文章编号:1004-0609(2014)10-2684-08
双极膜电去离子技术处理模拟低浓度含镍废水
尚广浩1, 2,张贵清1, 2,高从堦1
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 难冶有色金属资源高效利用国家工程实验室,长沙 410083)
摘 要:采用双极膜电去离子技术(EDI-BP)处理低浓度模拟含镍废水,研究了Ni(OH)2沉淀产生的原因及消除措施。结果表明:沿膜器高度的电流密度分布不均匀;浓水室靠近产水出水端阴膜面产生的Ni(OH)2 沉淀由于局部Ni2+、OH-离子浓度过高造成。第一脱盐室进水端阳膜面产生的Ni(OH)2沉淀由水解离造成。采用降低原水pH、浓水pH等措施能够有效地避免沉淀的产生;在原水Ni2+浓度30 mg/L、流速0.317 cm/s、pH值2.77,浓水pH 1.18和电流密度9.5 mA/cm2的条件下进行浓缩试验,试验稳定运行285 h,得到的产水电导率约为1.5 μS/cm,产水中未检测出Ni2+离子,浓水中Ni2+浓度可达2.7 g/L,浓缩倍数达90倍。
关键词:电去离子;双极膜;镍;废水;沉淀
中图分类号:X758 文献标志码:A
Treatment of dilute Ni2+-containing wastewater by electrodeionization with bipolar membrane: Precipitation
SHANG Guang-hao1, 2, ZHANG Gui-qing1, 2, GAO Cong-jie1
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. National Engineering Laboratory for Efficient Utilization of Refractory Non-ferrous Metals Resources, Central South University, Changsha 410083, China)
Abstract: The generation and avoidance of Ni(OH)2 precipitation during the treatment of the simulated dilute Ni2+-containing wastewater were investigated by electrodeionization with bipolar membrane. The results show that, the current density distribution along the height of EDI-BP stack is uneven. The reason of Ni(OH)2 precipitates on the surface of anion membrane near the outlet of feed in concentrate compartment is high local concentration of Ni2+ and OH-. Ni(OH)2 precipitating on the surface of the cation membrane near the inlet of first dilute compartment due to the splitting of water. By lowering the pH value of concentrate and feed, the generation of Ni(OH)2 precipitate can be avoided. Under the condition of Ni2+ concentration, flowrate, pH value of feed, pH value of concentration and the current density are 30 mg/L, 0.317 cm/s, 2.77, 1.18 and 9.5 mA/cm2, respectively, the concentration experiment carries stably for 285 h. The diluate is Ni2+-free at the conductivity of 1.5 μS/cm, and the concentration of Ni2+ in concentrate reaches 2.7 g/L which is 90 times of that in feed.
