文章编号:1004-0609(2008)S1-0422-06
粗TiCl4铜丝塔除钒废水沉淀泥浆综合回收工艺
王学文1,张力萍1,肖连生1,张贵清1,袁继维2,龚仕成2
(1. 中南大学 冶金科学与工程学院,长沙410083;
2. 遵义钛业股份有限公司,遵义563004)
摘 要:介绍了一种粗TiCl4铜丝塔除钒废水沉淀泥浆综合回收新工艺。该工艺由沉淀泥浆自氧化、碱洗脱氯、脱氯渣一次酸浸生产硫酸铜、一次酸浸渣苏打焙烧提钒和提钒渣二次酸浸5个主要工序组成。实验结果表明,粗TiCl4铜丝塔除钒废水沉淀泥浆在空气中能自氧化。沉淀泥浆在空气中堆放1个月,接近90%的金属铜变成CuCl2·2H2O,Cu2Cl(OH)3和Cu2(OH)3Cl;这些铜的氯氧化合物在碱性溶液中容易转化成Cu(OH)2;在控制液固比4?1,pH值为 11,温度为80 ℃的条件下搅拌1 h,转化率达96%。当酸浸液的pH值为2.0~2.5时,Fe、V、Ti等杂质留在渣中,浸出液蒸发浓缩至密度为1.38 g/cm3,冷却结晶得到的硫酸铜产品符合国标GB437—93的质量要求。酸浸渣按化学计量的2.5倍加苏打后在700 ℃焙烧3 h,焙烧后按液固比3?1加水在70 ℃搅拌1 h浸钒,水浸液按化学计量的3倍加氯化铵沉淀偏钒酸铵,偏钒酸铵在550 ℃热解2 h得到纯度为98.61%的V2O5。提钒渣再经二次酸浸。整个工艺过程铜和钒的总回收率分别达到98.63%和95.65%。
关键词:粗TiCl4精制;铜丝;除钒;综合回收
中图分类号:TQ 134.1; TF 841 文献标识码:A
Comprehensive recovery of precipitate of wastewater in removing vanadium from raw TiCl4 with copper-wire
WANG Xue-wen1, ZHANG Li-ping1, XIAO Lian-sheng1, ZHANG Gui-qing1, YUAN Ji-wei2, GONG Shi-cheng2
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Zunyi Titanium Industry Co. Ltd, Zunyi 563004, China)
Abstract: A new method was presented to recover copper and vanadium from the precipitate formed in the waste water after vanadium removal from raw TiCl4 with copper-wire. The recovery process consists of five major procedures, namely, the self-oxidization of precipitate, the removal of chlorine with sodium hydroxide solution, the first-stage leaching of copper with sulphuric acid and recovering vanadium by roasting the first leached residue with sodium carbonate, the leaching with water and the leaching copper with sulphuric acid once again. It is found that the precipitate can self-oxidize in air. After stacking for one month in air, about 90% metallic copper contained in the precipitate turns into CuCl2·2H2O, Cu2Cl(OH)3 and Cu2(OH)3Cl. The copper oxychlorides can easily convert to Cu(OH)2, and the conversion rate is over 96% under liquid-to-solid ratio 4?1 and pH 11 at 80 ℃ by stirring for 1 h. When pH value is maintained in the range of 2.0-2.5 during the leaching of sulfuric acid, the impurities of Fe, Ti and V are remained in the leached residue. And then the leaching liquor is concentrated to 1.38 g/cm3 by evaporation and cooled to obtain the product of CuSO4×5H2O, which is in accordance with the standard of GB437─93. After adding sodium carbonate under the stoichiometric proportion of 2.5 the residue is roasted at 700 ℃ for 3 h. The calcined product is leached with water under liquid-to-solid ratio 3?1 at 70 ℃ by stirring for 1 h. NH4Cl is then added in the leaching liquor containing vanadium according to the stoichiometric proportion of 3 to obtain the precipitate of NH4VO3. When NH4VO3 is thermolysized at 550 ℃for 2 h, V2O5 with the purity grade of 98.61% is produced. After vanadium removal, the residue is leached once again with sulfuric acid. The total recoveries of copper and vanadium are 98.63% and 95.65%, respectively.
