DOI:10.19476/j.ysxb.1004.0609.2018.03.23
FeSO4-H2O体系中水热赤铁矿沉铁及亚稳态铁物相转变行为
李存兄,魏 昶,邓志敢,李兴彬,樊 刚,王益昭,易烁文,李旻廷
(昆明理工大学 冶金与能源工程学院,昆明 650093)
摘 要:赤铁矿渣由于铁含量高,存在极大潜力作为副产品销售给钢铁、水泥制造以及涂料行业,从而实现铁资源化利用,硫含量是影响赤铁矿品质并实现其资源化利用的重要因素之一。赤铁矿渣中硫主要以亚稳态的铁矾、SO42-的不可逆吸附以及碱式硫酸铁等形式存在,其中铁矾是硫的主要存在形态。FeSO4-H2O体系中Fe(II)氧化水热水解赤铁矿沉铁及过程亚稳态铁物相转化研究表明:温度是影响亚稳态铁矾物相形成和转化的关键因素,升高温度亚稳态铁矾的热力学稳定性变差,有利于其向赤铁矿转变。降低初始硫酸浓度、延长反应时间、添加晶种均会促进Fe(II)氧化水解,也有利于亚稳态铁矾物相向赤铁矿转变,从而获得铁含量高、硫含量低的高品质沉铁渣。在初始Fe2+浓度30 g/L、Na2SO4浓度0.15 mol/L、初始酸浓度1.6×10-4 mol/L、温度200 ℃、氧分压 400 kPa、反应时间180 min、搅拌转速500 r/min的优化技术参数下,Fe(II)氧化水热水解赤铁矿沉铁过程中沉铁率为95%,获得不含亚稳态铁矾的赤铁矿沉铁渣,其中铁、硫和钠硫含量分别为66.6%、0.92%和0.033%(质量分数)。
关键词:沉铁;Fe(II)氧化水解;亚稳态铁矾转变;赤铁矿;硫含量;水热冶金
文章编号:1004-0609(2018)-03-0628-09 中图分类号:TF813 文献标志码:A
长期以来,硫化锌精矿是提取金属锌的最主要原料。铁是硫化锌精矿中除硫以外含量最高且较难脱出的杂质,锌铁分离是锌冶炼的重要组成部分[1-5]。在湿法炼锌过程中为获得高质量阴极锌及高电流效率必须将浸出液中的铁离子除掉,高铁硫酸锌溶液沉铁过程中根据铁入渣的形态不同,分为黄钾铁矾法、针铁矿法和赤铁矿法。
尽管黄钾铁矾法和针铁矿在世界范围内被广泛采用,但生成的渣含铁量相对较低且各种杂质元素含量高,都不具备潜在的应用价值,且近年来随着环保、资源高效综合利用要求的提高和铁渣处理成本的上升,问题日益突出[6]。而采用赤铁矿法获得的赤铁矿渣稳定性强、渣铁含量高(55%~65%),存在极大潜力作为副产品销售给钢铁、水泥制造以及涂料行业,从而实现铁资源化利用。
赤铁矿法除铁是在溶液pH为2.0~4.5,高温(180~200 ℃)、氧压(1.2~2.0 MPa)的操作条件下,使溶液中的Fe(II)氧化水解后以赤铁矿形式沉淀,涉及的化学反应如下[7]:
Fe(II)的氧化水解,
2FeSO4+0.5O2+2H2O=Fe2O3+2H2SO4 (1)
反应式(1)主要由Fe(II)氧化和Fe(III)水解两部分组成:
2FeSO4+0.5O2+H2SO4=Fe2(SO4)3+H2O (2)
Fe2(SO4)3+3H2O=Fe2O3+3H2SO4 (3)
溶液中的H+浓度将决定Fe(III)水解产物的类型[8-11],低酸浓度下的水解产物是Fe2O3,中等酸浓度下水解产物是结晶水系碱式硫酸铁:
Fe2(SO4)3+4H2O=2Fe(OH)SO4·H2O+H2SO4 (4)
高酸浓度下水解产物是碱式硫酸铁,发生如下反应:
Fe2(SO4)3+2H2O=2FeOHSO4+H2SO4 (5)
当溶液中有Na+、K+、NH 4 +等离子存在时生成铁矾:
M2SO4+3Fe2(SO4)3+12H2O=2MFe3(SO4)2(OH)6+6H2SO4(M=K, Na, NH4) (6)
日本秋田冶炼厂是目前全球唯一采用赤铁矿除铁的湿法炼锌企业,据研究报道[12-13],该厂将产生的赤铁矿渣全部作为副产品销售给水泥厂,实现了赤铁矿渣的资源化利用。由于该赤铁矿渣中一般含有2%~4%的硫,阻碍了其作为炼铁资源在钢铁企业资源化应用。
研究表明[14-16]:赤铁矿渣中硫的存在形态主要有3种:铁矾、SO42-的不可逆吸附以及碱式硫酸铁,而其中超过50%的硫以铁矾的形式存在。DUTRIZAC等[17-18]研究表明:在一定条件下,135~185 ℃的温度范围内,铁矾按如下反应转化成赤铁矿:
2MFe3(SO4)2(OH)6=3Fe2O3+M2SO4+3H2O+3H2SO4(M=K, Na, NH4) (7)
适当控制反应条件可抑制铁矾形成或使其向赤铁矿转变,从而降低赤铁矿渣中硫含量,获得高品质沉铁渣。因此,本文作者研究了FeSO4-H2O体系中Fe(II)氧化水热水解赤铁矿沉铁及过程亚稳态铁物相-铁矾的转化行为,从而获得低硫含量的高品质赤铁矿渣。
1 实验
1.1 实验原料
本实验中所用硫酸亚铁高铁酸性溶液是用分析纯七水硫酸亚铁(FeSO4·7H2O)和硫酸钠(Na2SO4)按一定浓度要求与稀酸溶液配置而成。赤铁矿晶种为分析纯三氧化二铁(Fe2O3)。Fe(II)氧化水热水解赤铁矿的氧化剂为工业氧气。
1.2 实验方法
采用2.0L-GSH型高压釜为反应容器,将1.5 L的硫酸亚铁高铁溶液加入高压釜内并根据需要添加适量晶种,密封高压釜后检查气密性。通氮气2~3 min,排出釜内残余氧气后开始加热,待温度升至设定值后开启搅拌并将其固定在500 r/min,通入氧气并调节氧分压为0.4 MPa,同时开始计时。反应过程中根据需要每隔半小时取一次样,并快速进行固液分离,到达反应时间后快速降温冷却。所有矿浆经液固分离后获得滤液和滤渣;滤液经测量其体积后送样分析,取等滤液体积的去离子水(80 ℃)洗滤渣4遍,滤渣用电热鼓风干燥箱烘干后制样供检测分析。
为考察Fe(II)氧化水热水解赤铁矿沉铁条件对沉铁率、沉铁渣物相及成分、亚稳态铁物相向赤铁矿转变的影响规律,本研究中采用控制变量法系统地研究了温度、初始硫酸浓度、时间、晶种等因素的影响。 根据前期大量探索实验结果,设定初始条件为:沉铁前液中初始Fe2+浓度30 g/L、Na2SO4 浓度0.15 mol/L、温度200℃、初始硫酸浓度1.6×10-4 mol/L、氧分压 400 kPa、反应时间180 min、搅拌转速500 r/min,在进行单因素实验时除考察因素变化外其他条件均为该初始值。
1.3 分析与检测
分别采用X射线衍射仪(D/max-2200)、扫描电子显微镜(VEGA 3 SBH)、马尔文激光粒度仪(ZEN3690)和红外光谱仪(Bruker Tensor27)对部分固体渣样中的物相组成、形貌及能谱特征、粒度分布及硫的吸附形态进行检测分析。并对赤铁矿渣和滤液中的铁、硫、钠等相关元素含量进行分析检测,以进行除铁率的计算及元素走向的分析,采用式(8)计算过程除铁率:
η=[1-ρ2V2/(ρ1V1)]×100% (8)
