DOI:10.19476/j.ysxb.1004.0609.2018.06.20
盐酸体系中辉锑矿的臭氧协同氧化浸出动力学
田庆华1, 2, 3,王浩1,王恒利1,辛云涛1,郭学益1, 2, 3
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 有色金属资源循环利用湖南省重点实验室,长沙 410083;
3. 中南大学 有色金属资源循环利用湖南省工程研究中心,长沙 410083)
摘 要:采用动力学研究的方法对盐酸体系中辉锑矿的臭氧协同氧化浸出过程进行了研究,分别探索了温度、盐酸浓度、气体流量和搅拌速度等因素对该过程中锑浸出率的影响;在此基础上,对该浸出过程进行动力学计算。结果表明:在温度75 ℃、盐酸浓度4.50 mol/L、气体流量2.0 L/min和搅拌速度500 r/min的条件下浸出50 min后,锑浸出率可以达到59.13 %。辉锑矿的臭氧协同氧化浸出符合收缩核模型,过程反应速率受混合过程控制,计算得到表观活化能为15.98 kJ/mol,并最终建立了该浸出过程的动力学总方程式。
关键词:盐酸;辉锑矿;臭氧;浸出;动力学
文章编号:1004-0609(2018)-06-1242-08 中图分类号:TF818 文献标志码:A
我国的锑资源十分丰富,锑的储量、产量均居世界首位[1]。工业上,从硫化锑矿中提取金属锑的方法主要有火法和湿法。传统火法炼锑主要包括鼓风炉挥发熔炼生产三氧化二锑和反射炉还原熔炼生产粗锑两个工序[2]。但是火法炼锑过程中会产生SO2,严重污染环境。近些年来,研究者将低温碱性熔炼技术应用到锑的提炼中[3],有效地减少了SO2的排放,但是,该方法因成本问题,目前还没有在工业上得以推广应用。湿法炼锑又分为碱法与酸法。碱性湿法炼锑是一种在工业上可以实现的冶炼方法,美国,澳大利亚和国内等都建立了相关工艺的冶炼厂[4]。碱法炼锑通过硫化锑与碱性介质反应生成易溶的亚硫代锑酸钠,从而达到浸出锑的目的[5-7]。该工艺锑回收率高,污染小,但是其生产成本比较高[6]。酸法炼锑包括氯化法[8]、新氯化法[9-10]和氯化锑氧化法[11]。由于酸法工艺具有产品(锑白)用量大、后续处理方便等优点,得到了众多研究者的关注。
臭氧是一种绿色强氧化剂,在水中的氧化还原电位(2.07 V)仅次于氟而居第二位,可以氧化大多数的金属及其化合物[12]。冶金工作者将臭氧引入湿法冶金的硫化物浸出和氧化物制备工艺中。李青翠等[13]开展了臭氧氧化浸出黄铁矿型金矿和氰化尾渣的系统研究。湛雪辉等[14]探索了盐酸体系中,臭氧和过氧化氢联合浸出方铅矿精矿的工艺。UKASIK等[12]研究在盐酸体系中利用臭氧氧化浸出黝铜矿的方法。本文作者[15-16]研究了臭氧氧化沉淀溶液中二价钴离子制备四氧化三钴的工艺。但是,在现有研究中将臭氧应用于辉锑矿的氧化浸出过程的报道还比较少。
在硫化物浸出动力学方面,国内外的研究者做了大量的工作。薛娟琴等[17]研究了超声波辅助下的硫化镍矿氧化浸出动力学。徐志峰等[18]分析了硫化锌精矿常压直接浸出动力学。李宏煦等[19]探索了生物因素对次生硫化铜矿堆浸过程动力学的影响。QIU等[20]研究了低温低氧压条件下黄铜矿的氧化浸出动力学过程。HAVLIK等[21]研究了在硫酸体系中臭氧氧化浸出黄铜矿动力学。但是,至今还没有学者对盐酸体系中臭氧氧化浸出辉锑矿动力学进行分析。
本文作者在现有研究基础上,将臭氧/氧气作为协同氧化剂引入辉锑矿浸出工艺中,探索了温度、盐酸浓度、气体流量和搅拌速度对锑浸出率的影响,并对这些工艺条件下的动力学进行分析,为高效提取辉锑矿中的锑提供理论基础和技术指导。
1 实验
1.1 实验原料
实验所用辉锑矿来自于湖南某冶炼厂,在进行动力学实验前,对其进行研磨及筛分。本实验所采用的辉锑矿的粒度<74 μm,对其进行ICP、XRF分析,结果见表1。对辉锑矿精矿进行物相分析,其XRD分析结果见图1。由图1可知,辉锑矿精矿中主要物质为辉锑矿、黄铁矿和石英等。