Key words: electrodeionization; bipolar membrane; nickel; wastewater; precipitation
电镀、电池、电子等行业中,有大量低浓度重金属废水产生,其中重金属含量通常为几至几十mg/L。该类重金属废水具有离子浓度低,常规重金属废水处理放方法难于处理的特点[1-3],如化学沉淀法、电渗析法等均不适用,反渗透法也由于重金属浓度太低而在经济上不划算。目前,常用的离子交换法则存在生产不连续、再生使用酸碱会对环境造成二次污染等缺点。鉴于环境保护和资源综合利用要求日益提高,研究新的低浓度重金属废水处理方法具有重要意义。
电去离子技术(EDI)有机结合离子交换技术与电渗析技术的优点,使用过程中不产生二次污染,生产连续、能耗低[4]。自20世纪80年代末商业化运行以来,EDI技术在超纯水制备方面取得了极大的成功[5-11],众多研究者采用此技术研究各种EDI技术在处理低浓度重金属废水方面的应用情况[4, 12-21],如传统混床EDI(EDI-MB)和仅填充阳离子交换剂的EDI(EDI-CB)等。结果表明:各种结构的EDI膜堆均能实现废水中重金属离子的去除,并得到含重金属离子浓度较高的浓水回收液,但EDI-MB脱盐室中因混合填充了阴、阳离子交换树脂,一定条件下重金属离子会与OH-结合产生重金属氢氧化物沉淀,从而影响EDI过程的正常运行。同时,EDI-MB过程中,阴阳树脂的混合填充容易造成脱盐室中水解离生成的H+与OH-在非导电点的再结合,降低电流效率和除杂效果。EDI-CB过程虽可避免重金属氢氧化物沉淀的产生,获得高浓度的重金属离子浓缩液,但该过程不能除去废水中的阴离子,不能获得高品质的产水。
双极膜技术的发展为电渗析[22]与EDI[23-25]技术的应用提供了新的活力。张贵清等[26]在其制备超纯水的工作基础[5-7]之上提出了采用双极膜EDI(EDI-BP)处理低浓度重金属废水的设想:在该技术中,阳、阴离子交换树脂分置于双极膜两侧隔室,低浓度重金属废水依次通过阳离子脱盐室(第一脱盐室)和阴离子脱盐室(第二脱盐室),先后实现重金属阳离子和阴离子的去除;在直流电场作用下,水在双极膜中解离生成H+与OH-,从而实现脱盐室树脂的连续电再生,实验过程无须外加酸、碱。由于废水中的重金属阳离子在未接触阴离子交换树脂之前已在阳树脂室中预先除去,避免了金属阳离子与阴离子交换树脂的接触,控制适当条件就可以避免在树脂床中产生重金属氢氧化物沉淀。同时,EDI-BP结构中,阴阳树脂分置两个脱盐室,避免了双极膜水解离生成的H+与OH-在脱盐室中的再结合,因此,与EDI-MB相比,EDI-BP具有更高的电流效率和更小的树脂床电阻率;而与EDI-CB相比,不仅能得到高浓度的重金属离子浓缩液,还可以同时获得高品质的去离子水。
本文作者采用EDI-BP处理模拟低浓度含镍废水,对过程中重金属氢氧化物沉淀的产生原因进行分析,进而提出有效的沉淀消除措施;并在此基础上进行浓缩试验,可同时获得较高品质的去离子水与高倍的Ni2+离子浓缩液。
1 实验
1.1 EDI-BP结构与工作原理
试验采用的EDI-BP结构与工作原理如图1所示,膜堆电极采用了分段电极。阳、阴离子交换树脂分别置于双极膜两侧隔室,试验过程中低浓度含镍废水(原水)依次通过第一脱盐室和第二脱盐室。在第一脱盐室中,原水中的Ni2+离子不断吸附至阳离子交换树脂上,并在电场的作用下通过阳树脂和阳膜不断迁入浓水室;经过第一脱盐室酸化后的溶液流经第二脱盐室时,原水中的阴离子不断吸附至阴树脂上,并在电场的作用下通过阴树脂和阴膜不断迁入浓水室,从而实现原水中杂质阴离子的脱除和浓缩。在外加直流电场作用下,双极膜中水解离生成的H+与OH-分别透过阳、阴离子交换膜迁入第一脱盐室与第二脱盐室,并不断再生阳离子与阴离子交换树脂。当原水经过第一脱盐室酸化后流经第二脱盐室时,双极膜水解离产生的OH-与溶液中的H+中和。