式中:η为除铁率,%;ρ1为沉铁前液中铁离子浓度,g/L;ρ2为沉铁后液中铁离子浓度,g/L;V1为沉铁前液体积,L;V2为沉铁后液体积,L。
2 结果与讨论
2.1 反应温度的影响
本研究中参照赤铁矿沉铁的工业生产操技术参数在170~200 ℃的温度范围内研究了反应温度对Fe(II)氧化水热水解赤铁矿沉铁过程的沉铁率、沉铁渣物相及化学组成、沉铁渣粒度及形貌的影响规律,实验结果分别如图1、图2、表1、图3和图4所示。
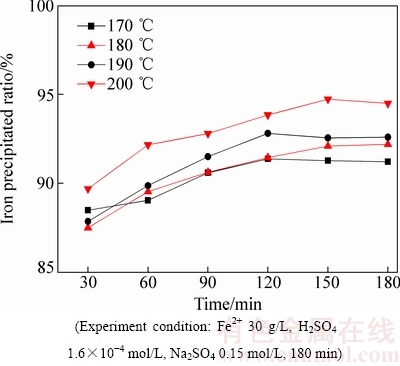
图1 反应温度对沉铁率的影响
Fig. 1 Effect of reaction temperature on amount of iron precipitated ratio
由图1可知,沉铁率随着反应温度的升高而缓慢增加,170 ℃和200 ℃下反应180 min后对应的沉铁率分别为91.22%和94.5%,说明反应温度对沉铁率的影响并不显著。然而,结合图2和表1可知,温度对赤铁矿沉铁渣的物相组成及铁、硫、钠含量的影响较为明显。在170 ℃时形成以铁矾含量为主、赤铁矿含量为辅的沉铁渣,随着反应温度的不断升高,沉铁渣中铁矾含量逐步降低,在200 ℃的XRD谱中只检测到赤铁矿物相。同时,沉铁渣中铁、硫、钠含量的变化也进一步证实了沉铁物相的转变。随着反应温度的升高赤铁矿沉铁渣中铁含量逐步升高而硫、钠含量却不断降低,170 ℃和200 ℃时渣中铁、硫、钠含量(质量分数)分别为46%、7.8%、 2.08%和66.6%、0.92%、0.033%。
在赤铁矿沉铁反应初期Fe(II)的氧化(耗酸反应2)和Fe(III)的水解(产酸反应3)同时进行使得反应溶液中酸度保持在较低范围内,从而在较低温度下(≤180 ℃)更利于铁矾的形成。随着沉铁反应的进行和反应温度的升高,Fe(II)氧化逐步完成,Fe(III)浓度进一步降低,亚稳态铁物相-铁矾的热力学稳定性降低并转变为赤铁矿。
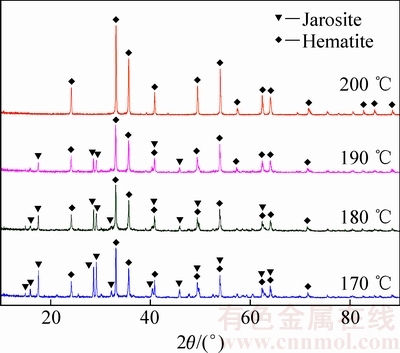
图2 不同反应温度下赤铁矿渣的XRD谱
Fig. 2 XRD patterns of precipitates at different temperatures
表1 反应温度对赤铁矿沉铁渣中铁、硫、钠含量的影响
Table 1 Effect of temperature on iron, sulfur and sodium content in precipitate
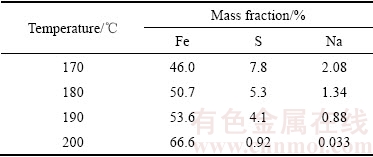
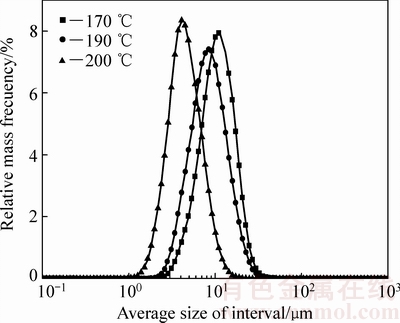
图3 170、190及200 ℃下赤铁矿沉铁渣粒度分布
Fig. 3 Size distribution of hematite precipitates obtained at 170, 190 and 200 ℃
由图3可知,温度对赤铁矿渣的粒度影响较为明显,赤铁矿沉铁渣的粒度随反应温度的升高而变小。由图4可以看出,在170 ℃和200 ℃下均得到形状不规则的赤铁矿晶体颗粒,200 ℃下的晶体颗粒发生明显的团聚现象且晶粒尺寸小于170 ℃下的赤铁矿渣颗粒。Fe(II)氧化水热水解赤铁矿沉铁伴随着赤铁矿晶核的形成、晶粒长大和晶粒团聚过程,随着反应温度的升高,反应溶液中Fe(II)氧化的氧化速率增大,Fe(III)的过饱和度增加,使得赤铁矿晶粒的成核速率大于其长大速率,从而在高温下获得晶粒细化的赤铁矿渣。综上所述,在Fe(II)氧化水热水解赤铁矿过程中相对较高的反应温度更有利于获得低硫含量的高品质赤铁矿沉铁渣。