表1 辉锑矿的主要化学成分
Table 1 Main chemical composition of stibnite concentrate (mass fraction,%)

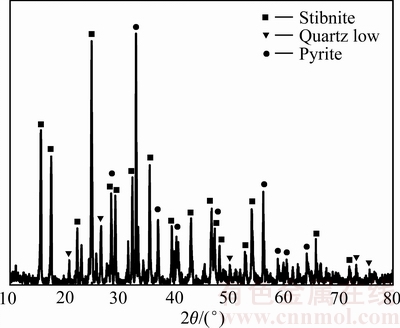
图1 辉锑矿精矿的XRD谱
Fig. 1 XRD pattern of stibnite concentrate
1.2 实验方法
本实验通过恒温磁力搅拌器加热,浸出反应在一个800 mL的烧杯中进行。配制一定浓度的盐酸400 mL,水浴加热到设定温度,然后称取4.00 g的辉锑矿加入烧杯中,向溶液中持续通入臭氧气体。实验过程中使用磁力搅拌器进行搅拌并控制搅拌速度,利用冷凝回流管减少溶液的挥发,反应时间50min,浸出过程中每10 min取一次样,稀释后对其进行ICP分析。实验结束后,进行液固分离,浸出液用量筒量取体积,浸出渣在70 ℃下烘干12 h,然后分别进行XRD、ICP及XRF分析,并由下式计算锑的浸出率:
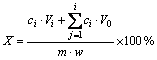 (1)
(1)
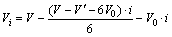 (2)
(2)
式中:X为锑的浸出率;i表示取样次数;ci为第i次浸出液中锑的浓度;V为浸出液初始体积;Vi为第i次浸出液的体积;V0为每次取样体积;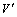 为浸出后液体积;m为加入的辉锑矿的质量;w为矿样中锑的质量分数,%。
为浸出后液体积;m为加入的辉锑矿的质量;w为矿样中锑的质量分数,%。
2 结果与讨论
2.1 温度对锑浸出行为的影响及锑的动力学模型
在盐酸浓度为4.50 mol/L、气体流量为2.0 L/min、搅拌速度500 r/min、温度分别为35、45、55、65、75和85 ℃的条件下,进行臭氧氧化浸出辉锑矿的实验,结果如图2所示。由图2可知,当温度从35 ℃升高到85 ℃时,浸出50 min后,锑浸出率从37.02%提高到65.38%,升高温度对辉锑矿浸出过程具有明显的促进作用。这是因为温度不仅与阿伦尼乌斯公式中的速率常数有关,而且还会影响反应物及生成物在溶液中的扩散。
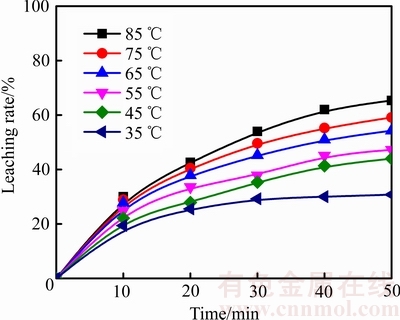
图2 不同温度下浸出率与时间的关系
Fig. 2 Relationships between leaching rate and time at different temperatures
由浸出渣的XRD谱(见图3)和ICP分析结果(见表2)可知:浸出渣主要物相包括单质硫、黄铁矿、辉锑矿和石英等;由SEM像(见图4)可以看出,矿物中的物相分布,单质硫作为固体产物,部分包裹在矿物表面,也有部分散乱分布。与原料成分进行对比,发现辉锑矿的含量大幅降低,黄铁矿也有少量参与反应;浸出过程中有单质硫生产并稳定存在。以硫酸高铈对浸出液进行滴定[22],测定其中锑的价态,发现浸出液中锑主要以三价锑离子形式存在。所以,在本实验过程中辉锑矿发生的主要化学反应为
Sb2S3+6HCl+3O3=2SbCl3+3S+3O2+3H2O (3)
表2 浸出渣的主要化学成分
Table 2 Main chemical composition of leaching residue (mass fraction,%)

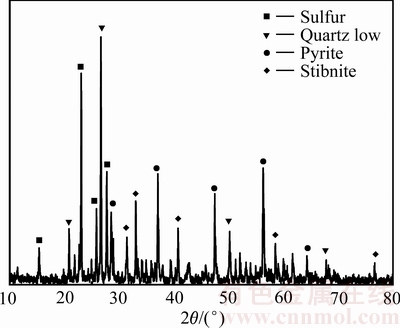
图3 浸出渣的XRD谱
Fig. 3 XRD pattern of leaching residue
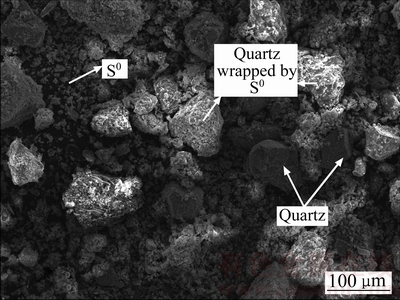
图4 浸出渣的SEM像
Fig. 4 SEM image of leaching residue