理论上,原水流经第一脱盐室后,杂质阳离子将被完全脱除,在第二脱盐室出水端将获得完全脱盐的中性纯水。然而,实际处理低浓度重金属废水过程中,由于浓水室离子浓度较高,为了避免在浓水室生成重金属氢氧化物沉淀,浓水pH值一般控制较低。由于隔开浓水室与脱盐室的离子交换膜具有一定的离子渗透性,得到的产水水质一般不高。为了提高产水水质,试验采用的EDI-BP结构在浓水室填充阴离子交换树脂,从而减弱重金属离子在电场作用下向阴极侧迁移的趋势,使得通过阴离子交换树脂向阳极侧传递的OH-能够充分地被浓水中的H+中和,从而降低浓缩室中重金属离子浓度较高时产生氢氧化物沉淀的可能。
试验采用的EDI-BP结构在阴极室填充阴离子交换树脂,阳极室填充阳离子交换树脂,阴极室与浓缩室之间采用阴离子交换膜,阳极室与浓缩室之间采用阳离子交换膜。当极水室通入纯水时,电极反应产生的H+与OH-通过极水室的相应树脂快速迁入相邻浓水室,与浓水室中的OH-或H+中和;而浓水室中的重金属离子向阴极方向迁移时则被阴膜所阻挡,彻底解决了处理重金属废水过程中阴极室金属还原问题。
1.2 试剂与原料
模拟低浓度含镍废水(原水)和浓水均为六水硫酸镍(NiSO4·6H2O)溶解在二级反渗透水中配制而成,其中原水中含Ni2+ 30 mg/L;试验过程中按一定比例补入原水、浓水储槽硫酸(H2SO4)调整原水、浓水pH值;极水为二级反渗透水;二级反渗透水电导率为2.5 μS/cm;NiSO4·6H2O、H2SO4等均为分析纯试剂。
1.3 试验装置与操作方法
试验采用的EDI-BP系统装置各主要部件连接如图2所示。试验系统包括1对电极、1个EDI膜堆、2个400 L原水储槽、1个100 L极水储槽、1个20 L浓水储槽和1个20 L浓水补水槽。其中阴阳极板均采用钛镀铂电极,均等分为7块,每块电极面积20.3 cm2(2.9 cm×7cm),沿膜器高度电极间距为0.1 cm;EDI-BP膜堆隔板采用1 cm厚优质有机玻璃自制,有效膜面积147cm2(21cm ×7cm),隔板之间采用O型圈密封,各隔室溶液流向由下至上,可以减小进出水压力差,同时,有利于隔室内气体的排出(见图1);离子交换膜分别为日本ASTOM 生产的Neosepta BP-1双极膜、CMB阳膜与AHA阴膜[27],有效面积均为147 cm2 (21 cm×7 cm) ,使用前先用0.1 mol/L Na2SO4 溶液浸泡12 h,然后用去离子水冲洗干净备用;填充的离子交换树脂分别为Dowex 650C、Dowex 550A[28]、ZG D001 MB和ZG D201 MB[29],使用前根据不同需要转型为不同的离子型态,其中Na+型Dowex 650C树脂填充于第一脱盐室下部,H+型Dowex 650C树脂填充于第一脱盐室上部,OH-型Dowex 550A填充于第二脱盐室上部, 型Dowex 550A填充于浓水室及第二脱盐室下部,H+型ZG D001 MB树脂填充于阳极室,OH-型ZG D201 MB树脂填充于阴极室。
型Dowex 550A填充于浓水室及第二脱盐室下部,H+型ZG D001 MB树脂填充于阳极室,OH-型ZG D201 MB树脂填充于阴极室。

图1 基于分段电极的EDI-BP原理图
Fig. 1 Schematic diagram of segment-electrode-based EDI-BP

图2 EDI-BP试验装置连接图
Fig. 2 Schematic flow diagram of EDI-BP system