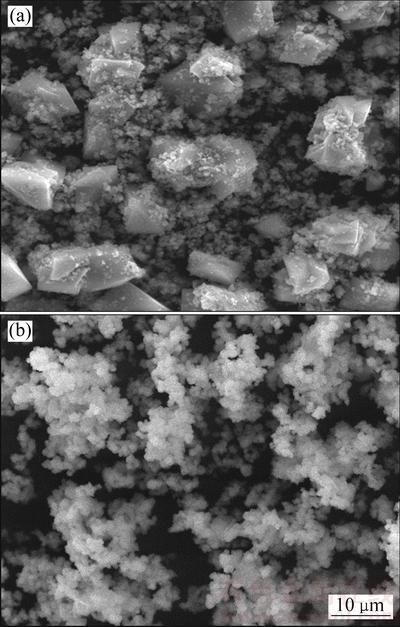
图4 170 ℃和200 ℃时赤铁矿沉铁渣的SEM像
Fig. 4 SEM images of hematite precipitates produced at 170 ℃ (a) and 200 ℃ (b)
2.2 初始硫酸浓度的影响
由图5可知,初始硫酸浓度对沉铁率影响较大。初始硫酸浓度由0增加至0.2 mol/L时,沉铁率由95.1%急剧降低至 78.6%。赤铁矿沉铁过程中反应溶液酸度过高导致生成赤铁矿的返溶,从而抑制了Fe(III)的水解。同时,由图6和表2可知,溶液酸度对沉铁渣的物相组成及渣中铁、硫、钠的含量有重要影响。当初始硫酸浓度1.6×10-4 mol/L时,沉铁渣中只检测到赤铁矿物相;当初始硫酸浓度增加至0.5 mol/L时,沉铁渣中除含有大量的赤铁矿外还有部分铁矾,并且铁矾含量随着初始硫酸浓度的升高而增加。铁矾含量的增加导致赤铁矿渣中铁含量降低,硫、钠含量升高,为控制赤铁矿渣中亚稳态铁物相-铁矾的含量应适当降低除铁前液中硫酸浓度或通过添加中和剂不断消耗赤铁矿沉铁过程产生的酸。
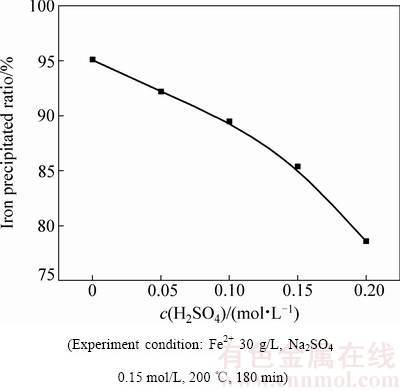
图5 初始硫酸浓度对沉铁率的影响
Fig. 5 Effect of initial sulfuric acid concentration on rate of iron precipitated
2.3 反应时间的影响
在30~180 min的范围内研究了反应时间对Fe(II)氧化水热水解赤铁矿沉铁过程的沉铁率、沉铁渣物相组成及转变的影响规律,实验结果分别如图7、图8和图9所示。
由图7可知,随着反应时间的延长沉铁率逐步增大,当反应时间由30 min 延长至180 min时,沉铁率由89.65%增大至94.5%。
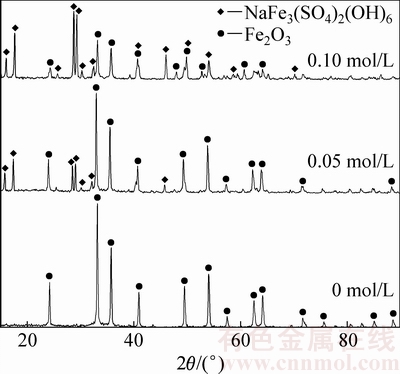
图6 不同初始硫酸浓度下赤铁矿渣的XRD谱
Fig. 6 XRD patterns of precipitates obtained at different initial sulfuric acid concentrations
表2 不同初始硫酸浓度下赤铁矿渣中铁、硫、钠的含量
Table 2 Content of Fe, S, and Na in hematite precipitate obtained at different initial sulfuric acid concentration
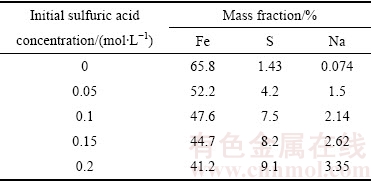
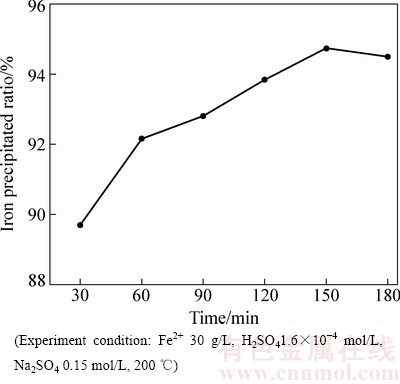
图7 反应时间对沉铁率的影响
Fig. 7 Effect of reaction time on rate of iron precipitated