由上述分析可知,臭氧氧化浸出辉锑矿是气-液-固多相反应。根据湿法冶金动力学中收缩核模型的相关理论可知,反应过程由反应物与生成物在液相中的外扩散、反应物与生成物在固相中的内扩散和反应物之间的界面化学反应等3个步骤组成,这3个步骤是连续进行的,浸出过程的总反应速率取决于最慢的环节,即控制步骤[23]。
由于在实验过程中,采用机械搅拌对浸出过程进行强化,液相扩散层的厚度减少,反应物和生成物在液相中的扩散非常迅速,在浸出过程中生成的固相膜层是阻止物质扩散的主要因素。综上所述,本反应的控制步骤可能是内扩散控制和界面化学反应控制。经验方程式如下[24]:其中式(4)表示化学反应控制,式(5)表示内扩散控制,式(6)表示混合过程控制,其中x为浸出率,ki为速率常数,t为反应时间,β为混合过程控制中两种控制步骤参与总控制的相关系数。
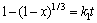 (4)
(4)
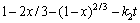 (5)
(5)
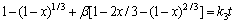 (6)
(6)
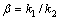
为了确定锑浸出过程的动力学参数和控制步骤,根据图2,将不同温度下所得到的锑浸出率x对时间t分别进行式(4)、(5)、(6)线性拟合,结果显示式(6)的线性相关系数较好,表明混合扩散控制可能适合于锑浸出。拟合曲线如图5所示,由此可得到不同温度时的k3。
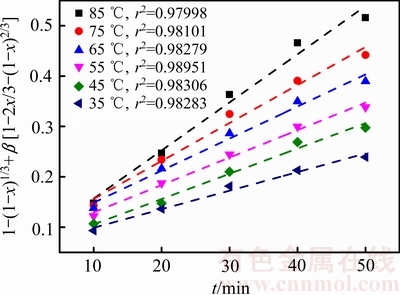
图5 不同温度下1-(1-x)1/3+β[1-2x/3-(1-x)2/3]与时间的关系
Fig. 5 Relationships between 1-(1-x)1/3+β[1-2x/3-(1-x)2/3] and time at different temperatures
根据阿伦尼乌斯方程,k可表示为
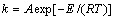 (7)
(7)
式中:k是反应速率常数;A为频率因子;E为表观活化能;R为摩尔气体常数;T为反应温度。为了计算反应表观活化能,两边同时取自然对数,得到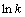 与1/T的关系式如式(8),所得拟合直线的斜率经过换算可得反应过程表观活化能。
与1/T的关系式如式(8),所得拟合直线的斜率经过换算可得反应过程表观活化能。
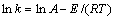 (8)
(8)
以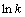 对1/T作图,结果见图6。
对1/T作图,结果见图6。
根据图5中所得ki值,通过拟合可得图6曲线,并根据图6中拟合曲线,得到速率常数(k, min-1)。结合阿仑尼乌斯公式可知,该反应的表观活化能为15.98 kJ/mol。一般的,扩散控制过程的表观活化能小于10 kJ/mol,化学反应控制过程的表观活化能则大于40 kJ/mol,混合过程控制的表观活化能介于两者之间。
综上所述,臭氧协同氧化浸出辉锑矿过程属于混合过程控制。
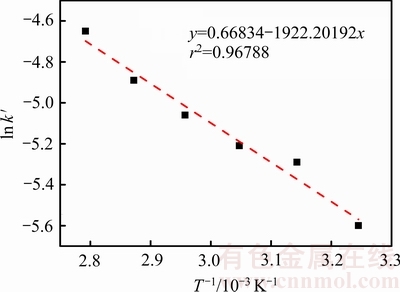
图6 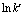 -
-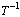 曲线
曲线
Fig. 6 Curve of 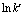 -
-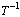
由图6并结合关系式(7)计算得到:频率因子A=1.95 s-1,表观活化能E=15.981 kJ/mol,所以臭氧氧化浸出辉锑矿的动力学方程为