试验启动时浓水外排,以消除第一脱盐室填充的Na+型树脂再生后产生的Na+的干扰,待产水电导率下降至基本稳定后浓水循环;极水为去离子水,循环使用。为保持浓水在循环过程中浓度不变,Ni2+达到一定浓度后,采用蠕动泵以一定流速外排回收,且根据试验情况采用蠕动泵以一定流速向浓水槽补充一定低浓度H2SO4溶液(H2SO4+去离子水);原水依次通过两个脱盐室后得到的产水外排回收。试验过程中随时记录溶液pH值、电导率、流量、压力、电流与电压;每隔一段时间从浓水、产水中分别取样测定其中的Ni2+和 浓度。其中Ni2+浓度采用ICP检测(Intrepid II XSP Radial, thermo electron corporation),
浓度。其中Ni2+浓度采用ICP检测(Intrepid II XSP Radial, thermo electron corporation), 浓度采用瑞士万通产861型双抑制型离子色谱检测;溶液pH值、电导率、流量、压力、电流与电压均采用在线仪表检测,并同步输入电脑存储。
浓度采用瑞士万通产861型双抑制型离子色谱检测;溶液pH值、电导率、流量、压力、电流与电压均采用在线仪表检测,并同步输入电脑存储。
2 结果与分析
2.1 探索性试验
试验条件:原水、浓水、极水流速分别为0.317、0.159与0.397 cm/s,原水pH=6.22,浓水启动液为二级反渗透水,随着实验的运行浓水中镍离子浓度逐步升高,浓水室与脱盐室压差为34.5 kPa,电流密度10.2 mA/cm2,实验共运行50 h。
系统运行时原水电导率(μCF)、产水电导率(μCP)随运行时间变化曲线如图3所示。过程中,原水电导率为115μS/cm,产水电导率为1.1 μS/cm左右。实验运行约45 h后,浓水室靠产水出水端阴膜面出现蓝绿色Ni(OH)2沉淀。可见,当原水和浓水pH值均较高时,可以得到高品质的脱盐水,但较易在浓缩室产生Ni(OH)2沉淀而使得试验难于进行。
行室行透水I-MB and EDI-BP
2.2 浓水室沉淀的产生原因及其消除措施
2.2.1 浓水室沉淀产生原因分析
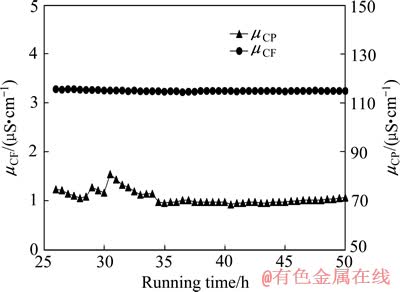
图3 原水与产水电导率随运行时间的变化
Fig. 3 Change of conductivity of feed and product with running time
Ni(OH)2沉淀的产生必然是Ni2+与OH-的离子积大于Ni(OH)2的溶度积造成。在图3所示的EDI-BP过程中,浓水室产生沉淀时浓水中Ni2+浓度小于1g/L,浓水pH值小于7,Ni2+与OH-的离子积小于1.7× 10-16。通过查询难溶化合物溶度积常数表可知,新生成Ni(OH)2的溶度积为2×10-15,理论上,试验条件下浓水室不应有Ni(OH)2沉淀生成。但实际上运行约45 h后,浓水室靠产水出水端阴膜面出现了蓝绿色Ni(OH)2沉淀,其位置如图4所示,该现象应与电流密度、Ni2+与OH-离子浓度在膜堆中的分布不均匀有关。
浓水室靠产水端阴膜面沉淀生成原理如图5所示。电场作用下,浓水中Ni2+具有向浓水室阴极侧迁移的趋势,进而造成浓水室Ni2+浓度自阴膜侧向阳膜侧逐步降低;同时,双极膜中的H2O解离生成OH-透过阴膜迁入浓水室时,由于浓水中H+的中和作用,浓水中OH-浓度自阴膜侧向阳膜侧逐步降低。
此外,Ni2+与阳离子交换树脂(膜)的亲和力较大,其在树脂中的电迁移性能较差,故负载重金属阳离子的阳树脂导电性能较差。由于负载Ni2+的阳树脂与负载H+的阳树脂导电性能相差巨大,因此,在EDI-BP过程中,沿EDI膜器高度的电流密度分布差别较大,若膜堆总电流不变,则脱盐室产水端的电流密度大于进水端的电流密度,透过阴膜迁入浓水室的OH-在阴膜表面的速率分布必然为脱盐室出水端高于进水端,造成浓水室接近阴膜侧pH值自脱盐室出水端向进水端逐步降低,自阴膜侧至阳膜侧逐渐降低。同理,接近阴膜面Ni2+浓度自脱盐室出水端向进水端逐步降低,自阴膜侧向阳膜侧逐渐降低。
因此,当浓水pH较高时,浓水中的H+不能及时中和透过阴膜迁入的OH-,当Ni2+与OH-的离子积大于Ni(OH)2溶度积时,在浓水室阴膜面产生Ni(OH)2沉淀,且沉淀容易在浓水室接近产水端产生。

图4 EDI-BP过程中Ni(OH)2沉淀出现位置