Key words: raw TiCl4 purifying; copper wire; vanadium removal; comprehensive recovery
TiCl4是钛冶金的一种重要中间产品,主要用于海绵钛和钛白的生产[1-2]。工业上多采用金红石或高钛渣加碳氯化制取TiCl4,这种方法得到的粗TiCl4含有FeCl3、AlCl3、TiOCl2、SiCl4、VOCl3、Cl2、HCl、VCl4等杂质[3]。粗TiCl4必须通过精制提纯后,才能用于海绵钛和钛白的生产。精馏可将粗TiCl4中的SiCl4、Cl2、HCl等低沸点杂质除去,蒸馏能分离FeCl3、TiOCl2等高沸点杂质,而VOCl3与TiCl4的蒸气压接近,只能采用化学法加以脱除[4]。常用的除钒方法有:硫化氢除钒法、有机物除钒法、铝粉除钒法和铜丝除钒法。硫化氢除钒法效果好,还原剂价格便宜,生产成本低,但工艺较复杂,且硫化氢属有毒气体,劳动条件差,我国工业上未采用。有机物除钒效果好,流程简单,生产成本低,但残渣黏度大,给后续工艺带来困难,这种方法目前在我国还处于试用阶段。铝粉除钒效果好,流程简单,生产成本低,但除钒渣处理比较困难,若对除钒过程形成的AlCl3分离不彻底将对产品质量有影响。铜丝除钒效果好,流程较简单,产品质量稳定,但要消耗大量价格高的铜,且清洗铜丝形成的废水对环境有污染,劳动强度大。铜丝除钒是我国独 有的一种除钒方法,它普遍应用于我国TiCl4生产企业[5-6]。
在除钒过程中铜丝参加还原反应,逐渐被消耗,形成VOCl2和CuCl覆盖在铜丝外表,使用一段时间后,铜丝的活性表面变小,除钒能力下降,此时为了确保TiCl4中钒含量不超标,必须用水冲洗铜丝表面的沉积物以恢复其还原活性[7]。冲洗铜丝塔得到的废水pH值为1.0~2.0。TiCl4生产企业一般是先将这种废水收集在地坑中加铁屑置换,然后定期清理地坑中的沉淀泥浆。收集得到的沉淀泥浆通常采用火法-湿法联合工艺回收其中的铜,而有价金属钒分散在烟气和炉渣中无法回收。沉淀泥浆中含有大量氯化物,在火法处理过程中氯化物大量挥发造成严重的环境污染。因此,本文作者针对粗TiCl4铜丝塔除钒废水沉淀泥浆处理工艺存在的问题,开发出沉淀泥浆经自氧化—铜钒分离—综合回收的处理新工艺,避免了因火法处理氯化物大量挥发对环境造成的污染,沉淀泥浆中的铜和钒都得到了有效回收,具有工艺简单、操作方便、金属回收率高、生产成本低、环境友好等优点。
1 实验
1.1 实验原料
实验原料为遵义钛厂粗TiCl4铜丝塔除钒废水经铁屑置换得到的沉淀泥浆,泥浆呈土灰色,其中含有大量铁屑置换下来的铜泥和碎铜丝。沉淀泥浆堆放在空气中逐渐变成绿色疏松粉状物,其主要化学成分如表1所示。
表1 沉淀泥浆自氧化后的化学成分
Table 1 Chemical compositions of precipitate after self- oxidization (mass fraction, %)

1.2 实验仪器和试剂
主要仪器:多磁头加热搅拌器(CJJ-931,金坛市晶玻实验仪器厂)、原子吸收光谱仪(AAnalyst100,美国)、pH计(Orion Model 410A,美国)。
试剂:氢氧化钠、碳酸钠、氯化铵、硫酸,均为分析纯。
1.3 实验方法
