KOH亚熔盐体系中用CuO催化氧化浸出铬铁矿
来源期刊:中国有色金属学报(英文版)2017年第4期
论文作者:刘龙杰 杜浩 张洋 郑诗礼 张懿
文章页码:891 - 900
关键词:铬铁矿;氧化铜;浸出;催化氧化
Key words:chromite ore; copper oxide; leaching; catalytic oxidation
摘 要:通过添加CuO催化剂来提高铬铁矿在KOH亚熔盐介质中的浸出率。研究反应体系温度、碱矿质量比,铜矿质量比和气体流速对铬浸出的影响。结果表明:CuO对提高铬铁矿在KOH亚熔盐介子的浸出率起着十分重要的作用。在反应温度为230 °C、碱矿质量比为6:1、搅拌转速为700 r/min、气体流速为1 L/min、反应时间为6 h的条件下铬的转化率在添加CuO时为98%,而未添加CuO时,铬的转化率仅为60.8%。动力学计算结果表明,添加CuO后在反应温度高于230 °C时,反应速度控制步骤为表面化学反应控制,反应活化能为15.79 kJ/mol;未添加CuO时,反应速度控制步骤为外扩散控制,反应活化能为38.01 kJ/mol。
Abstract: CuO was used as a catalyst in the concentrated KOH solution to enhance the leaching of chromium from the chromite ore. The impacts of temperature, KOH-to-chromite ore mass ratio, CuO-to-chromite ore mass ratio, and gas flow rate on the chromium leaching rate were investigated. The results indicated that CuO played an important role in improving the chromium leaching rate. The leaching rate reached 98% after leaching for 6 h when CuO was applied, whereas it was only 60.8% without CuO under the same reaction conditions: temperature 230 °C, KOH-to-ore mass ratio 6:1, stirring speed 700 r/min, gas flow rate 1 L/min. According to the kinetics study, the catalytic oxidation was controlled by surface chemical reaction and the activation energy was calculated to be 15.79 kJ/mol when the temperature was above 230 °C. In contrast, without CuO, the rate-determining step was external diffusion {Chen, 2013 #2}and the apparent activation energy was 38.01 kJ/mol.
Trans. Nonferrous Met. Soc. China 27(2017) 891-900
Long-jie LIU1,2, Hao DU1,2, Yang ZHANG1, Shi-li ZHENG1, Yi ZHANG1
1. Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
2. University of Chinese Academy of Sciences, Beijing 100049, China
Received 8 November 2015; accepted 13 June 2016
Abstract: CuO was used as a catalyst in the concentrated KOH solution to enhance the leaching of chromium from the chromite ore. The impacts of temperature, KOH-to-chromite ore mass ratio, CuO-to-chromite ore mass ratio, and gas flow rate on the chromium leaching rate were investigated. The results indicated that CuO played an important role in improving the chromium leaching rate. The leaching rate reached 98% after leaching for 6 h when CuO was applied, whereas it was only 60.8% without CuO under the same reaction conditions: temperature 230 °C, KOH-to-ore mass ratio 6:1, stirring speed 700 r/min, gas flow rate 1 L/min. According to the kinetics study, the catalytic oxidation was controlled by surface chemical reaction and the activation energy was calculated to be 15.79 kJ/mol when the temperature was above 230 °C. In contrast, without CuO, the rate-determining step was external diffusion {Chen, 2013 #2}and the apparent activation energy was 38.01 kJ/mol.
Key words: chromite ore; copper oxide; leaching; catalytic oxidation
1 Introduction
Chromate production from the chromite ore is an important metallurgical process but it is usually associated with gross pollution [1,2]. Nowadays, the alkali roasting method accounts for more than 90% of global chromate production [3]. In the typical alkali roasting method, chromite ore is calcined at about 1373 K in a kiln or rotary furnace with the addition of limestone and dolomite. And a large amount of CaCrO4 would generate as a chemical carcinogen, and would severely pollute the environment [4,5]. Efforts have been made to improve the chromate production process, and the liquid-phase oxidation (LO) method has become an attractive alternative for the chromate production [6,7]. In this method, chromite ore is converted to soluble chromate in alkali-hydroxide solution using oxygen or other oxidizing agents, and high chromium extraction can be achieved under moderate reaction conditions [6,8]. Further, only 0.5 t of chromium ore processing residue (COPR) will be generated per ton of chromate, whereas in the traditional alkali roasting method, the COPR is 3 t per ton of chromate [1]. In addition, the COPR obtained from LO process can be easily detoxified, and can be utilized as desulfur catalysts. This would significantly eliminate the environment pollution originated from the COPR [9]. However, in order to realize high recovery of chromium, chromite ore needs to be oxidized in 80% KOH (mass fraction) concentration at 320 °C for more than 6 h. During this procedure, extensive amount of energy would be consumed. In this regard, it is of crucial importance to develop a new method to decrease the LO reaction temperature and KOH concentration as well to control the cost for chromate production using this new process.