Fig. 4 Position of Ni(OH)2 precipitation appeared in EDI-BP process

图5 浓水室靠产水端阴膜面沉淀生成示意图
Fig. 5 Schematic diagram of Ni(OH)2 precipitation generated in concentrate chamber
2.2.2 浓水室沉淀的消除试验
根据浓水室沉淀产生的原因可以得出:降低浓水pH值或降低产水端电流密度有助于避免浓水室Ni(OH)2沉淀产生,本文作者采用降低浓水pH值的方法避免浓水室沉淀产生。
试验条件:原水、浓水、极水流速分别为0.198 、0.238和0.278 cm/s,原水pH=6.22,浓水中Ni2+浓度(670±30) mg/L,浓水pH=1.0左右,浓水室与脱盐室压差为98 kPa,电流密度16.0 mA/cm2,实验共运行279 h,膜堆电压(U)和产水电导率(μCF)随运行时间变化曲线如图6所示。在运行过程中,膜堆电压维持在17 V左右,产水电导率约为1.2 μS/cm。实验运行约270 h时,通过有机玻璃隔板观测,未发现浓缩室有沉淀产生。可见,降低浓水pH值后,透过阴膜迁入浓水室的OH-能够被浓水中的H+快速中和,有效地避免Ni(OH)2的生成。

图6 膜堆电压与产水电导率随运行时间的变化
Fig. 6 Changes of voltage of stack and conductivity of product with running time
2.3 脱盐室沉淀的产生原因及消除措施
2.3.1 第一脱盐室沉淀的产生原因分析
图6中所示的试验虽然成功避免了浓水室沉淀的产生,但经长时间运行后,第一脱盐室进水端接近阳膜表面生成新的Ni(OH)2沉淀(见图4中),最终导致实验无法进行。
由图6可以看出,第一脱盐室进水端阳膜表面Ni(OH)2沉淀的生成,导致脱盐室进水端电阻增加,膜堆总电阻相应增加,膜堆电压上升;脱盐室进水端导电能力进一步变差,沿膜器高度的电流分布差进一步加大,即进水端电流减小,出水端电流增大,出水端树脂的再生度提高,产水电导率下降。
分析第一脱盐室进水端阳膜面Ni(OH)2沉淀产生的原因,其原理如图7所示。第一脱盐室进水端,填充于隔室内的阳树脂逐步被原水中的Ni2+饱和。在较Ni2+与强酸强碱性阳树脂的亲和力比高pH值条件下的H+与阳树脂的亲和力大,故Ni2+在阳树脂上的电迁移性能也较H+的差,同时,负载Ni2+的阳树脂导电性能较负载H+的阳树脂导电性能差。第一脱盐室进水端阳树脂被Ni2+饱和时,阳树脂的导电性下降引起。此时,阳树脂的导电性与水溶液的导电性相比,差距不大,溶液和树脂的总导电性下降,溶液和树脂中的阳离子迁移速度不能满足电流迁移的需要。因此,在阳膜/阳树脂界面发生水的离解,故而在第一脱盐室进水端的树脂与阳膜的接触处有OH-产生,导致局部pH值升高,产生Ni(OH)2沉淀。该过程受到原水中Ni2+浓度及原水流量的影响,电流不变时,原水中Ni2+离子浓度越高,流速越快,第一脱盐室进水端靠阳膜面Ni(OH)2沉淀越易生成。
2.3.2 第一脱盐室沉淀的消除试验
根据离子交换平衡理论[30]及第一脱盐室进水端阳膜面沉淀产生的原因,可以通过降低第一脱盐室进水端阳树脂和膜中的Ni2+离子饱和度来增大阳树脂导电能力来避免浓差极化造成的水解离,避免Ni(OH)2沉淀生成。因此,可以在原水中加入在阳离子树脂上导电性较好、且不会生成氢氧化物沉淀的阳离子,通过加入阳离子的竞争吸附与传质作用,可以降低第一脱盐室进水端阳树脂与阳膜中的Ni2+离子饱和度,进而避免沉淀的生成,比如,提高原水中H+浓度以及降低原水pH值。
试验条件:原水、浓水、极水流速分别为0.238、0.238与0.278 cm/s,浓水中Ni2+浓度(550±50) mg/L,浓水pH=1.15左右,浓水室与脱盐室压差为78kPa,电流密度16.0mA/cm2,过程中控制原水中Ni2+浓度为30 mg/L不变,逐步减少原水中H2SO4 加入量,控制原水pH值分别为2.77、2.93、3.02与3.13。在不同原水pH条件下,实验共运行600 h。
