
Corrosion and high-temperature oxidation of AM60 magnesium alloy
LU Sheng(芦笙)1, ZHOU Xiao-yan(周晓燕)1, CHEN Jing(陈静)2, HOU Zhi-dan(侯志丹)1
1. Department of Materials Science and Engineering,
Jiangsu University of Science and Technology, Zhenjiang 212003, China;
2. Department of Mechanical Engineering,
Jiangsu University of Science and Technology, Zhenjiang 212003, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The corrosion and high-temperature oxidation characteristics of AM60 Mg alloy in different environments were investigated by immersion test, electrochemical polarizing analysis and differential thermal analysis (DTA)/thermo gravimetric (TG) experiments. The influence of aging heat treatment on the corrosion resistance of AM60 Mg alloy was studied. AM60 Mg alloy shows better corrosion resistance in sea water than in 3.5%(mass fraction) NaCl solution. The corrosion resistance after aging for 24 h is better than that of both as-cast and aging for 48 h. Corrosion resistance of Mg alloy is controled by microstructure, composition of α-matrix. Precipitation of β phase along the grain boundaries acts as a barrier that decreases corrosion rate, whereas the decrease of aluminum content of α phase causes an increase in the corrosion rate. The DTA and TG curves of heating process in air are characterized with combustion after 590 ℃. When heated in helium, the curves show two endothermic peaks and a remarkable evaporation of magnesium. As for isothermal DTA and TG experiments, mass increment caused by oxidation does not happen till 520 ℃.
Key words: AM60 magnesium alloy; corrosion; oxidation; microstructure; aging
1 Introduction
Magnesium alloys have been widely used in aerospace, automotive and electronics industries due to their unique properties, such as low density, good damping capacity and ease of manufacturing. In fact, the application of magnesium is further extended in cars to replace steel/cast iron components. Other than the automotive use of magnesium alloys, their ease in making castings with thin walls has made them attractive for applications in consumer electronics, mobile phones, and laptops[1-2].
It is also well known that magnesium is very active and its alloys are lack of corrosion resistance, resulting in the restricted application of unprotected magnesium alloys to exposure environment[3-4]. Magnesium alloys corrode easily when small amount of aggressive ions like chloride anions are present in the service medium[5-6]. Moreover, with the increase of oxidation temperature, magnesium oxide film changes into porous structure and thus loses its protective properties for oxidation[7-8]. Understanding the corrosion and oxidation characteristics of different Mg alloys is necessary and of industrial interest.
The corrosion resistance and oxidation characteristics of magnesium alloys have been widely reported[9-10]. Most of the researches have focused on pure magnesium and its alloys such as AZ91 and AZ31 magnesium alloys[11-12]. Though AM60 Mg alloy is also frequently found in application of automobile and 3C industries, but little work has been done on its corrosion and oxidation behavior.
In the present study, AM60 Mg alloys were investigated by immersion test and electrochemical method as well as thermo gravimetric (TG) method and differential thermal analysis (DTA). By changing immersion medium, aging temperature and oxidation temperature, the influences of aging treatment, medium and atmosphere temperature on corrosion and oxidation behavior of AM60 Mg alloy were studied.
2 Experimental
2.1 Corrosion experiment
Permanent mould cast AM60 ingot was used in this work. The samples were cut into coupons with certain dimensions by electrical discharge machine and then aged at 160 ℃ for 24 and 48 h, respectively. These as-cast and aged samples were prepared for immersion corrosion and electrochemical test. Samples were polished with 1.5 μm diamond paste and etched in a 10% nitric acid ethanol solution for microstructure observation with a MM6 optical microscope.
Two types of immersion mediums, man-made sea water and 3.5%NaCl solution were used for corrosion test at ambient temperature. For each condition, three replicated coupons were taken for test with 21 mm×17 mm×3 mm in size. The man-made sea water had a pH value around 6.5-7.5, which was prepared with demineralised water and consisted of 15 g/L NaCl, 1.2 g/L CaCl2, 15 g/L MgCl2?6H2O, 3 g/L MgSO4. The immersion test was taken for 24 h. After that, the corroded specimens were immersed in a chromate acid (200 g/L CrO3+10 g/L AgNO3 for 5 min at ambient temperature to remove the surface corrosion products and then were washed by flowing water, cleaned with acetone and absolute alcohol respectively for 2 min and cold dried again.
The mass loss was subsequently measured by an electronic balance with a precision of ±0.1 mg. The corrosion rate (g/(m2?h)) was calculated according to the difference (Δm) between the original mass (m0) and the final mass(mo).