As a clean and efficient method, catalytic wet oxidation (CWO) has shown obvious advantages in the treatment of industrial process water and wastewater. Its efficiency in removal of organic pollutants via complete oxidation of organic matters to carbon dioxide and water using air or oxygen has attracted much research attention [10-12]. It has been reported that CuO could catalyze the oxidation of the sodium salts of four hydroxyl carboxylic acids (citric, lactic, malic and tartaric) in NaOH solution in Bayer process. Further, catalysts based on copper oxides show high activities in the CWO of persistent organic pollutant in wastewater [10,11], while the lack of the knowledge in the catalytic mechanism, especially in the alkaline solutions, greatly governs the development of CWO.
In this work, the CWO of chromite ore using CuO as a catalyst was investigated in highly concentrated KOH solutions. The impacts of temperature, KOH-to-chromite ore mass ratio, CuO-to-chromite ore mass ratio, and gas flow rate on the chromium leaching were systematically investigated. The catalytic effect of CuO was discussed in detail. The results in this work would provide fundamental information for further development of CWO process in strong alkaline solutions.
2 Experimental
2.1 Minerals
The chromite ore sample purchased from South Africa was provided by Bluestar Yima Chrome Chemical Materials Co., Ltd., China. The chemical composition of typical samples was analyzed by ICP-OES (PE Optima 5300DV, PerkinElmer) and listed in Table 1. Before each experiment, the sample was dried overnight at 110 °C and sieved to obtain particles in size range of 38-63 μm. The reagents potassium hydroxide and copper(II) oxide powder used in this work were of analytical grade and were purchased from Xilong Chemical Co., Ltd., China. Commercial pure oxygen was obtained from Millennium Beijing Gas Scales Center. Ultrapure water was produced using the millipore and used in all the experiments.
Table 1 Chemical composition of South Africa chromite ore (mass fraction, %)

Figure 1 shows the XRD pattern of a chromite ore sample. Clearly, the chromium mostly presents in the form of (Mg, Fe)(Cr, Al)2O4 spinel. The SEM image of the chromite ore is shown in Fig. 2. It is found that the mineral particles have a compact surface.
2.2 Experimental apparatus and procedure
All experiments were performed in a 500 mL cylindrical stainless steel reactor under the atmospheric pressure. The diameter of the reactor was 82 mm and the height was 150 mm, as schematically illustrated in Fig. 3. The system was equipped with a temperature controller (CKW-2200) with a precision of ±2 °C, and a mechanical agitator (D-8401WZ) was used to keep the slurry suspended during the experiments. The agitator blade was propellant agitator with three paddles and its diameter was 54 mm. A peristaltic pump (YZ1515X) was used to pump water into the reactor during the experiments to make up for the loss of water due to evaporation. The oxygen was introduced from a gas cylinder with the flow rate controlled by a flow meter.
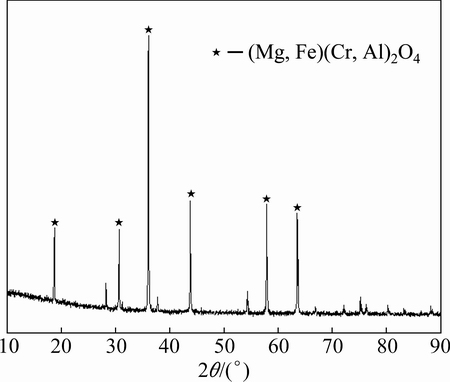
Fig. 1 XRD pattern of South Africa chromite ore

Fig. 2 SEM image of South Africa chromite ore

Fig. 3 Schematic diagram of experimental apparatus
Before each experiment, 282.35 g KOH, 40.00 g K2CO3, 15.92 g Al(OH)3 and 61.73 g deionized water were added to the reactor to obtain a slurry under constant stirring. After the slurry was heated to the desired reaction temperature, the chromite ore particles were added to the reactor, meanwhile, the oxygen continuously passed through the slurry. At certain reaction time, approximately 2 g of the reacting slurry sample was taken out and mixed with 80 mL of deionized water, followed by filtration and washing to obtain the tailings. After the reaction, the slurry was diluted with deionized water and then filtered to obtain the leaching solution and residue for further analysis.
The chromium leaching rate was calculated using the following equation:
η=[(1-w(Cr)r/w(Cr)0)]×100% (1)
where w(Cr)r and w(Cr)0 are the contents of chromium in the residues obtained from the reaction stage and that in the original chromite ore, respectively.
The compositions of chromite ore and residues were analyzed by ICP-OES. The mineralogical phases of chromite ore and residues were examined with X-ray diffraction (Empyrean, PANalytical B.V). The SEM-EDS images of the chromite ore and the residues were obtained with SEM (JSM-7001F+INCA, JEOL) equipment.
