳香酮与芳香胺、芳香醛及氨基酸的反应,无论是延长研磨时间还是延长放置时间,均不能得到产物,说明这种方法不适用于芳香酮与芳香胺及芳香醛与氨基酸的缩合反应。
表4 部分Schiff碱类化合物产率和反应时间
Table 4 Yield of Schiff bases and reaction time
3 结论
(1) 室温固相研磨法合成Schiff碱类化合物的最佳工艺条件是:n(芳醛):n(芳胺):n(对甲苯磺酸)为100:100:3,研磨时间为1~3 min,收率可达86.5%~95.5%。
(2) 对甲苯磺酸催化研磨法明显优于溶剂回流法和常规固相研磨法,具有反应时间短,收率高,能耗低,甚至使难以直接缩合的Schiff碱顺利合成等优势。
(3) 芳香醛环上的取代基对反应无影响,但芳胺环上邻、对位上的强吸电子基抑制反应进行。
参考文献:
[1] 王美怡, 李正名, 李永红. 微波及超声波辅助合成新型苯环5位Schiff 碱取代苯磺酰脲类化合物及除草活性研究[J]. 有机化学, 2010, 30(6): 883-887.
WANG Mei-yi, LI Zheng-ming, LI Yong-hong. Microwave and ultrasound irradiation-assisted synthesis of novel 5-schiff base substituted benzenesulfonylurea compounds[J]. Chinese J Org Chem, 2010, 30(6): 883-887.
[2] 金晓晓, 王江涛, 白洁. 壳聚糖与肉桂醛的缩合反应制备席夫碱及其抑菌活性研究[J]. 高校化学工程学报, 2010, 24(4): 645-650.
JIN Xiao-xiao, WANG Jiang-tao, BAI Jie. Synthesis of Schiff base from chitosan and cinnamaldehyde and its antimicrobial activity[J]. J Chem Eng of Chinese Univ, 2010, 24(4): 645-650.
[3] Suresh P, Srimurugan S, Babu B, et al. Asymmetric sulfoxidation of prochiral sulfides using aminoalcohol derived chiral C3-symmetric trinuclear vanadium Schiff base complexes[J]. Tetrahedron: Asymmetry, 2007, 18(23): 2820-2827.
[4] LIU Hai-bin, WANG Mei, WANG Ying, et al. Influence of substituents in the salicylaldehyde-derived Schiff bases onvanadium-catalyzed asymmetric oxidation of sulfides[J]. Appl Organomet Chem, 2008, 22(5): 253-257.
[5] 巩春侠, 魏小平, 李建平. 水杨醛缩邻苯二胺双席夫碱敏感膜Cu(Ⅱ)离子选择性电极及SCN-的测定[J]. 应用化学, 2010, 27(8): 950-954.
GONG Chun-xia, WEI Xiao-ping, LI Jian-ping. Fabrication and application in SCN~-detection of copper(Ⅱ) ionic selective electrode based on salicylaldehyde-1,2-phenylenediamine sensitive membrane[J]. Chinese J Appl Chem, 2010, 27(8): 950-954.
[6] 程青芳, 许兴友, 王启发, 等. 新型吡唑Schiff 碱及金属配合物的合成和抑菌活性[J]. 有机化学, 2009, 29(9): 1387-1391.
CHENG Qing-fang, XU Xing-you, WANG Qi-fa, et al. Synthesis and antibacterial activities of novel pyrazole schiff[J]. Chinese J Org Chem, 2009, 29(9): 1387-1391.
[7] Anchal K, Shipra B. Microwave promoted synthesis of some schiff bases[J]. Archives of Applied Science Research, 2010, 2(3): 221-224.
[8] 王春, 张英群, 李敬慈, 等. 取代芳基的室温研磨合成及其紫外可见吸收光谱的研究[J]. 有机化学, 2005, 25(9): 1135-1137.
WANG Chun, ZHANG Ying-qun, LI Jing-ci, et al. Study of the synthesis of substituted aromatic schiff base at room temperature under grinding and their ultraviolet spectral absorption[J]. Chinese J Org Chem, 2005, 25(9): 1135-1137.