The electrochemical experiment of polarization curves was conducted in a conventional three-electrode electrochemical cell with a Pt electrode as the counter and a saturated calomel electrode as the reference. The experimental samples were used as the working electrodes with dimensions of 10 mm×10 mm×3 mm. The working electrode was welded with copper wire and then sealed with epoxy with a 10 mm×10 mm unsealed area exposed for measurement. A Par M283 electrochemical measurement system and a M325 constant potential device were used to record the polarization curves in 3.5%NaCl solution and man-made sea water by potentiodynamic method. The polarization started from a cathodic potential of -300 mV to the corrosion potential and stopped at an anodic potential of 300 mV to the corrosion potential. The scanning rate was controled at 1.0 mV/s.
2.2 Oxidation experiment
As-cast AM60 samples (around 8 mg) were used to conduct the oxidation test by a set of Perkineler instrument, Pyris Diamond thermogravimetric/ differential thermal analyzer (TG/DTA).Two types of TG/DTA experiments were carried out to measure the oxidation characteristics of as-AM60 Mg alloy. The first one was taken during a heating process in helium and air atmosphere respectively with heating rate of 50 ℃/min and gas flowing rate of 500 mL/min. The second one was isothermal TG/DTA experiment with the sample exposed at 200, 250, 290, 330, 380, 430, 480 and 520 ℃ for 1 h, respectively. The gas flowing rate was also controled at 500 mL/min. The heating rate before reaching the isothermal condition was 100 ℃/min. By analyzing the characteristics of TG and DTA, the oxidation dynamics was investigated and discussed.
3 Results and discussion
3.1 Microstructure
As seen in Fig.1(a), the microstructure of the as-cast AM60 alloy is mainly composed of primary α-Mg matrix and little eutectic β phase(Mg17Al12).
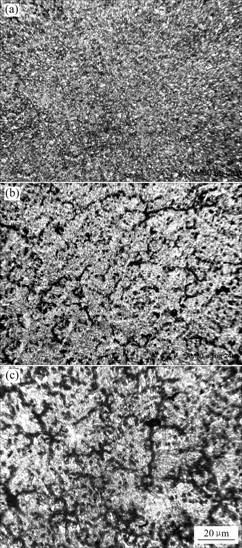
Fig.1 Microstructures of different samples: (a) As-cast; (b) Aged at 160 ℃ for 24 h; (c) Aged at 160 ℃ for 48 h
According to the Mg-Al binary diagram, AM60 Mg alloy is in the (α+β) region at 160 ℃. During the aging process at 160 ℃, precipitation of β phase takes place in continuous and discontinuous ways. At the initial stage, precipitation occurs along the grain boundaries so that the amount of β phase increases to form a continuous network. With extended aging, rod-like β phase precipitates toward the grain interiors gradually. After holding long enough at the aging temperature, the precipitation process of β phase slows down due to the decrease of the Al content among the α-matrix and the movement of rod-like β phase toward grain interiors. The microstructure changes of AM60 Mg alloy during aging are shown in Figs.1(b) and (c). Compared with the sample aged for 24 h, there are more network β phases precipitated along the grain boundaries and rod-like β phase in interior grains after aged for 48 h.
3.2 Corrosion rate
During immersion tests, it was observed that bubbles emerged from the AM 60 Mg alloy specimens. The corrosion surface looked like honeycomb in 3.5%NaCl solution while pitting appeared to be the main corrosion model in man-made sea water. The influence of ageing time on corrosion rate is shown in Fig.2. It is obvious that AM60 Mg alloy shows good corrosion resistance in man-made sea water. On the other hand, AM60 alloy corroded markedly when exposed to 3.5%NaCl solution. It may result from the fact that more chloride ions are present in 3.5%NaCl solution than in sea water. With strong abilities of penetration and hydration adsorption, chloride ions accelerate the quick movement of metal cations toward the medium, thus leading to an increase of corrosion rate.
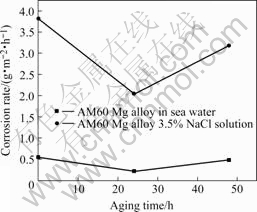
Fig.2 Corrosion rate in sea water and 3.5%NaCl solution
3.3 Polarization curves
The corrosion process of magnesium and its alloys was dominated by the galvanic corrosion. In neutral and alkaline media, magnesium acts as anodes by losing electrons that transfer to cathodes to produce hydrogen. The electrochemical parameters obtained from polarization curves are shown in Table 1.
Table 1 Data from polarizing curves
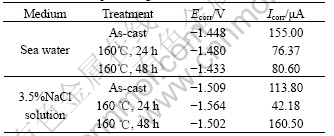
It is indicated that AM60 Mg alloy has more negative free-corrosion potential, Ecorr, in 3.5%NaCl solution than in sea water. The more serious negation the potential, the more corrosive the metal or alloy. This is consistent with results of the immersion test and well explains the fact that the corrosion resistance of magnesium alloys in sea water is better than that in 3.5%NaCl solution.