由图8可知,在200 ℃下反应30 min时获得以赤铁矿含量为主、铁矾含量为辅的沉铁渣,随着反应时间的延长亚稳态的铁矾不断向赤铁矿转变,至90 min时沉铁渣中只检测到赤铁矿物相。说明在反应初期有铁矾形成,随着赤铁矿沉铁反应的进行铁矾完全转化为赤铁矿。200 ℃下反应1、2和3 h后获得赤铁矿渣的红外光谱图(见图9)中S的存在形态及含量变化进一步验证了这一推断。在图9中,在900~1300 cm-1范围内的3个红外波段是SO42-的震动吸收峰,其对应着S—O键的拉伸震动。随着反应时间的延长,3个波段的透过率明显减弱,说明SO42-在渣中的含量随着反应时间延长而降低。由此可得,在高温下适当延长反应时间有利于亚稳态铁矾向赤铁矿的转化,从而降低赤铁矿沉铁渣中硫含量。
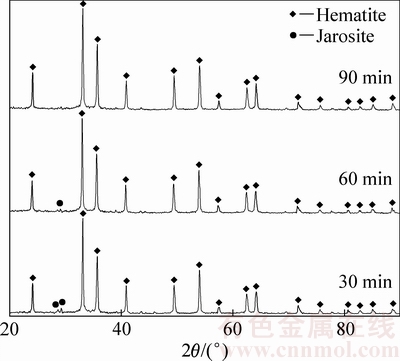
图8 不同反应时间下赤铁矿渣的XRD谱
Fig. 8 XRD patterns of precipitates obtained at different reaction time
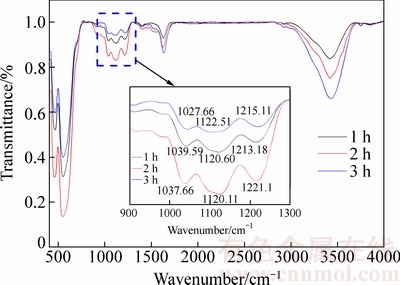
图9 1、2和3 h时赤铁矿沉铁渣的红外光谱图
Fig. 9 FT-IR spectra of hematite precipitates obtained at 1, 2 and 3 h
2.4 Fe2O3晶种的影响
在Fe(II)氧化水热水解赤铁矿沉铁过程中,只有当溶液中的Fe(III) 浓度超过均相成核的临界饱和度时,才会从溶液中形成晶核析出;而晶种的存在为Fe(III)析出提供了大量的核心,使得Fe(III)在未达到均相成核的临界饱和度之前,就已扩散至晶种表面,并在晶种表面发育长大成晶型颗粒。图10所示为180 ℃时晶种对沉铁率的影响。由图10可知,添加适量晶种可提高除铁率。
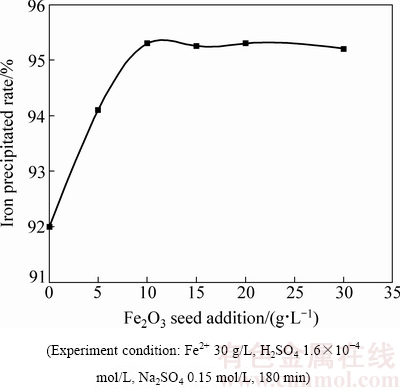
图10 180 ℃下晶种对沉铁率的影响
Fig. 10 Effects of hematite seed addition on iron precipitated at 180 ℃
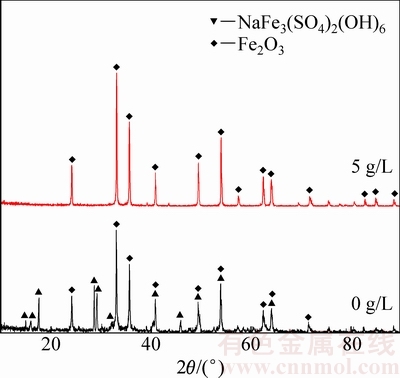
图11 180 ℃下不同晶种浓度时赤铁矿渣的XRD谱
Fig. 11 XRD patterns of hematite obtained at different amounts of hematite seed at 180 ℃
表3 180 ℃下晶种浓度对赤铁矿沉铁渣中铁、硫、钠含量的影响
Table 3 Content of Fe, S, and Na in hematite precipitate obtained at different concentrations of hematite seed at 180 ℃
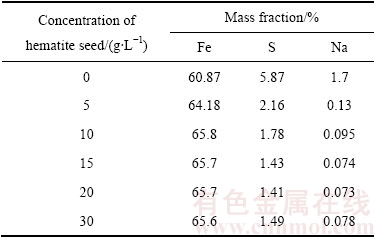
图11所示为不同晶种浓度时赤铁矿渣的XRD谱。由图11可知,在180 ℃下不添加晶种时,获得的沉铁渣为赤铁矿和铁矾的混合物;当晶种用量为5 g/L 时,可得到纯净的赤铁矿渣。而且由表3可知,晶种用量由0增大至10 g/L的过程中沉铁渣中铁含量显著增加,硫、钠含量明显降低。由2.1和2.3节可知,虽然在高温下延长反应时间可有效抑制铁矾的形成或使其向赤铁矿转变,但反应温度过高,反应时间过长致使湿法炼锌企业的生产成本增加,而在赤铁矿沉铁生产过程中通过添加晶种可有效缓解这一矛盾。