膜堆电压(U)和产水电导率(μCF)随运行时间变化曲线如图8所示。由图8可看出,原水pH值由低到高变化,各pH值条件下分别运行约110~200 h。各隔室均未发现Ni(OH)2 沉淀产生,可见,降低原水pH值可以有效地避免脱盐室沉淀的产生。并在此过程中,膜堆电压稳定至14 V左右,产水电导率维持在5 μS/cm左右,产水中未检测出Ni2+。
2.4 典型条件下的EDI-BP浓缩试验
前述实验通过适当降低原水与浓水pH值可以有效地避免了Ni(OH)2沉淀的产生,但是存在产水电导率较高与浓水中Ni2+浓度较低的问题。因此,在避免沉淀产生的前提下,以获取优质产水和较高Ni2+浓缩倍数为目的进行了浓缩实验,实验过程中提高了产水室与浓缩室之间的压差,降低了膜堆电流,且适当提高了原水流量。

图7 第一脱盐室进水端阳膜面沉淀生成示意图
Fig. 7 Schematic diagram of Ni(OH)2 precipitation generated in first dilute chamber

图8 膜堆电压与产水电导率随运行时间的变化
Fig. 8 Changes of voltage of stack and conductivity of product and feed with running time
试验条件:原水、浓水、极水流速分别为0.317、0.317与0.357 cm/s,原水pH=2.77,浓水pH=1.18,浓水室与脱盐室压差为93 kPa,电流密度为13.6 mA/cm2,实验共运行285 h,膜堆电压约为20 V。
浓水中Ni2+浓度(ρNi)与产水电导率(μCP)随运行时间变化曲线如图9所示。在运行过程中,浓水中Ni2+浓度逐步升高并接近2.7 g/L,产水电导率约为1.5 μS/cm。
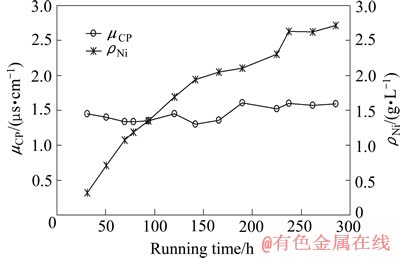
图9 膜堆电压、浓水中Ni2+浓度与产水电导率随运行时间的变化
Fig. 9 Changes of voltage of stack and concentration of Ni2+ in concentrate and conductivity of product with running time
对比实验参数接近时的产水电导率,图9中Ni2+浓度为2.7 g/L时,产水电导率为1.5 μS/cm;图8所示过程中pH=2.77时,产水电导率为5 μS/cm。二者脱盐室与浓水室压力差分别为93 kPa和78 kPa。在如图6所示的实验过程,产水电导率为1.2 μS/cm,脱盐室与浓水室压力差为98 kPa。可见,产水电导率随着压差的增大而降低,压差对产水电导率有着显著的影响,压差的增大有助于产水水质的提高。
实验过程中为保持浓水体积不变,采用蠕动泵定量排出浓水,当蠕动泵流量控制在88 mL/h时,浓水体积平衡,根据相关实验参数计算得出该实验浓水中Ni2+的理论浓度约为2.7 g/L,与原水比浓缩倍数高达90倍。实验进行至230 h时,浓水中Ni2+浓度接近理论值;继续运行约55 h后,各项参数稳定,未发现有沉淀产生。
3 结论
1) 采用EDI-BP技术处理模拟低浓度模拟含镍废水,对电解过程中沉淀的产生原因、影响因素和消除措施进行研究。结果表明:操作条件不当时,浓水室靠产水出水端阴膜面因为局部Ni2+离子与OH-离子浓度过高易生成Ni(OH)2沉淀,第一脱盐室进水端阳膜面因为水的解离而生成沉淀,二者均可以通过相应隔室溶液pH的降低得以避免。
2) 降低原水与浓水pH值,在原水Ni2+离子浓度30 mg/L、原水流速0.317 cm/s、原水pH=2.77、浓水pH=1.18和电流密度9.5 mA/cm2的条件下进行浓缩试验,试验稳定运行285 h,得到的产水电导率约为1.5 μS/cm,产水中未检测出Ni2+离子,且浓水中Ni2+离子浓度可达2.7 g/L,浓缩倍数达90倍。
REFERENCES
[1] 苗 雨, 闵小波, 柴立元, 尹一男. 铁基生物絮凝剂去除废水中的氟和铅[J]. 中国有色金属学报, 2012, 22(8): 2366-2373.