3 Results and discussion
This new method is based on the sub-molten salt method which has been described in detail elsewhere [1,8,13]. The flow sheet of the chromate production process is illustrated in Fig. 4, and can be briefly described as follows: chromite ore was oxidized in the sub-molten salt medium in the loop airlift reactor, after the leaching process, the slurry was diluted for the separation of K2CrO4 and ferrite-enriched residue; then the diluted alkaline solution was evaporated and concentrated for recycling. The leaching of chromium was operated in 80% KOH (mass fraction) sub-molten salt solutions at 320 °C, consuming extensive amount of energy due to the evaporation and concentration of the KOH solution. In this regard, in order to optimize the current process, it was of critical importance to decrease the LO temperature and the KOH concentration.
The leaching process of chromite ore is a typical LO reaction, and the oxygen mass transfer in the KOH sub-molten salt solution is a vital factor. In order to intensify the reaction, the catalytic effect of potassium nitrate was examined in previous studies [8]. It was found that the introduction of potassium nitrate could decrease the KOH-to-ore mass ratio from 4:1 to 2:1 and reduce the energy consumption significantly. However, the leaching temperature had to be raised to 320 °C for potassium nitrate to be recycled to function. The selection of alternative catalysts to decrease the operation temperature was therefore of great importance. It was reported that in the Bayer liquor, CuO was the most active catalyst for the oxidation of four hydroxyl carboxylic acids (citric, lactic, malic and tartaric) to carbon dioxide and water in comparison with other transition metal oxide such as Fe2O3, MnO2, Co3O4, and Ni2O3 [10]. Catalytic oxidation of copper oxide for chromite ore in concentrated KOH solutions was therefore systematically studied in the following sections.
In the industrial production, K2CO3 is usually generated due to continuous blowing of air into the reactor, and there is about 10% K2CO3 (mass fraction) in the reaction system on average. Furthermore, aluminum compounds will be leached out in the form of KAlO2 during the reaction, therefore, the reaction medium is mainly composed of KOH, K2CO3, KAlO2 and K2CrO4.

Fig. 4 Illustrative flow sheet of chromate cleaner production process in demonstration plant
Consequently, the effects of K2CO3 and KAlO2 on the boiling point and the viscosity of the system were examined, and the results are shown in Fig. 5. It was found that the boiling point of 60% KOH could be significantly increased from 180 to 290 °C with the addition of K2CO3 and KAlO2. In the industrial practice, after the recycle, the mother liquor of KOH sub-molten system contains about 10% of K2CO3 and 5% KAlO2. Thus, in this work, the addition of K2CO3 and KAlO2 was controlled to be the same as that in the industrial production and the content of KOH was selected to be 60%.
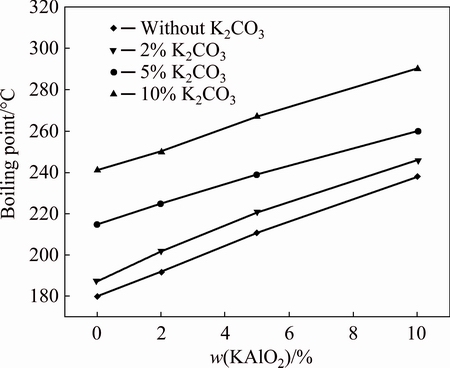
Fig. 5 Effect of K2CO3 and KAlO2contents on boiling point of 60% KOH solution
3.1 Effect of CuO-to-chromite ore mass ratio
The possibility of CuO acting as catalyst was investigated by examining the effect of CuO-to-chromite ore mass ratio on chromium conversion, and the results are summarized in Fig. 6. The reaction conditions were controlled to be temperature of 230 °C, stirring speed of 700 r/min, KOH-to-chromite ore mass ratio of 6:1, and gas flow rate of 1 L/min. As presented in Figs. 6 and 7, the chromium leaching rate initially increases significantly with the increase in CuO loading (the mass fraction of CuO in KOH sub-molten medium), and reaches a plateau due to the solubility limit of CuO. Similar phenomenon has also been observed in the CuO catalytic wet oxidation in Bayer liquor (highly alkaline) [10]. It has been established that the dissolution of CuO could form soluble copper hydroxyl. The copper hydroxyl could help to promote the generation of free radical intermediates [11], and thus intensifying the oxidation of chromite ore. According to the study in the catalytic wet oxidation of sodium citrate, lactate, malate and tartrate in high alkaline solution, the catalytic oxidation pathway can be proposed as follows:
1) Dissolution of CuO
CuIIO+nOH-=[CuII(OH)n]2-n (2)
2) Chromite ore oxidation
2Cr2O3·FeO+22OH-+14[CuII(OH)n]2-n=11H2O+ +Fe2O3+14[CuI(OH)n]1-n (3)
+Fe2O3+14[CuI(OH)n]1-n (3)
3) Super oxide formation
[CuI(OH)n]1-n+O2=[CuII(OH)n]2-n+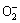 (4)
(4)
The catalytic effect of CuO was realized by the dissolution of CuO to [CuII(OH)n]2-n, which was dependent on the solubility of CuO. And it can be deduced that the decomposition of chromite ore could be enhanced due to the formation of superoxide anion 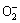 . As a result, the CuO-to-chromite ore mass ratio was chosen to be 0.1:1.