From Table 1, it can be found that, among three treatment conditions for AM60 Mg alloy, the electrochemical corrosion current, Icorr, shows the lowest value when aged for 24 h. All these data are consistent with the results of immersion test.
LUNDER et al[13] have compared the corrosion resistance of the as-cast AZ91 Mg alloy in states of solution heat treated (T4), and solution heat treated and then aged (T6) and the related the corrosion behaviors with the amount of β phase. SONG et al[14] have considered that the barrier effect of β phase only dominates the corrosion process when β precipitates are finely distributed, building up a certain degree of continuity in the barriers. From Fig.1, it can be found that the amount and continuity of β phase increase with aging time. For AM60 phase, at the initial stage of aging from 0 to 24 h, the decrease of corrosion rate can be interpreted by the barrier effect of β phase. After aging for 48 h, the fact that the corrosion rate increases again could not be explained just by such a barrier effect. According to the barrier mechanism, the corrosion process should be retarded because more β phase appears in the microstructure. To get better understanding about the aforesaid phenomena, it should be noted that the composition of α-matrix plays a critical role in the corrosion resistance of magnesium alloys. It has been reported that the corrosion rate of α phase decreases with the increase of the concentration of Al[15-16]. As the aging time extends, it is believed that β phase (Mg17Al12) will precipitate, resulting in a decrease of Al level in α phase.
To conclude the result from the corrosion experiment for AM60 Mg alloy, ageing effect on corrosion shows two adverse tendencies. As the aging prolongs, β phase precipitates along grain boundaries to enhance the barrier effect of β phase. On the other hand, the Al concentration of α matrix decreases, thus weakens the corrosion resistance. Therefore, the corrosion rate depends on the integrated influence of β barriers and the Al content in α matrix. For AM60 Mg alloy, the concentration of Al is about 6.11%(mass fraction), which leads to less amount of β phase in as-cast samples. After aged for 24 h, with more amount of β phase distributed along the grain boundaries, the barrier effect of β phase exceeds the negative influence of less concentration of Al in α matrix and the corrosion resistance gets improved. This has been proved by immersion test and polarization analysis in this work. When aged for 48 h, on the contrary, the remarkable decrease of Al in α matrix dominates the corrosion process and the sample aged for 48 h corrodes more easily than samples as-cast and aged for 24 h.
3.4 High-temperature oxidation
Figs.3 (a) and (b) show the TG curves of AM60 Mg alloy during the heating process in air and helium atmosphere, respectively. It seems that there is a slight trend of mass loss before temperature reaches 590 ℃ in air. After that TG curve goes up and combustion takes place. A number of white MgO particles were observed after the experiment.
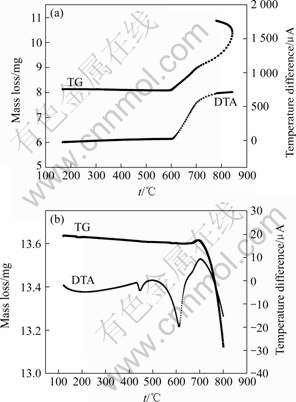
Fig.3 TG and DTA curves of AM60 Mg alloy during heating process in air (a) and helium atmosphere (b)
The TG and DTA curves in helium atmosphere in Fig.3(b) are different from those in Fig.3(a). Although mass loss also appears before around 600 ℃, a small endothermic peak can be found at around 440 ℃. Another large endothermic peak appears at 630 ℃. According to the Mg-Al binary diagram, the latter is the melting endothermic peak, while the former is consistent with the eutectic temperature, a mass gain in the heating period from 640 to 700 ℃, a mass loss follows a mass increment. The mass increment phenomenon can be explained by the combustion of magnesium due to the existence of little air in the open system. As temperature goes higher, magnesium vapor gets away, resulting in mass loss.
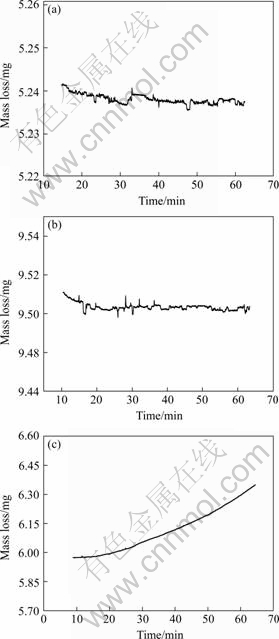
Fig.4 TG curves of isothermal experiment for AM60 Mg alloy held at (a) 330 ℃, (b) 430 ℃ and (c) 520 ℃
The typical isothermal TG curves of AM60 Mg alloy in air are shown in Fig.4. When held at 200 and 250 ℃, nothing happens in the curves. Fig.4 (a) indicates that while held at 290 and 330 ℃, a slight mass loss occurs due to the evaporation of magnesium. For the isothermal experiments at 380, 430 and 480 ℃, TG curves tend to smooth again because of the balance of oxidation and evaporation. It is very interesting that the mass increment from oxidation happens till 520 ℃.