MIAO Yu, MIN Xiao-bo, CHAI Li-yuan, YIN Yi-nan. Romoval of fluoride and lead in wastewater with iron-bioflocculant[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(8): 2366-2373.
[2] 杨津津, 徐晓军, 王 刚, 王 盼, 韩振宇, 管堂珍, 田 蕊. 微电解絮凝耦合技术处理含重金属铅锌冶炼废水[J]. 中国有色金属学报, 2012(7): 2125-2132.
YANG Jin-jin, XU Xiao-jun, WANG Gang, WANG Pan, HAN Zhen-yu, GUAN Tang-zhen, TIAN Rui. Treatment of zinc and lead smelting wastewater containing heavy metals by combined process of micro-electrolysis with flocculation[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(7): 2125-2132.
[3] 钱 勇. 工业废水中重金属离子的常见处理方法[J]. 广州化工, 2011(5): 130-131, 138.
QIAN Yong. The common processing method of heavy metal ion in industrial wastewater[J]. Guangdong Chemical Industry, 2011(5): 130-131, 138.
[4] YEON K H, SONG J H, MOON S H. A study on stack configuration of continuous electrodeionisation for removal of heavy metal ions from the primary coolant of a nuclear power plant[J]. Water Research, 2004, 38(7): 1911-1921.
[5] ZHANG Gui-qing, GRABOWSKI A, STRATHMANN H, EIGENBERGER G. Production of high-purity water by a new scheme of CEDI with bipolar membrane[C]// International Congress on Membranes and Membrane Processes 2005, vol. II. Seoul: The Membrane Society of Korea, 2005: 1407-1408.
[6] GRABOWSKI A, ZHANG G, STRATHMANN H, EIGENBERGER G. The production of high purity water by continuous electrodeionisation with bipolar membranes: Influence of the anion-exchange membrane permselectivity[J]. Journal of Membrane Science, 2006, 281(1): 297-306.
[7] GRABOWSKI A, ZHANG G, STRATHMANN H, EIGENBERGER G. Production of high-purity water by continuous electrodeionisation with bipolar membranes: influence of concentrate and protection compartment[J]. Separation and Purification Technology, 2008, 60(1): 86-95.
[8] WOOD J, GIFFORD J, ARBA J, SHAW M. Production of ultrapure water by continuous electrodeionisation[J]. Desalination, 2010, 250(3): 973-976.
[9] ALMARZOOQI F A, ALGHAFERI A A, SAADAT I, HILAL N. Application of capacitive deionisation in water desalination: A review[J].Desalination,2014, 342: 3-15.
[10]  M. Various applications of electrodeionization (EDI) method for water treatment: A short review[J].Desalination,2014, 342: 16-22.
M. Various applications of electrodeionization (EDI) method for water treatment: A short review[J].Desalination,2014, 342: 16-22.
[11] ALVARADO L, CHEN A. Electrodeionization: Principles, strategies and applications[J].Electrochimica Acta,2014, 132: 583-597.
[12] DZYAZKO Y S, PONOMARYOVA L N, ROZHDESTVENSKAYA L M, VASILYUK S L, BELYAKOV V N. Electrodeionization of low-concentrated multicomponent Ni2+-containing solutions using organic-inorganic ion-exchanger[J].Desalination,2014, 342: 43-51.
[13] LU H, WANG J, BU S, FU L. Influence of resin particle size distribution on the performance of electrodeionisation process for Ni2+ removal from synthetic wastewater[J]. Separation Science and Technology, 2011, 46(3): 404-408.
[14]  M. Removal of Cu2+ ions by a micro-flow electrodeionisation (EDI) system[J]. Desalination, 2011, 277(1): 296-300.