2.5 沉铁渣中S的存在形态分析
本实验条件下赤铁矿沉铁渣中的硫主要来源有3个方面,分别来自铁矾、硫酸盐夹带和SO42-的不可逆吸附。为消除硫酸盐夹带的影响,对获得的沉铁渣进行了充分洗涤。从而认为沉铁渣中的总硫含量(wt(S))主要是主要包括铁矾中硫含量(w(S)jar)和SO42-的不可逆吸附硫含量(w(S)ads),即:
wt(S)=w(S)jar+w(S)ads
采用扫描电镜能谱对170 ℃下赤铁矿沉铁渣中Fe, S,Na及O元素进行能谱分析,结果如图12所示。由图12可知,铁、硫、钠及氧元素存在于EDS面扫描图中的同一区域,证明了铁矾的存在。
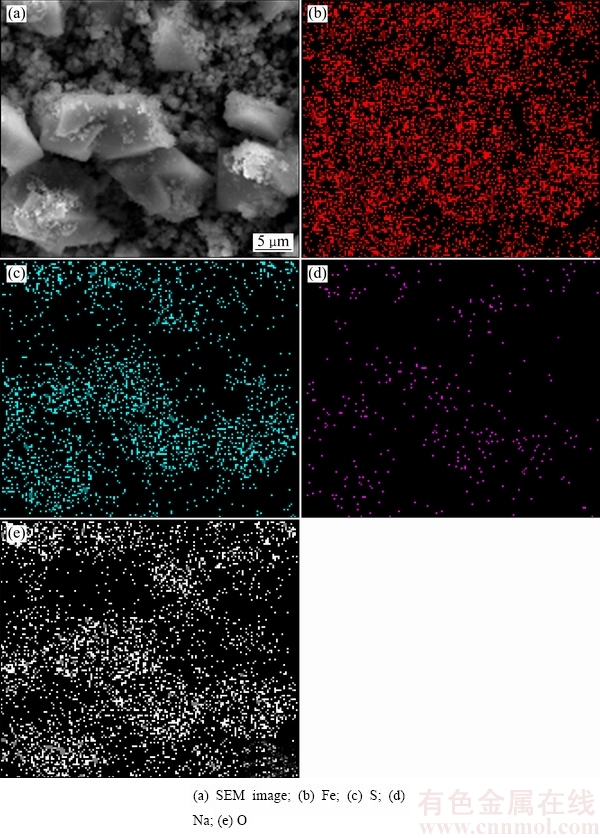
图12 170 ℃下赤铁矿沉铁渣的SEM像及EDS面扫描图
Fig. 12 SEM image and EDS maps showing element distribution of precipitates obtained at 170 ℃
表4 不同温度和时间下赤铁矿渣中S的存在形态
Table 4 Sulphur distribution in precipitated solid at different temperature or time
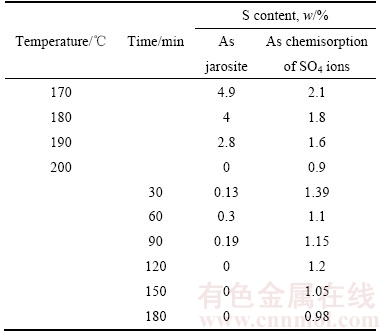
本实验中分别对反应时间为180 min、170~200 ℃下和温度为200 ℃、30~180 min下获得的赤铁矿沉铁渣中以铁矾形式和SO42-不可逆吸附形式存在的硫含量进行了检测,结果如表4所示。结合表1,表4可知,反应温度由170 ℃升高至200 ℃时,沉铁渣中以铁矾形式存在的硫由4.9%急剧降低为0,以SO42-不可逆吸附形式存在的硫含量由2.1%缓慢降低为0.9%。可见,温度对赤铁矿沉铁渣中硫含量,特别是以铁矾形式存在的硫含量影响显著。在200 ℃下,随着反应时间的延长,以铁矾形式存在的硫含量先增大后降低为0,以SO42-不可逆吸附形式存在的硫含量由1.39%逐步降低至0.98%。
3 结论
1) 温度是影响亚稳态铁矾物相形成和转化的关键因素。在本研究条件下的170~190 ℃温度范围内反应180 min后获得的沉铁渣为赤铁矿和铁矾的混合物,在200 ℃下反应90 min后获得纯净的赤铁矿渣。升高温度亚稳态铁矾的热稳定性变差,有利于其向赤铁矿转变,从而可获得高铁、低硫含量的高品质赤铁矿沉铁渣。
2) 降低初始硫酸浓度、延长反应时间、添加晶种均会促进Fe(II)氧化水解,也有利于亚稳态铁矾物相向赤铁矿转变。由XRD谱和沉铁渣中铁、硫、钠含量分析可知,200 ℃下当初始硫酸浓度为1.6×10-4 mol/L或者在180 ℃下添加5 g/L晶种时,所得沉铁渣中只检测到性质稳定的赤铁矿物相。
3) 在初始Fe2+ 浓度30 g/L、Na2SO4 浓度0.15 mol/L、初始硫酸浓度1.6×10-4 mol/L、温度200 ℃、氧分压 400 kPa、反应时间180 min、搅拌转速500 r/min的优化技术参数下,Fe(II)氧化水热水解赤铁矿沉铁过程中沉铁率为95%,获得不含亚稳态铁矾的赤铁矿沉铁渣,其中铁、硫、钠硫含量分别为66.6%、0.92%和0.033%。
REFERENCES
[1] 魏 昶, 李存兄. 新提取冶金学[M]. 北京: 冶金工业出版社, 2013: 10-68.