沉淀泥浆采用如图1所示的工艺处理,将地坑中掏出的泥浆自然渗干后,堆放在空气中,待其自然氧化变成疏松绿色粉状物后,加入NaOH控制溶液pH值大于11脱氯,加热搅拌。脱氯液用于中和铁屑置换液,脱氯渣按化学计量1.0~1.2倍加稀硫酸在70 ℃搅拌1 h浸铜,浸铜液浓缩至密度为1.38 g/cm3,冷却结晶得到硫酸铜晶体。硫酸铜结晶母液返回铁屑置换工序。酸浸渣按化学计量的2.5倍加苏打焙烧,焙烧料按液固比3?1水浸提钒,水浸液按化学计量的3倍加氯化铵沉淀偏钒酸铵,水浸渣酸溶再回收铜。

图1 沉淀泥浆综合回收工艺流程图
Fig.1 Flowsheet of vanadium and copper recovery from precipitate
1.4 分析方法
实验样品中钒含量的测定采用钒渣的标准分析方法,即硫酸亚铁铵滴定法,铜含量的分析用原子吸收分光光度法,氯离子浓度采用硝酸银溶液滴定法,溶液的pH值用pH计测定。
2 结果与讨论
2.1 沉淀泥浆自氧化
沉淀泥浆自然渗干后堆放在海拔约35 m,相对湿度为50%~80%,平均气温为15 ℃的空气中,1个月后表面形成大量绿色疏松的粉状物。图2所示是粉状物的X射线衍射的分析结果。从图2可以看出,这种粉状物的主要成分是CuCl2·2H2O、Cu2Cl(OH)3和Cu2(OH)3Cl。然而,虽然表1中,Ti含量接近7%,但X射线衍射分析未检测出钛的物相,这说明Ti(OH)2Cl2和TiO(OH)2都是无定型结构。
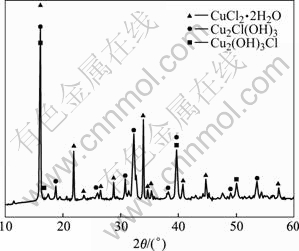
图2 沉淀泥浆自氧化后XRD谱
Fig. 2 XRD pattern of precipitate after self-oxidization
由表1可以看出,沉淀泥浆中含有大量的Ti(OH)2Cl2,当铜丝表面的沉积物用水冲洗时,其中残留的TiCl4水解生成Ti(OH)2Cl2和盐酸[6],CuCl歧化成Cu和CuCl2。随着铁屑置换的进行,废水的pH值降低,Ti(OH)2Cl2进一步水解或脱水生成Ti(OH)3Cl或TiOCl2。在潮湿的空气中,由于Cl-离子的参与,加速了泥浆中Cu的自氧化过程。

无论是CuCl2·2H2O还是Cu2Cl(OH)3和Cu2(OH)3- Cl在酸性溶液中都是可溶的,因此,铜的浸出率直接反映泥浆的自氧化程度。图3所示是沉淀泥浆中铜的浸出率与其自氧化时间的关系。由图3可以看出,沉淀泥浆从地坑中掏出后,随其在空气中堆放时间的延长,铜的浸出率迅速增高。当堆放时间达到1个月左右时,铜的浸出率达90%。继续延长沉淀泥浆在空气中堆放时间,铜的浸出率变化不大。这主要是CuCl2·2H2O,Cu2Cl(OH)3及Cu2(OH)3Cl的大量形成,阻碍了泥浆中未被氧化的金属铜与空气中的氧气和水分接触。实验发现,沉淀泥浆在空气中堆放1个月后,加硫酸浸出,浸出渣加入粗TiCl4铜丝塔除钒废水中浸泡后渗干,其中的金属铜在空气中自氧化速度也很慢,这可能是渣中残余的金属铜被TiO(OH)2和Fe2O3等包裹。

图3 沉淀泥浆自氧化时间与铜的浸出率的关系
Fig.3 Relationship between self-oxidization time of precipitate and copper leaching rate
2.2 自氧化泥浆碱洗脱氯
表1显示,沉淀泥浆中含有大量的Cl,直接用作生产硫酸铜的原料,不仅对产品质量有影响,而且会造成生产设备严重腐蚀。因此,在沉淀泥浆自氧化后,要先脱氯。脱除CuCl2·2H2O、Cu2Cl(OH)3及Cu2(OH)3Cl中的氯的最简便方法是碱洗:

自氧化后的沉淀泥浆脱氯在NaOH溶液中进行。实验结果表明,沉淀泥浆碱洗脱氯主要受液固比的影响。在80 ℃搅拌1 h,溶液pH值为11,碱洗脱氯液固比与自氧化泥浆脱氯效果的关系如图4所示。随着液固比增大,氯的脱除率迅速升高,当液固比增大到4时,氯的脱除率已接近97%,继续增大液固比,氯的脱除率变化不大。因此,沉淀泥浆碱洗脱氯的最佳工艺条件可确定为:液固比为4?1,pH值为11,在80 ℃搅拌1 h。

图4 液固比与脱氯效率的关系
Fig.4 Relationship between liquid-to-solid ratio and dechlorination rate
沉淀泥浆脱氯液用于中和铁屑置换后液的目的是,降低置换后溶液的pH值,减小V(IV)在溶液中的溶解度,提高有价金属钒的回收率。
2.3 铜与钒的综合回收
脱氯渣和提钒渣均按液固比4?1加入水和硫酸,80 ℃搅拌1.5 h浸出铜。pH值对脱氯渣铜浸出率的影响如图5所示。图5显示,酸浸溶液的pH值对铜的浸出率影响很大,随着溶液pH值的降低,铜的浸出率升高。当溶液pH值降至2.5时,铜的浸出率上升到88%,继续增大溶液的酸度,铜的浸出率增长缓慢。这是因为当溶液pH值为2.5时,脱氯渣中大部分铜被浸出,而Ti、Fe等杂质留在渣中,这些杂质对铜的浸出起阻碍作用[7-8]。要进一步提高铜的浸出率就得增大溶液的酸度,从而造成V、Fe等溶解,这不仅影响硫酸铜产品的质量,而且不利于钒的回收。因此,脱氯渣铜浸出液的pH值控制在2.0~2.5较为适宜。控制铜浸出液较高pH值的另一个原因是,一次酸浸渣提钒后还要进行二次酸浸(见图1),当二次酸浸液中游离酸控制在20~40 g/L H2SO4时,二次酸浸渣中Cu、V的含量分别可降至0.5%和0.3%以下[9-12]。当二次酸浸液返回用于铜的一次酸浸液,且pH值控制在2.0~2.5时,Cu留在溶液中,V则以铜钒化合物的形式进入一次酸浸渣中。整个工艺过程铜的总回收率达98.61%,钒的总回收率为95.65%。
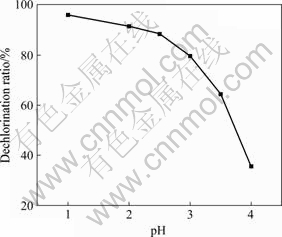
图5 pH值对铜浸出率的影响
Fig. 5 Effect of pH value on copper leaching rate
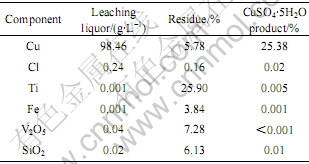
将脱氯渣回收铜按液固比4?1加入水和硫酸,在80 ℃控制溶液pH值为2.5,搅拌浸出1.5 h,浸出液浓缩至密度为1.38 g/cm3冷却结晶制备硫酸铜的实验结果如表2所示。从表2可以看出,沉淀泥浆经上述工艺得到的硫酸铜产品质量达到国家标准GB437─93。
表2 脱氯渣回收铜的实验结果
Table 2 Experimental results of copper recovery
由表2可以看出,沉淀泥浆经自氧化—碱洗脱氯—酸浸后钒富集在酸浸渣中。提取酸浸渣中的钒既 可采用氯化钠焙烧-水浸,也可采用碳酸钠焙烧-水浸[13-16]。由于氯化钠焙烧产生HCl等有害气体,实验选用碳酸钠焙烧-水浸工艺回收酸浸渣中的钒。向酸浸渣中按化学计量的2.5倍加苏打在700 ℃焙烧3 h,焙烧料按液固比3?1加水在70 ℃搅拌1 h,水浸液再按化学计量的3倍加氯化铵沉淀偏钒酸铵,偏钒酸铵在550 ℃热解2 h,实验结果如表3所示。表3显示,实验得到的V2O5的质量达到GB3283—87标准。