 (9)
(9)
2.2 盐酸浓度、气体流量及搅拌速度对锑浸出行为的影响
2.2.1 盐酸浓度的影响
在温度为75 ℃、气体流量为2.0 L/min、搅拌速度500 r/min、盐酸浓度分别为3.00、3.75、4.50和5.25 mol/L的条件下,探索盐酸浓度对锑浸出率的影响,结果如图7所示。由图7可知,盐酸浓度是影响锑浸出的一个重要因素,锑浸出率随着盐酸浓度的增大而升高。这是因为当浸出剂体积一定时,提高浸出剂浓度,辉锑矿颗粒周围的盐酸分子的量增大,盐酸与辉锑矿接触几率增大,从而达到促进浸出的效果。而且,随着盐酸浓度的提高,溶液中氯离子浓度升高,锑在溶液液中的溶解度增大[25],浸出过程也就更加容易发生。
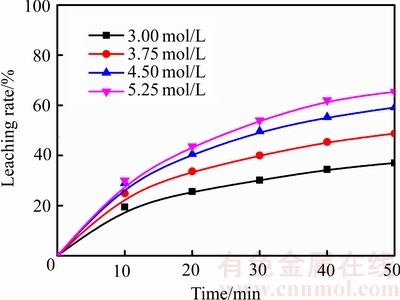
图7 不同盐酸浓度下浸出率与时间的关系
Fig. 7 Relationship between leaching rate and time at different hydrochloric acid concentrations
根据图7中浸出率x与时间t的关系,在浸出剂浓度 3.00~5.25 mol/L的条件下进行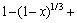
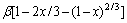 与时间进行线性拟合,得到不同浸出条件下
与时间进行线性拟合,得到不同浸出条件下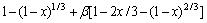 与时间的关系(见图8)。
与时间的关系(见图8)。
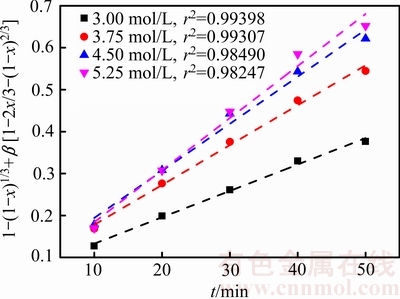
图8 不同盐酸浓度下1-(1-x)1/3+β[1-2x/3-(1-x)2/3]与时间的关系
Fig. 8 Relationships between 1-(1-x)1/3+β[1-2x/3-(1-x)2/3] and time at different hydrochloric acid concentrations
2.2.2 气体流量的影响
在温度为75 ℃、盐酸浓度为4.50 mol/L、搅拌速度500 r/min、气体流量分别为1.0、1.5、2.0和2.5 L/min的条件下,探索气体流量对锑浸出率的影响,结果如图9所示。由图9可知,当流量在1.0~2.0 L/min的范围内时,气体流量对辉锑矿的浸出过程有明显的促进作用,而当流量超过2.0 L/min后,继续增大气体流量对锑的浸出速率几乎没有影响。这是由于在一定程度内增大气体流量不仅使更多的气体参与反应,而且可以对浸出体系造成一定的搅动,增加浸出剂与辉锑矿接触几率,从而加快反应的进行。但是,当流量超过2.0 L/min后,臭氧等反应物之间的接触已经比较充分,所以,继续增大气体流量的效果不明显。
根据图9中浸出率x与时间t的关系,在气体流量 1.0~2.5 L/min的条件下进行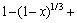
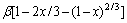 与时间线性拟合,得到不同浸出条件下
与时间线性拟合,得到不同浸出条件下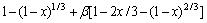 与时间的关系 (见图10)。
与时间的关系 (见图10)。
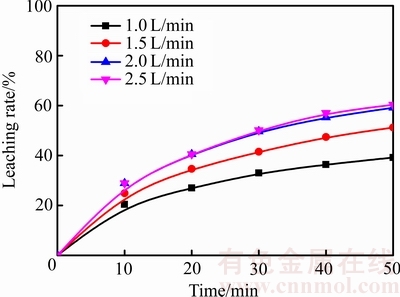
图9 不同气体流量下浸出率与时间的关系
Fig. 9 Relationship between leaching rate and time at different gas flow rates
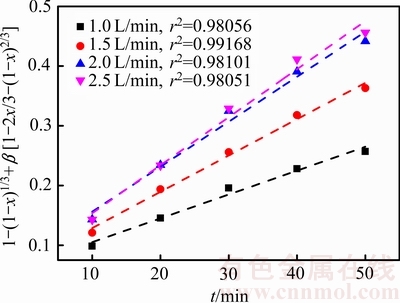