WEI Chang, LI Cun-xiong. Extractive metallurgy of zinc[M]. Beijing: Metallurgical Industry Press, 2013: 10-68.
[2] ZHANG Fan, WEI Chang, DENG Zhi-gan, LI Xing-bin, LI Cun-xiong, LI Min-ting. Reductive leaching of indium-bearing zinc residue in sulfuric acid using sphalerite concentrate as reductant[J]. Hydrometallurgy, 2016, 161: 102-106.
[3] ZHANG Fan, WEI Chang, DENG Zhi-gan, LI Cun-xiong, LI Xing-bin, LI Min-ting. Reductive leaching of zinc and indium from industrial zinc ferrite particulates in sulphuric acid media[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(9): 2495-2501.
[4] 彭 兵, 林冬红, 刘 恢, 彭 宁, 陈 栋, 李燕春, 袁莹珍. 高铁锌焙砂还原焙烧-碱浸工艺[J]. 中国有色金属学报, 2017, 27(2): 424-429.
PENG Bing, LIN Dong-hong, LIU Hui, PENG Ning, CHEN Dong, LI Yan-chun, YUAN Ying-zhen. Alkaline leaching zinc from high iron-bearing zinc calcine after reduction roasting[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(2): 424-429.
[5] 刘 洋, 谭 军, 刘常青, 尹周澜, 陈启元, 张平民, 廖 舟. 碳还原锌浸出渣炼铁过程的热力学分析[J]. 中国有色金属学报, 2015, 25(7): 1979-1986.
LIU Yang, TAN Jun, LIU Chang-qing, YIN Zhou-lan, CHEN Qi-yuan, ZHANG Ping-min, LIAO Zhou. Thermodynamic analysis on iron-making process of zinc leaching residue by carbon reduction[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(7): 1979-1986.
[6] 陈家镛, 余淑秋, 伍志春. 湿法冶金中铁的分离与利用[M]. 北京: 冶金工业出版社, 1991: 112-135.
CHEN Jia-yong, YU Shu-qiu, WU Zhi-chun. Separation and utilization of iron in hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 1991: 112-135.
[7] DREISINGER D B, PETERS E. The oxidation of ferrous sulfate by molecular oxygen under zinc pressure leach conditions[J]. Hydrometallurgy, 1989, 22(1): 101-119.
[8] CHENG T C, DEMOPOULOS G P. Hydrolysis of ferric sulfate in the presence of zinc sulfate at 200 ℃: precipitation kinetics and product characterization[J]. Industrial & Engineering Chemistry Research, 2004, 43(20): 6299-6308.
[9] UMETSU Y, TOZAWA K, SASAKI K. The hydrolysis of ferric sulphate solutions at elevated temperatures[J]. Canadian Metallurgical Quarterly, 1977, 16(1): 111-117.
[10] VOIGT B, GOBLER A. Formation of pure hematite by hydrolysis of iron(III) salt solutions under hydrothermal conditions[J]. Crystal Research and Technology, 1986, 21(9): 1177-1183.
[11] 岳 明, 孙宁磊, 邹 兴, 邵建春, 刘金山, 王魁珽, 陆业大. 锌浸出液三价铁直接水解赤铁矿法除铁的探讨[J]. 中国有色冶金, 2012, 41(4): 80-85.