. As a result, the CuO-to-chromite ore mass ratio was chosen to be 0.1:1.
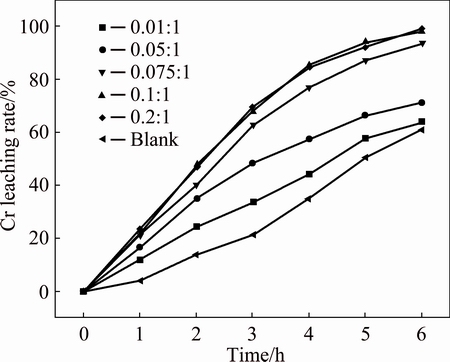
Fig. 6 Effect of CuO-to-chromite ore mass ratio on chromium leaching rate
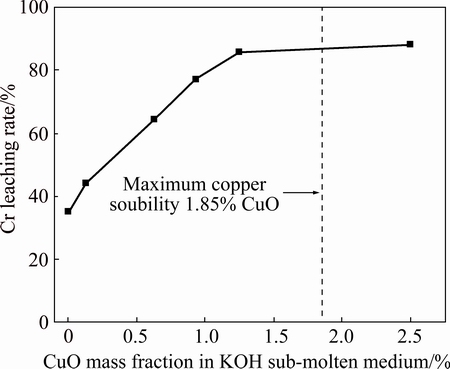
Fig. 7 Effect of mass fraction of CuO in KOH sub-molten medium on chromium leaching rate (Reaction time: 4 h)
3.2 Effect of CuO addition with various temperatures
The effect of temperature on the chromium leaching was studied under reaction conditions controlled to be CuO-to-chromite ore mass ratio of 0.1:1, stirring speed of 700 r/min, gas flow rate of 1 L/min, and KOH-to- chromite ore mass ratio of 6:1, with the results shown in Fig. 8. From Fig. 8, it is observed that when the temperature is lower than 210 °C, the addition of CuO has a negative effect on the chromium leaching. That is because the viscosity of sub-molten system at low temperature is very high, leading to poor performance in the mass transfer. Further, it is known that low temperature is unfavorable for CuO dissolution, and the excess CuO may scavenge superoxide intermediates and further hinder the oxidation of chromite ore [10]. At 230 °C, the addition of CuO significantly promotes the leaching of chromium, and similar results are observed at 250 °C. Thus, it is concluded that the addition of CuO could enhance the oxidative conversion of chromium and increase the chromium leaching ratio when the temperature is above 230 °C.
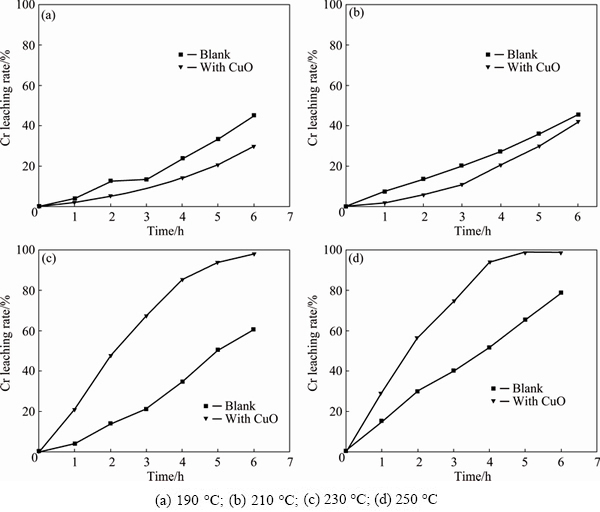
Fig. 8 Influence of Cu addition on chromium leaching rate compared with blank at different temperatures
3.3 Effect of alkali-to-chromite ore mass ratio
The alkali-to-chromite ore mass ratio is important in influencing the mass transportation as well as reaction media recycling economy in the whole process [8,14]. The effect of alkali-to-chromite ore mass ratio (ATO) was investigated under the reaction conditions of temperature 230 °C, stirring speed 700 r/min, CuO-to- chromite ore mass ratio 0.1:1 and gas flow rate 1 L/min, and the results are shown in Fig. 9. From Fig. 9, it can be seen that the chromium leaching rate increased significantly from 85% to 98% with ATO increasing from 4:1 to 6:1. This is because with the increase in ATO, the mass transfer becomes better due to improving in the medium fluidity, and the mineral particles could be efficiently mixed with the reaction medium [15].