图10 不同气体流速下1-(1-x)1/3+β[1-2x/3-(1-x)2/3]与时间的关系
Fig. 10 Relationship between 1-(1-x)1/3+β[1-2x/3-(1-x)2/3] and time at different gas flow rates
2.2.3 搅拌速度的影响
在温度为75 ℃、盐酸浓度为4.50 mol/L、气体流量为2.0 L/min的条件下,考察了搅拌速度分别为300、400、500和600 r/min时对锑浸出率的影响,结果见图11。由图11可知,随着搅拌速度的加快,锑的浸出速率逐渐升高。但是当搅拌速度高于500 r/min后,继续加快搅拌速度,锑浸出率的变化将不明显。增大搅拌速度可以使反应物接触更充分,同时也有利于反应物从产物层向溶液中的扩散,所以在一定程度上可以加快锑的浸出过程。
根据图11中浸出率x与时间t的关系,在搅拌速度 300~600 r/min的条件下进行1-(1-x)1/3+ β[1-2x/3-(1-x)2/3]与时间线性拟合,得到不同浸出条件下1-(1-x)1/3+β[1-2x/3-(1-x)2/3]与时间的关系(见图12)。
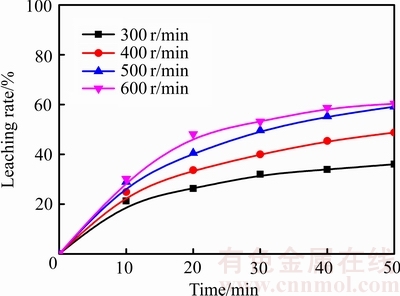
图11 不同搅拌速度下1-(1-x)1/3+β[1-2x/3-(1-x)2/3]与时间的关系
Fig. 11 Relationship between 1-(1-x)1/3+β[1-2x/3-(1-x)2/3] and time at different stirring speeds
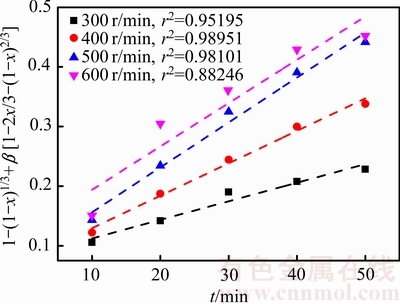
图12 不同搅拌速度下浸出率与时间的关系
Fig. 12 Relationship between leaching rate and time at different stirring speeds
2.2.4 动力学总方程式的建立
由图7~12可知,不同盐酸浓度、气体流量和搅拌速度条件下,臭氧氧化浸出辉锑矿过程都比较符合内扩散控制模型,而且该浸出反应的反应速率在一定条件下和浸出剂浓度c(3.00~5.25 mol/L)、气体流量Q(1.0~2.0 L/min)、搅拌速度v(300~600 r/min)都有关系。在考虑这3个影响因素的条件下,辉锑矿臭氧氧化浸出动力学总方程式可以表示为[26]
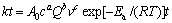 (10)
(10)
式中:A0为频率因子;a、b、c为常数。
根据式(10),在不同浸出条件下,分别进行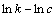 (3.00~5.25 mol/L),
(3.00~5.25 mol/L),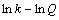 (1.0~2.0 L/min),
(1.0~2.0 L/min),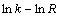 (300~600 r/min)线性拟合,其线性方程式与线性相关系数见表3。
(300~600 r/min)线性拟合,其线性方程式与线性相关系数见表3。
表3 不同条件下线性方程式和线性相关系数
Table 3 Linear equations and linear correlation coefficients at different conditions

由表3可知:a=2.28,b=0.78,c=1.29。对比式(7)与式(10),可知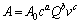 (c=4.50,Q=2.0,v=500 r/min),带入数值得到:A0=1.214×10-5 s-1,则该浸出反应的动力学总方程式为
(c=4.50,Q=2.0,v=500 r/min),带入数值得到:A0=1.214×10-5 s-1,则该浸出反应的动力学总方程式为