表3 酸浸渣提钒实验结果
Table 3 Experimental result of vanadium recovery

3 结论
1) 粗TiCl4塔除钒废水沉淀泥浆在空气中堆放1个月,不需要氧化焙烧,其中的金属铜接近90%转变成CuCl2·2H2O、Cu2Cl(OH)3和Cu2(OH)3Cl。沉淀泥浆在空气中自氧化既节能高效又清洁环保。
2) 沉淀泥浆自氧化后,在液固比为4?1,pH 值为11的碱性溶液中于80 ℃搅拌1 h,可将其中的氯氧化合物转化成Cu(OH)2。含Cu(OH)2的脱氯渣再 按液固比4?1在硫酸溶液中搅拌浸出,控制浸出终点pH 值为2.0~2.5,浸出液浓缩结晶可得到符合国标 GB437—93质量要求的硫酸铜产品。
3) 沉淀泥浆酸浸渣经苏打焙烧后,焙砂水浸,浸出液加氯化铵沉淀偏钒酸铵,偏钒酸铵再经热解能得到纯度在98.5%以上的V2O5产品。粗TiCl4铜丝塔除钒废水沉淀泥浆经空气自氧化、碱洗脱氯、酸浸回收铜、苏打焙烧提钒,其中的铜和钒都得到有效回收。
REFERENCES
[1] 王向东, 逯福生, 贾 翃, 郝 斌, 马云风. 2006年中国钛工业发展报告[J]. 钛工业进展, 2007, 23(2): 1-5.
WANG Xiang-dong, LU Fu-sheng, JIA Hong, HAO Bing, MA Yun-feng. Development report on titanium industry in 2006[J]. Titanium Industry Progress, 2007, 23(2): 1-5.
[2] 张 健, 吴 贤. 国内外海绵钛生产工艺现状[J]. 钛工业进展, 2006(2): 7-14.
ZHANG Jian, WU Xian. Development of process of sponge titanium[J]. Titanium Industry Progress, 2006(2): 7-14.
[3] 莫 未, 邓国珠, 罗方承. 钛冶金[M]. 第2版. 北京: 冶金工业出版社, 1998: 248-266.
MO Wei, DENG Guo-zhu, LUO Fang-cheng. Titanium metallurgy[M]. 2nd ed. Beijing: Metallurgical Industry Press, 1998: 248-266.
[4] 李亚军, 孙虎民, 许伟春. 粗四氯化钛除钒工艺现状及发展趋势[J]. 现代化工, 2007, 27(6): 24-26.
LI Ya-jun, SUN Hu-min, XU Wei-chun. Status of process for removing vanadium from tetrachloride titanium and its development trends[J]. Modern Chemical Industry, 2007, 27(6): 24-26.
[5] XU Cong. Preparation of TiCl4 with the titanium slag containing magnesia and calcia in a combined bed[J]. Chinese J Chem Eng, 2006, 14(3): 281-288.