Fig. 9 Effect of KOH-to-ore mass ratio on chromium leaching rate
3.4 Effect of gas flow rate
Figure 10 shows the influence of gas flow rate on the leaching rate of chromium under conditions of temperature 230 °C, stirring speed 700 r/min, KOH-to- chromite ore mass ratio 6:1, CuO-to-chromite ore mass ratio 0.1:1. It is clear that the gas flow rate does not exhibit significant effect on the chromium recovery. The reason is that the high agitation speed during the reaction can provide sufficient dispersion of gases into the liquid phase even the gas flow is small. Therefore, the gas flow rate was kept to be 1 L/min in the following experiments.

Fig. 10 Effect of gas flow rate on chromium leaching rate
3.5 Effect of temperature
The effect of temperature ranging from 190 to 270 °C was studied with the other reaction conditions fixed as stirring speed of 700 r/min, gas flow of 1.0 L/min, KOH-to-chromite ore mass ratio of 6:1, CuO-to-chromite ore mass ratio of 0.1:1. The results are presented in Fig. 11. In Fig. 11, it is observed that the chromium leaching rate obviously increases with the increase of temperature, and a maximum of 99% chromium leaching rate was obtained at 270 °C after 4 h reaction. This is because the decomposition of chromite spinel and the dissolution of CuO can be significantly enhanced with increasing the reaction temperature.
The liquid oxidization methods for chromite ore oxidation reported in the literatures usually operated at temperatures higher than 300 °C in KOH solutions with content more than 70%. Due to the catalytic oxidation effect of CuO, the temperature and KOH content are significantly decreased in this new method, thus the energy consumption and operation cost could be reduced greatly in comparison with those of LO methods.

Fig. 11 Effect of temperature on chromium leaching rate
3.6 Morphology of residues
The morphologies of the leaching residues obtained under the conditions of reaction temperature 250 °C, initial KOH content 60%, CuO-to-chromite ore mass ratio 0.1:1 and the oxygen flow rate 1 L/min were analyzed by using the XRD method. The results are shown in Fig. 12. It is observed that after reacting for 4 h, no obvious peak could be observed from the XRD pattern, indicating that the residue is in amorphous state.

Fig. 12 XRD patterns of residues for different leaching time
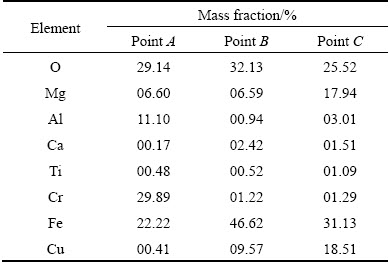
In order to examine the microstructure of the partially reacted chromite ore, the cross-section of the leaching residue was polished and examined. The backscattered electron image and the related EDS results of the residue’s cross-section are given in Fig. 13 and Table 2, respectively. The EDS results show that the central part of the particle (point A) mainly consists of Cr, Fe, Mg, Al and O elements, and the outer-ring (points B and C) is rich in Fe, O and Cu elements. Obviously, the chromium in the surface layer of the chromite mineral has been extracted, and the unreacted chromite core is surrounded by the Fe-Cu-rich oxide product layer. The compositional change of the cross-section indicates that solid product is formed as the ash layer. Other researchers also discovered the phenomenon that chromite residue was covered by the “ash” layer. The existence of the product layer has a strong effect on the reaction process, and it is proposed that the chromium dissolution process might be controlled by diffusion in the reaction product layer, and the existence of Cu species might have an effect on the oxidation of Cr(III) to Cr(VI).

Fig. 13 Backscattered electron image of process residue leached at 250 °C
Table 2 EDS results of process residue leached at 250 °C for 3 h (points A, B and C shown in Fig. 13)
3.7 Kinetics analysis
The influence of temperature on chromite ore decomposition can be used to calculate the apparent activation energy, the reaction rate, and analyze the decomposition mechanism.
During reaction, chromite ore was attacked by KOH with progressive erosion of the spinel structure and produced an amorphous-like residue. The XRD patterns are presented in Fig. 12, and the backscattered electron image and the related EDS results of the residue’s cross-section are given in Fig. 13 and Table 2, respectively. According to Fig. 13 and Table 2, as well as previous work regarding the chromite ore processing, it was concluded that the chromite ore decomposition in KOH sub-molten medium could be described using the shrinking core model.
The reaction rate is generally controlled by the following step: the diffusion of reactants through the ash layer, or chemical reaction on the surface of the core of un-reacted materials [1,8].