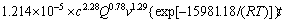 (11)
(11)
为了验证上述动力学总方程式的适用性,以1-(1-x)1/3+β[1-2x/3-(1-x)2/3]的实验结果对1-(1-x)1/3+ β[1-2x/3-(1-x)2/3]的理论计算值进行拟合,其结果见图13,图中横、纵坐标分别为动力学方程式的理论值和实际实验值。由图13可知,拟合直线的斜率及线性相关系数都接近1。因此,上述动力学总方程式适用于盐酸体系中辉锑矿臭氧氧化浸出过程。
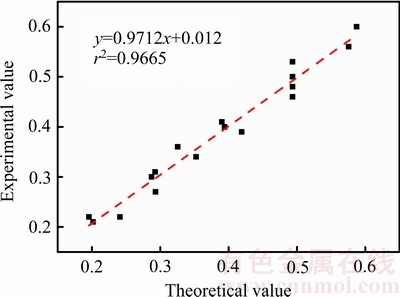
图13 动力学总方程式验证图
Fig. 13 Verification plot of overall dynamic equations
3 结论
1) 辉锑矿臭氧氧化浸出过程中,锑的浸出率与温度、浸出剂浓度、气体流量和搅拌速度有关,随着温度升高、浓度增大、流量增大(1.0~2.0 L/min)和搅拌速度增大(300~600 r/min),锑浸出速率增大。
2) 在温度75 ℃、盐酸浓度4.50 mol/L、气体流量2.0 L/min和搅拌速度500 r/min的条件下浸出50 min后,锑浸出率达到了59.13%。
3) 该浸出过程在25~75 ℃内符合收缩核模型,过程反应速率受混合扩散控制。该反应的表观活化能为15.98 kJ/mol,动力学总方程式为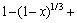
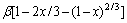 =
=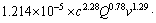
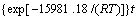 。
。
REFERENCES
[1] TIAN Qing-hua, WANG Heng-li, XIN Yu-tao, GUO Xue-yi. Effect of selected parameters on stibnite concentrates leaching by ozone[J]. Hydrometallurgy, 2016, 165: 295-299.
[2] 陈永明, 黄 潮, 唐谟堂, 姚维义, 唐朝波, 皮关华. 硫化锑精矿还原造锍熔炼一步炼锑[J]. 中国有色金属学报, 2005, 15(8): 1311-1316.
CHEN Yong-ming, HUANG Chao, TANG Mo-tang, YAO Wei-yi, TAO Chao-bo, PI Guan-hua. Production of antimony by directly reducing-matting smelting of stibnite concentrate[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(8): 1311-1316.
[3] 叶龙刚, 唐朝波, 唐谟堂, 杨建广, 陈永明, 杨声海, 何 静. 硫化锑精矿低温熔炼新工艺[J]. 中南大学学报(自然科学版), 2012, 43(9): 3338-3343.
YE Long-gang, TANG Chao-bo, TANG Mo-tang, YANG Jian-guang, CHEN Yong-ming, YANG Sheng-hai, HE Jing. Separation antimony from stibnite concentrate through a low temperature smelting[J]. Journal of Central South University (Science and Technology), 2012, 43(9): 3338-3343.