[6] 李定元. 我国四氯化钛的铜丝除钒工艺[J]. 钛工业进展, 2000(4): 12-14.
LI Ding-yuan. Process of removal vanadium from raw titanium tetrachloride with copper wire in China[J]. Development for Titanium Industry, 2000(4): 12-14.
[7] 邓 彤, 文 震, 刘 东. 硫酸介质中氯化物参与下氧化浸出铜渣过程[J]. 中国有色金属学报, 2001, 11(2): 302-306.
DENG Tong, WEN Zhen, LIU Dong. Leaching of copper residue with oxygen in sulfuric acid with participation of chloride[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(2): 302-306.
[8] OWUSU G. Selective extraction of copper from acidic zinc sulfate leach solution using LIX622[J]. Hydrometallurgy, 1999, 51(1): 1-8.
[9] 烟 伟, 蔡万玲. 从混合铜矿混酸浸出液中除去Ca和Fe结晶制备CuSO4·5H2O工艺研究[J]. 湿法冶金, 2000, 29(l): 16-20.
YAN Wei, CAI Wang-ling. Study on removing Fe and Ca and crystallizing CuSO4·5H2O from leaching solution of mixed copper ore with mixed acid[J]. Hydrometallurgy of China, 2000, 29(l): 16-20.
[10] 刘承先. 含铜污泥中铜的回收及污泥无害化处理[J]. 辽宁化工, 2001(6): 248-249.
LIU Cheng-xian. Recovery of copper from copper-containing sludge and innocent treatment of sludge[J]. Liaoning Chemical Industry, 2001(6): 248-249.
[11] 曾青云, 杨 丹, 刘永平. 用低品位铜矿石浸出液制备硫酸铜溶液[J]. 湿法冶金, 2006, 25(3): 141-144.
ZENG Qing-yun, YANG Dan, LIU Yong-ping. Preparation of copper sulfate solution using leaching solution of low grade copper ore[J]. Hydrometallurgy of China, 2006, 25(3): 141-144.
[12] 何 耀. 利用废铜渣生产硫酸铜及回收有价金属的研究[J]. 有色冶炼, 1999, 28(4): 38-39.
HE Yao. Producing copper sulfate and recovering valuable metals from waste copper slag[J]. Non-Ferrous Smelting, 1999, 28(4): 38-39.
[13] 梁建龙, 刘慧娟, 史文革, 胡鄂明, 李熙琪, 彭 军. 湿法冶金提钒浸出新工艺[J]. 中国矿业, 2006, 15(7): 64-66.
LIANG Jian-long, LIU Hui-juan, SHI Wen-ge, HU E-ming, LI Xi-qi, PENG Jun. A study of a new technology leaching of vanadium from vanadium ores with hydrometallurgy[J]. China Mining Magazine, 2006, 15(7): 64-66.
[14] 张 萍, 蒋馥华. 苛化泥为焙烧添加剂从石煤提取五氧化二钒[J]. 稀有金属, 2000, 24(2): 115-118.
ZHANG Ping, JIANG Fu-hua. Extraction of vanadium pentoxide from stone coal by using causticizing mud as roasting additive[J]. Chinese Journal of Rare Metals, 2000, 24(2): 115-118.
[15] LOZANO L J, JUAN D. Leaching of vanadium from spent sulphuric acid catalysts[J]. Minerals Engineering, 2001, 14(5): 543-546.
[16] 刘公召, 隋智通. HDS失活催化剂焙烧提钒的动力学[J]. 中国有色金属学报, 2002 , 12(5): 1065-1068.
LIU Gong-zhao, SUI Zhi-tong. Kinetics of extracting vanadium from HDS spent catalyst by alkali leaching[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(5): 1065-1068.
通讯作者:张力萍,硕士研究生;电话:0731-8830143;E-mail: zhangliping0123@126.com
(编辑 杨 华)