Suppose that the chromite ore particles have a spherical geometry and the process is controlled by liquid boundary layer diffusion control (external diffusion), the shrinking core model can be described as follows:
x=kit (5)
where x is the leaching rate, ki is the apparent constant and t is the reaction time.
When the surface reaction is the controlling step, the kinetics of the process is simplified as follows:
1-(1-x)1/3=krt (6)
If the diffusion of the reagents through the product layer is the controlling step (internal diffusion), the kinetics of the process should be calculated by the following equation:
1+2(1-x)-3(1-x)2/3=kdt (7)
In order to determine the kinetic parameters and the rate-determining step in the leaching process, the experimental data were put into the above integral rate equations. The left sides of these expressions were plotted with respect to the leaching time, and the dependency of these models on the kinetic data was evaluated using correlation coefficient values. The slopes of these plots were the apparent rate constants (ki, kr and kd).
3.7.1 Kinetics analysis of chromium leaching without CuO
The rate-determining step of the reactions without the addition of CuO was analyzed, and the chromite leaching rate at 230 °C was fitted into Eqs. (5)-(7), and the results are summarized in Fig. 14. From Fig. 14, it can be seen that Eq. (5) fits the experiment data perfectly with a Pearson R correlation of 0.98846, indicating that the kinetics of the chromium leaching process without CuO is controlled by external diffusion.
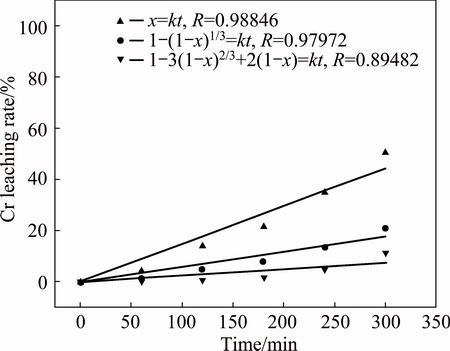
Fig. 14 Chromium leaching kinetics without CuO at 230 °C
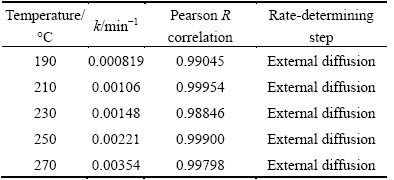
The fitting of chromium leaching rates over time at various temperatures from 190 to 270 °C was performed, and the results are summarized in Fig. 15 and Table 3.
It can be seen from Table 3 that the reaction rate constant k exhibits parabolic features as a function of temperature. As the temperature increases from 190 to 210 °C, k decreases slightly. Further increasing temperature from 210 to 270 °C, significant increase in k is observed. In addition, it is concluded that the chromium leaching is controlled by external diffusion at various temperatures without the addition of CuO. Thus, the chromium leaching process without CuO would be promoted by optimizing the mass transfer properties of the reaction media.

Fig. 15 Chromium leaching kinetics without CuO at various temperatures
Table 3 Chromium leaching kinetic parameters without CuO
The apparent activation energy for chromium leaching from the chromite ore was calculated using the Arrhenius equation (Eq. (8)), and the result was presented in Fig. 16.
 (8)
(8)
where k, Ea, A, R and T are kinetics constant, apparent activation energy, pre-exponential factor, molar gas constant, and thermodynamic temperature, respectively.
From Fig. 16, the apparent activation energy for chromium leaching from the chromite ore was calculated to be 38.01 kJ/mol, and the chromium leaching kinetics was expressed as Eq. (9).
x=14.47exp(-4571.93/T)t (9)
3.7.2 Kinetics analysis of chromium leaching with CuO
To reveal the rate-determining step of the of chromite leaching from the chromite ore when CuO was added, the chromite leaching rate at 230 °C with addition of CuO was fitted into the above kinetic equations, with the results presented in Fig. 17. It can be seen that Eq. (6) fits the experiment data best with a Pearson R correlation of 0.99664, indicating that the kinetics of chromite leaching process is controlled by the surface reaction.

Fig. 16 Arrhenius plot for leaching process of chromite ore without addition of CuO
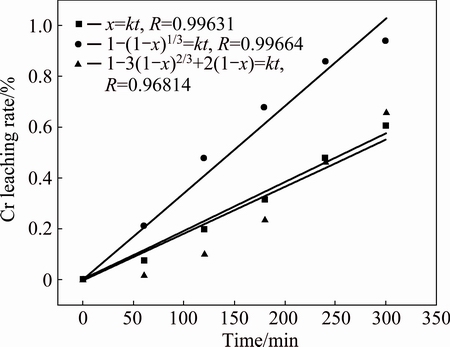
Fig. 17 Chromium leaching kinetics with addition of CuO at 230 °C
The fitting of chromium leaching rates over time at different temperatures was carried out, and the results are summarized in Fig. 18 and Table 4.