[4] ANDERSON C G. The metallurgy of antimony[J]. Chemie der Erde-Geochemistry, 2012, 72: 3-8.
[5] RASCHMAN P, SMINCAKOVA E. Kinetics of leaching of stibnite by mixed Na2S and NaOH solutions[J]. Hydrometallurgy, 2012, 113: 60-66.
[6] UBALDINI S, VEGLIO F, FORNARI P, ABBRUZZESE C. Process flow-sheet for gold and antimony recovery from stibnite[J]. Hydrometallurgy, 2000, 57(3): 187-199.
[7] 刘小文, 杨建广, 伍永田, 李 静, 唐朝波, 陈永明, 唐谟堂. 由辉锑矿低温固硫熔炼制取粗锑[J]. 中国有色金属学报, 2012, 22(10): 2896-2901.
LIU Xiao-wen,YANG Jian-guang,WU Yong-tian, LI Jing, TANG Chao-bo, CHEN Yong-ming, TANG Mo-tang. Separation of needle antimony from stibnite through low-temperature sulfur-fixing smelting process[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(10): 2896-2901.
[8] LOUTFY H M, IBRAHIM A S. Electrolytic recovery of antimony from nature stibnite ore[J]. Hydrometallurgy, 1996, 43(1/3): 265-275.
[9] GUDYANGA F P, MAHLANGU T, CHIFAMBA J, SIMBI D J. Reductive-oxidative pretreatment of a stibnite flotation concentrate: Thermodynamic and kinetic considerations[J]. Minerals Engineering, 1998, 11(6): 563-580.
[10] 唐谟堂, 赵天从, 鲁君乐. 新氯化-水解法的原理和应用[J]. 中南大学学报(自然科学版), 1992, 23(4): 405-411.
TANG Mo-tang, ZHAO Tian-cong, LU Jun-le. Principle and application of the new chlorination-hydrolization process[J]. Journal of Central South University(Science and Technology), 1992, 23(4): 405-411.
[11] YANG Jian-guang, WU Yong-tian. A hydrometallurgical process for the separation and recovery of antimony[J]. Hydrometallurgy, 2014, 143: 68-74.
[12] UKASIK M, HAVLIK T. Effect of selected parameters on tetrahedrite leaching by ozone[J]. Hydrometallurgy, 2005, 77(1): 139-145.
[13] LI Qing-cui, LI Deng-xin, QIAN Fang-jun. Pre-oxidation of high-sulfur and high-arsenic refractory gold concentrate by ozone and ferric ions in acidic media[J]. Hydrometallurgy, 2009, 97: 61-66.
[14] 湛雪辉, 李朝辉, 湛含辉, 李 飞, 曹 芬, 李 侠. 臭氧-过氧化氢联合浸出方铅矿[J]. 中南大学学报(自然科学版), 2012, 43(5): 1651-1655.
ZHAN Xue-hui, LI Zhao-hui, ZHAN Han-hui, LI Fei, CAO Fen, LI Xia. Combined leaching of galena by ozone-hydrogen peroxide solution[J]. Journal of Central South University (Science and Technology), 2012, 43(5): 1651-1655.
[15] TIAN Qing-hua, XIN Yun-tao, JIAO Cui-yan, GUO Xue-yi. Recovery of cobalt from cobalt chloride solution under the action of ozone[J]. Advanced Materials Research, 2012, 460: 317-320.
[16] TIAN Qing-hua, GUO Xue-yi, YI Yu, LI Zhi-hai. Kinetics of oxidation-precipitation of cobalt (Ⅱ) from solution by ozone[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 42-45.
[17] 薛娟琴, 毛维博, 卢 曦, 李京仙, 王玉洁, 吴 明. 超声波辅助硫化镍矿氧化浸出动力学[J]. 中国有色金属学报, 2010, 20(5): 1013-1020.
XUE Juan-qin, MAO Wei-bo, LU Xi, LI Jing-xian, WANG Yu-jie, WU Ming. Dynamics of ultrasound-assisted oxidation leaching of nickel sulfide concentrate[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(5): 1013-1020.
[18] 徐志峰, 朱 辉, 王成彦. 富氧硫酸体系中硫化锌精矿的常压直接浸出动力学[J]. 中国有色金属学报, 2013, 23(12): 3340-3347.