[9] 周益民, 叶向荣, 忻新泉. 无溶剂合成希夫碱. CN 1220988A[P]. 1999-06-30.
ZHOU Yi-min, YE Xiang-rong, XIN Xin-quan. Solvent-free synthesis of Schiff bases. CN 1220988A[P]. 1999-06-30.
[10] 李贵深, 王春, 李敬慈. 芳香醛与噻唑酮衍生物的固相缩合反应[J]. 应用化学, 2004, 21(10): 1069-1071.
LI Gui-shen, WANG Chun, LI Jing-ci. Solid state condensation of aromatic aldehydes and thiazolineone derivatives[J]. Chinese J Appl Chem, 2004, 21(10): 1069-1071.
[11] 刘锦贵, 邓林, 党珊. 取代-3-甲酰色酮与(硫代)巴比妥酸的固相缩合反应[J]. 合成化学, 2008, 16(1): 93-95.
LIU Jin-gui, DENG Lin, DANG Shan. Solid phase condensation between substituted 3-formyl chromone and barbituric acid or thiobarbituric acid[J]. Chinese J Syn Chem, 2008, 16(1): 93-95.
[12] 李云辉, 李海燕, 潘利华, 等. 4,4’-二溴-6,6’-二( N,N-二(乙氧基羰甲基氨甲基)-2, 2’-联吡啶的合成与表征[J]. 应用化学, 2010, 27(9): 1008-1011.
LI Yun-hui, LI Hai-yan, PAN Li-hua, et al. Preparation and Characterization of 4,4’-Dibromo-6,6’-bis(N,N-bis (ethoxycarbonylmethyl) amino methyl)-2,2’-bipyridine[J]. Chinese J Appl Chem, 2010, 27(9): 1008-1011.
[13] 李记太, 孙明轩, 何根业. 对甲苯磺酸催化研磨法合成5-芳亚甲基巴比妥酸[J]. 有机化学, 2011, 31(1) 123-125.
LI Ji-tai, SUN Ming-xuan, HE Gen-ye. Synthesis of 5-arylmethylene barbituric Acid Catalyzed by p-Toluene Sulfonic Acid Using Grinding Method[J]. Chinese J Org Chem, 2011, 31(1) 123-125.
[14] 陈兴, 祁楠, 程侣柏. 希夫碱系化合物的合成及SHG效应[J]. 大连理工大学学报, 1994, 34(1): 31-37.
CHEN Xing, QI Nan, CHENG Lü-bai. Synthesis of Schiff bases organic compounds and investigation of SHG activity[J]. J Dalian Uinv of Technology 1994, 34(1): 31-37.
[15] 田作霖, 张新. Schiff碱化合物的合成及IR, 1HNMR波谱性质[J]. 长春光学精密机械学院学报, 1995, 18(4): 42-45.
TIAN Zuo-lin, ZHANG Xin. Syntheses and IR, H-NMR spectra of schiff base compounds[J]. J Changchun Institute of Optics and Fine Mechanic, 1995, 18(4): 42-45.
[16] 彭海静. 苯甲醛缩苯胺衍生物的合成与晶体化学研究[D]. 苏州: 苏州大学材料与化学化工学部, 2011: 5.
PENG Hai-jing. Synthesis and study of crystal chemistry on benzylidene-aniline derivatives[D]. Suzhou: Suzhou University. School of Material, Chemistry and Chemical Engineering, 2011: 5.
[17] Singh N B, Singh R J, Singh N P. Organic solid state reactivity[ J] . Tetrahedron, 1994, 50(22): 6441-6493.
(编辑 陈灿华)
收稿日期:2011-05-20;修回日期:2011-07-28
基金项目:国家自然科学基金资助项目(51074069);湖南省科技计划项目(2009SK4027)
通信作者:文瑞明(1964-),男,湖南益阳人,教授,从事有机合成研究;电话:0737-6353218;E-mail:wenruiming@sohu.com