Differing from that of the chromium leaching kinetics without CuO, the rate-determining step of this process with the addition of CuO gradually shifts from external diffusion control to surface chemical reaction control as the temperature increases from 190 to 230 °C. Above 230 °C, the addition of CuO accelerates the leaching of chromium, and the rate-determining step changes to surface chemical reaction. Thus, the addition of CuO can improve the chromium extraction of rate substantially.
The apparent activation energy for chromium leaching from chromite ore was also calculated using Eq. (8), with results presented in Fig. 19. When the temperature was above 230 oC, the activation energy was calculated to be 15.79 kJ/mol. And the activation energy for temperatures under 210°C was not calculated due to less data to be fitted. The chromium leaching kinetics can be described as follows:
1-(1-x)1/3=0.086exp(-1899.93/T)t (10)

Fig. 18 Chromium leaching kinetics with addition of CuO at various temperatures
Table 4 Chromium leaching kinetic parameters with addition of CuO


Fig. 19 Arrhenius plot for leaching process of chromite ore with addition of CuO
3.8 Decomposition mechanism of chromite ore with CuO addition in KOH system
Chromite is a spinel structure in which Fe2+ occupies the tetrahedral positions and Cr3+ occupies the octahedral positions. At the reaction temperature of 230 °C, the diffusion of Fe2+ and Cr3+ can be neglected. In highly concentrated KOH solution, O2- can be formed for the acid-base equilibrium shown in Eq. (11) [8]:
2OH-=H2O+O2- (11)
In the soda ash roasting process, the oxidation of chromite ore can be accelerated with the new O2- generated along the vacancies of cations [5]. In our system, the decomposition of chromite ore is a solid-liquid-gas three-phase reaction and the O2- can be changed into 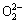 when O2 was introduced into the KOH system due to Eq. (12):
when O2 was introduced into the KOH system due to Eq. (12):
1/2O2+O2-=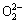 (12)
(12)
Reaction (12) indicates that the increase of oxygen dissolution can accelerate the decomposition of chromite ore, which is in accordance with the report that KOH system combining with oxygen shows strong decomposition characters. Also, 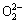 and
and 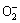 can be transformed when CuO dissolves in the solution by Eq. (13):
can be transformed when CuO dissolves in the solution by Eq. (13):
[CuII(OH)n]2-n+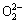
 [CuI(OH)n]1-n+
[CuI(OH)n]1-n+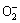 (13)
(13)
In the leaching process of chromite ore, O2- attacks chromite ore surface and makes it easier for spinel structure destruction. And the dissolution of oxygen and the formation of 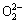 and
and 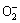 can speed up the aberration of spinel. Therefore, the addition of CuO could improve the oxygen dissolution and act as transportation media.
can speed up the aberration of spinel. Therefore, the addition of CuO could improve the oxygen dissolution and act as transportation media.
It should be noted that soluble copper is an active catalyst for the leaching of chromium from chromite ore in highly alkaline solutions, the removal and recovery of this catalyst, its cost and potential environmental effects should be considered for its production use. 90% of CuO is leached with the slag discharged, and others can be recycled in the mother liquid solution. The CuO leached with the slag can be separated with gravity sedimentation method due to different densities of CuO and chromite ore residue.
4 Conclusions
1) The addition of CuO can accelerate the leaching of chromium in KOH medium, the leaching rate of chromium reaches 98% with CuO after reaction for 6 h, while the leaching rate of chromium is only 60.8% without CuO. Therefore, CuO plays an important role in improving the chromium leaching in the system.
2) The effect of the CuO addition mainly contributed to the dissolution of CuO into the medium, and the chromium leaching rate increased significantly with the increase in CuO loading, and reached a plateau at the solubility of the CuO in the solution. At temperatures of 190 to 210 °C, the addition of CuO may slow down the leaching of chromium, while at temperatures of 230 and 250 °C, the addition of CuO can accelerate the leaching of chromium and improve the oxygen mass transfer in the medium.
3) The kinetics study indicates that the shrinking core model is suitable to describe the leaching process. Without the addition of CuO, the rate-determining step could be determined to be external diffusion and the apparent activation is 38.01 kJ/mol. While with the addition of CuO, the rate-determining step shifts from external diffusion to surface chemical reaction and the apparent activation is 15.79 kJ/mol when the temperature is above 230 °C. The addition of CuO improves the leaching of chromium and decreases the apparent activation energy.
References
[1] CHEN Gang, WANG Jia-jun, WANG Xiao-hui, ZHENG Shi-Li, DU Hao, ZHANG Yi. An investigation on the kinetics of chromium dissolution from Philippine chromite ore at high oxygen pressure in KOH sub-molten salt solution [J]. Hydrometallurgy, 2013, 139: 46-53.
[2] ZHANG Yang, ZHENG Shi-li, DU Hao, XU Hong-bin, ZHANG Yi. Effect of mechanical activation on alkali leaching of chromite ore [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 888-891.