4 Conclusions
1) The corrosion resistances of AM60 Mg alloy in sea water is superior to that in 3.5%NaCl solution. The corrosion rate of sample aged for 24 h is lower than that of samples as-cast and aged for 48 h.
2) Ageing treatment, shows two adverse corrosion effects for magnesium alloys. On the one hand, β phase precipitates along grain boundaries, which enhances the barrier effect of β phase. On the other hand, the Al concentration of α matrix decreases, thus weakens the corrosion resistance. Therefore the corrosion performance of AM60 Mg alloy depends on the integrated influence of β barriers and the Al content in α matrix.
3) The DTA and TG curves of heating process in air are characterized with combustion after 590 ℃. While heating in helium, the curves show two endothermic peaks and a remarkable evaporation of magnesium.
4) From isothermal DTA and TG curves, mass increment resulting from oxidation happens till 520 ℃.
References
[1] ZENG R C. Recent development and application of magnesium alloys[J]. Acta Metallurgic Sinica, 2001, 37(7): 673-685.
[2] THARUMARAJAH A, KOLTUN P. Is there an environmental advantage of using magnesium components for light-weighting cars?[J]. Journal of Cleaner Production, 2007, 15: 1007-1013.
[3] CHEN J, WANG J, HAN E, KE W. In-situ observation of formation and spreading of micro-droplets on magnesium and its alloy under cyclic wet-dry conditions[J]. Corrosion Science, 2007, 49: 1625-1634.
[4] MCINTYRE N S, CHEN C. Role of impurities on Mg surfaces under ambient exposure conditions[J]. Corrosion Science, 1998, 40(10): 1697-1709.
[5] LINDSTROM R, JOHANSSON L G, SVENSSON J E. The influence of NaCl and CO2 on the atmospheric corrosion of magnesium alloy AZ91[J]. Materials and Corrosion, 2003, 54: 587-594.
[6] CHEN J, WANG J, HAN E H. AC impedance spectroscopy study of the corrosion behavior of an AZ91 magnesium alloy in 0.1M sodium sulfate solution[J]. Electrochimica Acta, 2007, 52: 3299-3309.
[7] SHIH T S, LIU J B, WEI P S. Oxide films on magnesium and magnesium alloys[J]. Materials Chemistry and Physics, 2007, 104: 497-504.
[8] YOU B S, PARK W W, CHUNG I S. The effect of calcium additions on the oxidation behavior in magnesium alloys[J]. Scripta Mater, 2000, 42: 1089-1094.
[9] STAROSTIN M, TAMIR S. New engine coolant for corrosion protection of magnesium alloys[J]. Materials and Corrosion, 2006, 57(4): 345-348.
[10] BLAWERT C, DIETZEL W, E GHALI, SONG G. Anodizing treatments for magnesium alloys and their effect on corrosion resistance in various environments[J]. Advanced Engineering Materials, 2006, 8(6): 511-533.
[11] MATHIEU S, RAPIN C, STEINMETZ J. A corrosion study of the main constituent phases of AZ91 magnesium alloys[J]. Corrosion Science, 2003, 45: 2741-2755.
[12] SONG G, STRENS S. Understanding magnesium corrosion—a frame work for improved alloy performance[J]. Advanced Engineering Materials, 2003, 5(12): 837-858.
[13] LUNDER O, VIDEM M, NISANCIOGLU K. Corrosion resistant magnesium alloys[J]. Mater Manufacturing, 1995, 104: 352-357.
[14] SONG G, ATRENS A, Dargusch M. Influence of microstructure on the corrosion of die cast AZ91 D[J]. Corrosion Science, 1999, 41: 249-273.
[15] AMBAT R, AUNG N N, ZHOU W. Evaluation of microstructural effects on corrosion behavior of AZ91D magnesium alloy[J]. Corrosion Science, 2000, 42: 1433-1455.
[16] SONG G, BOWLES A L, St JOHN D H. Corrosion resistance of aged die cast magnesium alloy AZ91D[J]. Mater Sci Eng A, 2004, A366: 74-86.
Foundation item: Project (JSAWT-06-08) supported by the Key Laboratory of Advanced Welding Technology of Jiangsu Province, China
Corresponding author: LU Sheng; Tel: +86-511-84407569; E-mail: lusheng119@yahoo.com.cn
(Edited by CHEN Wei-ping)