YUE Ming, SUN Ning-lei, ZOU Xing, SHAO Jian-chun, LIU Jin-shan, WANG Kui-ting, LU Ye-da. The discussion on hydrolysis precipitation of ferric oxide directly from ferric-ion rich zinc leachate[J]. China Nonferrous Metallurgy, 2012, 41(4): 80-85.
[12] CHENG T C. Production of hematite in acidic zinc sulphate media[D]. Canada: Materials Engineering McGill University, 2002: 56-104.
[13] YAMADA T, KURAMOCHI S, SATO S, SHIBACHI Y. The Recent operation of the hematite process at the IIJIMA refinery[C]// DUTRIZAC J E, GONZALEZ J A, BOLTON G L, HANCOCK P. Zinc and Lead Processing. Montreal, Canada: CIM, 1998: 627-638.
[14] 杨 凡, 邓志敢, 魏 昶, 李存兄, 李兴彬. 采用赤铁矿去除高铁闪锌矿浸出液中的铁[J]. 中国有色金属学报, 2014, 24(9): 2387-2391.
YANG Fan, DENG Zhi-gan, WEI Chang, LI Cun-xiong, LI Xing-bin. Iron-removal by hematite from leaching liquor of high iron sphalerite[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2387-2391.
[15] RUIZ M C, ZAPATA J, PADILLA R. Effect of variables on the quality of hematite precipitated from sulfate solutions[J]. Hydrometallurgy, 2007, 89(1): 32-39.
[16] 杨 凡, 邓志敢, 魏 昶, 李兴彬, 李存兄, 邱伟佳, 朱如龙. 高铁闪锌矿赤铁矿法除铁中锌离子和镁离子的影响及存在机理[J]. 中国有色金属学报, 2014, 24(11): 2906-2912.
YANG Fan, DENG Zhi gan, WEI Chang, LI Xing bin, LI Cun xiong, QIU Wei jia, ZHU Ru-long. Effectand existencemechanismofzincion andmagnesium ion in iron removal from high iron sphalerite by hematite process[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(11): 2906-2912.
[17] DUTRIZAC J E, SUNYER A. Hematite formation from jarosite type compounds by hydrothermal conversion[J]. Canadian Metallurgical Quarterly, 2012, 51(1): 11-23.
[18] CLAASSEN J O, SANDENBERGH R F. Particle growth parameters in the precipitation of metastable iron phases from zinc-rich solutions[J]. Hydrometallurgy, 2006, 84(3/4): 165-174.
Hydrothermal hematite precipitation and conversion behavior of metastable iron phase in FeSO4-H2O system
LI Cun-xiong, WEI Chang, DENG Zhi-gan, LI Xing-bin, FAN Gang, WANG Yi-zhao, YI Shuo-wen, LI Min-ting
(Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: Because of high iron content, hematite precipitate is a potential marketable byproduct as a material in the steel making, cement and pigment industries. While, sulfur content is a key factor which affects the hematite quality and its utilization as a resource. The residual sulfur in hematite precipitate is attributed to the part existing as metastable jarosite, irreversible absorption of SO42- ions and basic ferric sulfate, and jarosite appears to be the main sulfate-contaminating phase. The results shows that temperature is the key factor which affects the formation and conversion of metastable jarosite. Therefore, jarosite becomes thermodynamically unstable and to converses into hematite with increasing temperature. It is advantage for metastable jarosite conversion and obtaining high quality hematite precipitate with higher iron content and lower sulfur content by decreasing initial sulfuric acid concentration, prolonging retention time and addition of seed. High quality hematite precipitate with Fe, S and Na content of 66.6%, 0.92% and 0.033%, respectively, and with iron precipitation ratio of 95% are obtained under the condition as follows: initial solution containing 30 g/L ferrous ion, 0.15 mol/L sodium sulphate and 1.6×10-4 mol/L initial acid, temperature of 200 ℃, oxygen partial pressure of 400 kPa, reaction time of 180 min and agitation speed of 500 r/min.
Key words: hematite precipitation; ferrous ion oxydrolysis; metastable conversion; hematitie; sulfur content; hydrothermal metallurgy
Foundation item: Projects(51474117, 51664038, 51364022, 51564030) supported by the National Natural Science Foundation; Project(ZD2014003) supported by the Major Scientific Research Fund of Yunnan Ministry of Education, China
Received date: 2016-12-21; Accepted date: 2017-04-05
Corresponding author: LI Cun-xiong; Tel: +86+871-65188819; E-mail: licunxiong@126.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51474117,51664038,51364022,51564030);云南省教育厅科学研究基金重大专项项目(ZD2014003)
收稿日期:2016-12-21;修订日期:2017-04-05
通信作者:李存兄,教授,博士;电话:0871-65188819;E-mail:licunxiong@126.com