[3] ZHENG Shi-li, ZHANG Yi, LI Zuo-hu, QI Tao, LI Hui-quan, XU Hong-bin. Green metallurgical processing of chromite [J]. Hydrometallurgy, 2006, 82: 157-163.
[4] COSTA M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans [J]. Critical Reviews in Toxicology, 1997, 27: 431-442.
[5] HU Guo-rong, WANG Jia-liang, PENG Zhong-dong, DU Ke, WANG Wei-gang, JIANG Qing-lai. Preparation of potassium chromate by roasting of carbon ferrochrome [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 966-972.
[6] XU Hong-bin, ZHENG Shi-li, ZHANG Yi, LI Zuo-hu, WANG Zhi-kuan. Oxidative leaching of a Vietnamese chromite ore in highly concentrated potassium hydroxide aqueous solution at 300 °C and atmospheric pressure [J]. Minerals Engineering, 2005, 18: 527-535.
[7] ZHANG Yang, ZHENG Shi-li, XU Hong-bin, DU Hao, ZHANG Yi. Decomposition of chromite ore by oxygen in molten NaOH-NaNO3 [J]. International Journal of Mineral Processing, 2010, 95: 10-17.
[8] SUN Zhi, ZHANG Yi, ZHENG Shi-Li, ZHANG Yang. A new method of potassium chromate production from chromite and KOH-KNO3-H2O binary submolten salt system [J]. AIChE Journal, 2009, 55: 2646-2656.
[9] ZHANG Yi, LI Zuo-hu, QI Tao, WANG Zhi-kuan, ZHENG Shi-li. Green chemistry of chromate cleaner production [J]. Chin J Chem, 1999, 17: 258-266.
[10] TARDIO J, BHARGAVA S, PRASAD J, AKOLEKAR D B. Catalytic wet oxidation of the sodium salts of citric, lactic, malic and tartaric acids in highly alkaline, high ionic strength solution [J]. Topics in Catalysis, 2005, 33: 193-199.
[11] ONDA A, SUZUKI Y, TAKEMASA S, KAJIYOSHI K, YANAGISAWA K. Catalytic wet oxidations of aromatic compounds over supported copper oxides [J]. J Mater Sci, 2008, 43: 4230-4235.
[12] LEE D K, KIM D S. Catalytic wet air oxidation of carboxylic acids at atmospheric pressure [J]. Catalysis Today, 2000, 63: 249-255.
[13] CHEN Gang, WANG Xiao-hui, WANG Jia-jun, DU Hao, ZHANG Ying, ZHENG Shi-li, ZHANG Yi. A new metallurgical process for the clean utilization of chromite ore [J]. International Journal of Mineral Processing, 2014, 131: 58-68.
[14] SUN Zhi, ZHENG Shi-li, ZHANG Yi. Thermodynamics study on the decomposition of chromite with KOH [J]. Acta Metallurgica Sinica, 2007, 20: 187-192.
[15] WANG Zhong-hang, ZHENG Shi-li, WANG Shao-na, QIN Ya-ling, DU Hao, ZHANG Yi. Electrochemical decomposition of vanadium slag in concentrated NaOH solution [J]. Hydrometallurgy, 2015, 151: 51-55.
刘龙杰1,2,杜 浩1,2,张 洋1,郑诗礼1,张 懿1
1. 中国科学院 过程工程研究所 绿色过程与工程重点实验室,北京 100190;
2. 中国科学院大学,北京 100049
摘 要:通过添加CuO催化剂来提高铬铁矿在KOH亚熔盐介质中的浸出率。研究反应体系温度、碱矿质量比,铜矿质量比和气体流速对铬浸出的影响。结果表明:CuO对提高铬铁矿在KOH亚熔盐介子的浸出率起着十分重要的作用。在反应温度为230 °C、碱矿质量比为6:1、搅拌转速为700 r/min、气体流速为1 L/min、反应时间为6 h的条件下铬的转化率在添加CuO时为98%,而未添加CuO时,铬的转化率仅为60.8%。动力学计算结果表明,添加CuO后在反应温度高于230 °C时,反应速度控制步骤为表面化学反应控制,反应活化能为15.79 kJ/mol;未添加CuO时,反应速度控制步骤为外扩散控制,反应活化能为38.01 kJ/mol。
关键词:铬铁矿;氧化铜;浸出;催化氧化
(Edited by Wei-ping CHEN)
Foundation item: Project (2013CB632601) supported by the National Basic Research of China; Projects (91634111, 51404227) supported by the National Natural Science Foundation of China
Corresponding author: Hao DU; Tel: +86-10-82544856; E-mail: duhao121@hotmail.com
DOI: 10.1016/S1003-6326(17)60103-1