M. Removal of Cu2+ ions by a micro-flow electrodeionisation (EDI) system[J]. Desalination, 2011, 277(1): 296-300.
[15] MAHMOUD A, HOADLEY A F A. An evaluation of a hybrid ion exchange electrodialysis process in the recovery of heavy metals from simulated dilute industrial wastewater[J]. Water Research, 2012, 46(10): 3364-3376.
[16] SPOOR P B, KOENE L, TER VEEN W R, JANSSEN L J J. Electrodeionisation 3: The removal of nickel ions from dilute solutions[J]. Journal of Applied Electrochemistry, 2002, 32(1): 1-10.
[17] SPOOR P B, KOENE L, TER VEEN W R, JANSSEN L J J. Continuous deionization of a dilute nickel solution[J]. Chemical Engineering Journal, 2002, 85(2): 127-135.
[18] SPOOR P B, GRABOVSKA L, KOENE L, JANSSEN L J J, TER VEEN W R.Pilot scale deionisation of a galvanic nickel solution using a hybrid ion-exchange/electrodialysis system[J]. Chemical Engineering Journal, 2002, 89(1): 193-202.
[19] FENG X, GAO J, WU Z. Removal of copper ions from electroplating rinse water using electrodeionisation[J]. Journal of Zhejiang University: Science A, 2008, 9(9): 1283-1287.
[20] XING Y, CHEN X, WANG D. Variable effects on the performance of continuous electrodeionisation for the removal of Cr(Ⅵ) from wastewater[J]. Separation and Purification Technology, 2009, 68(3): 357-362.
[21] LU H, WANG J, YAN B, BU S. Recovery of nickel ions from simulated electroplating rinse water by electrodeionisation process[J]. Water Science & Technology, 2010, 61(3): 729-735.
[22] 徐铜文, 何炳林. 双极膜—新的工业革命[J]. 世界科技研究与发展, 2000, 22(3): 19-27.
XU Tong-wen, HE Bing-lin. Bipolar membranes: Prospects and opportunities[J]. Dev Res World Sci Technol, 2000, 22(3): 19-27.
[23] PARSI E J. Apparatus for the removal of dissolved solids from liquids using bipolar membranes: U.S 4871431[P]. 1989-10-03.
[24] THATE S, SPECOGNA N, EIGENBERGER G. A comparison of different EDI concepts used for the production of high-purity water[J]. Ultrapure Water, 1999, 16: 42-57.
[25] THATE S, EIGENBERGER G. Untersuchung der elektro- chemischen deionisation zur reinstwasser-herstellung[J]. Vom Wasser, 2003, 101: 243-247.
[26] 张贵清, 肖连生, 张启修. 电去离子技术处理低浓度重金属废水的研究进展[C]// 第二届全国(中国)膜技术在冶金中应用研讨会论文集. 南京: 南京工业大学化学膜科学技术研究所, 2006: 161-167.
ZHANG Gui-qing, XIAO Lian-sheng, ZHANG Qi-xiu. Research progress about the treatment of dilute heavy metal wastewater using electrodeionisation[C]// The Second National (China) Symposiumon the Application of Membrane Technology in Metallurgy. Nanjing: Membrane Science & Technology Research Center, Nanjing University of Technology, 2006: 161-167.
[27] Products of Ion Exchange Membrane. ASTOM Corporation [EB/OL]. [2013-11]. Available: http://www.astom-corp.jp/ en/en-main2.html.
[28] Dow Water & Process Solutions. The Dow Chemical Company [EB/OL]. [2013-11]. Ailable: http://www.dowwaterandprocess. com
[29] 浙江争光实业股份有限公司. 离子交换树脂[EB/OL]. http://www.chinaresin.com/pro1/typeid/11.html. 2014-03-31.
Zhejiang Zhengguang Industrial Co., Ltd.Ion exchange resin [EB/OL]. [2014-03-31]. Ailable: http://www.chinaresin. com/ pro1/typeid/11.html.
[30] 张启修. 冶金分离科学与工程[M]. 北京: 科学出版社, 2004: 118-120.
ZHANG Qi-xiu. Metallurgical separation science and engineering[M]. Beijing: Science Press, 2004: 118-120.
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(20876177)
收稿日期:2013-03-31;修订日期:2014-08-25
通信作者:张贵清,副教授,博士;电话:0731-88830472;E-mail: gq_zhang@163.com