XU Zhi-feng, ZHU Hui, WANG Cheng-yan. Atmospheric direct leaching kinetics of zinc sulfide concentrate in oxygen-rich sulfuric acid system[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(12): 3340-3347.
[19] 李宏煦, 苍大强, 陈景河, 邱冠周. 生物因素对次生硫化铜矿堆浸过程动力学的影响[J]. 中国有色金属学报, 2007, 17(2): 331-335.
LI Hong-xu, CANG Da-qiang, CHEN Jing-he, QIU Guan-zhou. Effect of biological factors on heap bioleaching kinetics of secondary copper sulfide[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(2): 331-335.
[20] QIU Ting-sheng, NIE Guang-hua, WANG Jun-feng, CUI Li-feng. Kinetic process of oxidative leaching of chalcopyrite under low oxygen pressure and low temperature[J]. Transactions of Nonferrous Metals Society of China, 2007, 17(2): 418-422.
[21] HAVLIK T, DVORSCIKOVA J, LVANOVA Z, KAMMEL R. Sulphuric acid chalcopyrite leaching using ozone as oxidant[J]. Metall, 1999, 53(1): 57-60.
[22] 周宗侯, 廖 星. 溶液中锑铁物相分析的研究[J]. 冶金分析, 1990, 10(1): 29-32.
ZHOU Zong-hou, LIAO Xing. Investigation on chemical phase analysis of antimony and iron in the solution[J]. Metallurgical Analysis, 1990, 10(1): 29-32.
[23] 郑雅杰, 陈昆昆. 采用Na2SO3溶液从硒渣中选择性浸出Se及其动力学[J]. 中国有色金属学报, 2012, 22(2): 585-591.
ZHENG Ya-jie, CHEN Kun-kun. Selective leaching Se from selenium residue by Na2SO3 solutions and leaching kinetics[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(2): 585-591.
[24] AWE S A, SAMUELSSON C, SANDSTROM A. Dissolution kinetics of tetrahedrite mineral in alkaline sulphide media[J]. Hydrometallurgy, 2010, 103: 167-172.
[25] TIAN Qing-hua, WANG Heng-li, XIN Yun-tao, LI Dong, GUO Xue-yi. Ozonation leaching of a complex sulfidic antimony ore in hydrochloric acid solution[J]. Hydrometallurgy, 2016, 159: 126-131.
[26] ZHENG Ya-jie, CHEN Kun-kun. Leaching kinetics of selenium from selenium-tellurium-rich materials in sodium sulfite solutions[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 536-543.
Ozonation synergistic leaching kinetics of stibnite in system of hydrochloric acid
TIAN Qing-hua1, 2, 3, WANG Hao1, WANG Heng-li1, XIN Yun-tao1, GUO Xue-yi1, 2, 3
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Hunan Key Laboratory of Nonferrous Metal Resources Recycling, Central South University, Changsha 410083, China;
3. Hunan Engineering Research Center of Nonferrous Metal Resources Recycling, Central South University, Changsha 410083, China)
Abstract: A kinetic study method was used to study the synergistic oxidation and leaching of lanthanide with ozone in the hydrochloric acid system. The factors affecting the leaching rate such as temperature, concentration of hydrochloric acid, gas flow rate and stirring speed were investigated. On this basis, the kinetics of the leaching process was calculated. The results show that the antimony extraction is 59.13% after leaching for 50min at the conditions of temperature 75 ℃, hydrochloric acid concentration 4.50 mol/L, gas flow rate 2.0 L/min, and stirring speed 500 r/min. The process is consistent with the shrinkage model, the process reaction rate is controlled by the mixing process, the apparent activation energy calculated is 15.98 kJ/mol, and the general equation of dynamics of the leaching process is finally established.
Key words: hydrochloric acid; stibnite; ozone; leaching; kinetics
Foundation item: Project(51474257) supported by the National Natural Science Foundation of China
Received date: 2017-04-18; Accepted date: 2017-07-04
Corresponding author: GUO Xue-yi; Tel: +86-731-88876275; E mail: xyguo@csu.edu.cn
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(51474257)
收稿日期:2017-04-18;修订日期:2017-07-04
通信作者:郭学益,教授,博士;电话:0731-88876275;E mail: xyguo@csu.edu.cn