J. Cent. South Univ. Technol. (2010) 17: 985-990
DOI: 10.1007/s11771-010-0588-z
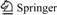
Catalytic hydrolysis of carbonyl sulfide over modified coal-based activated carbons by loading metal
YI Hong-hong(易红宏), YU Li-li(于丽丽), TANG Xiao-long (唐晓龙),
NING Ping(宁平), LI Hua(李华), WANG Hong-yan(王红妍), YANG Li-na(杨丽娜)
Faculty of Environmental Science and Engineering,
Kunming University of Science and Technology, Kunming 650093, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: A novel type of metal oxide/activated carbon catalyst was prepared by sol-gel method for the hydrolysis of carbonyl sulfide (COS). The influences of the calcination temperature, additive content (2.5%-10.0% Fe2O3, mass fraction) and the basic density of the activation process were thoroughly investigated. The surface of catalysts was characterized by Boehm titration. The products were characterized by scanning electron microscopy (SEM), X-ray diffractometry (XRD) and X-ray photoelectron spectroscopy (XPS). The results show that catalysts with 2.5%-5.0% Fe2O3 after calcining at 500 ℃ have superior activity. The conversion rate of COS increases with increasing the relative density of basic capacity loaded onto activated carbon(AC), and the activity follows the order: KOH>Na2CO3>NaHCO3. Boehm titration data clearly show that the total acidity increases (from 0.06 to 0.48 mmol/g) and the basic groups decrease (from 0.78 to 0.56 mmol/g) after COS hydrolysis and H2S adsorption. The XPS results show that the product of H2S may be absorbed by the interaction with metal compounds and O2 to form sulfate (171.28 eV) and element sulfur (164.44 eV), which lead to catalysts poisoning.
Key words: carbonyl sulfide; activated carbon; metal oxide; hydrolysis
1 Introduction
Air pollution, as other global environmental problems, is one of the crucial issues nowadays. Coal, natural gas and petroleum, as the major energy sources in chemical industry, which contain sulfur compounds, account for most of the emissions of toxic and harmful gas. Controlling sulfur-containing compound gases is of major importance in all countries as they participate in chemical and physical cycles, which leads to crucial environmental problems such as air pollution and acid rain [1-3]. In chemical industry, the presence of sulfur-containing compounds leads to corrosion problems and catalysts poisoning [4-5].
The removal of H2S was described in Refs.[6-9]. However, the removal of carbonyl sulfide (COS) has not been considered yet as much as H2S because COS is relatively inactive compared with H2S [10-14]. An effective way of removing COS is the catalytic hydrolysis due to the mild reaction condition and high conversion rate [1, 3-4].
Recently, the application of activated carbons (AC) for air purification has become more popular. This is due to their large surface area and porous structure. For physical adsorption the sizes and volumes of pores are important, whereas for special adsorption, surface reaction and chemisorptions surface chemistry play a significant role. Catalytic properties of AC surfaces are enhanced by additional modification [6-9]. SAKANISHI et al [5] reported that the impregnation of Fe on the AC greatly enhanced the removal of both COS and H2S at 300-450 ℃, which was disadvantageous for the high temperature. The supported metal oxides (where the support is typically alumina or titania) were also studied at relatively high temperatures, and H2S required the second treatment [10-14]. The layered double hydroxides (LDHs) of type [MZMgYAlX(OH)2](CO3)X/2·
0.5H2O (where M=Ni or Co, and X+Y+Z=1) were prepared by adsorbing COS at 20 ℃, but the adsorbents were easy to sinter [15-16].
Therefore, in this work, the efficiency of AC for COS removal at low temperature (50 ℃) and their performance as catalyst carrier to their surface characteristics were investigated. The effects of metal species and content, and the basic density were also discussed. Published results indicated the important effects of dynamic conditions of the experiment and H2O content [3, 11-15, 17]. So, far little attention was paid to the importance of carbon surface chemical feature for COS hydrolysis and basic strength.
2 Experimental
2.1 Catalyst preparation
The activated carbons (LJ-40 commercial), which were crushed and sieved to of 0.25-0.42 mm, were used as a matrix. The surface areas of the fresh AC were measured on a NOVA2000e (Quantachrome instruments) surface characterization system. The fresh AC were first washed 3-4 times with tap water and 3-4 times with distilled water, and then boiled in 1 mol/L KOH for 1.5 h and washed with distilled water to a constant pH, and finally dried at 120 ℃ for 4 h. Metal oxide was supported by sol-gel method. Fe(NO3)3·6H2O, Al(NO3)3·6H2O and Zn(NO3)2·6H2O were used as precursors to formulate the metal oxides. The activated carbon catalysts were supported by desired proportion (mass fraction of MOX is 2.5%-10.0%). The prepared M/AC catalysts were dried to remove moisture at 120 ℃ for 3 h. The products were then calcined at 400-600 ℃ for 3 h with a heat rate of 2 ℃/min under atmospheric condition. They were impregnated by 8% (mass fraction) Na2CO3, NaHCO3, or KOH (when used), and finally dried at 120 ℃ for 3 h. These materials are designated as M (Fe, Zn, Al)-(SC, SB, CP)/AC respectively.
2.2 Characterization
2.2.1 Scanning electron microscopy (SEM)
XL30ESEM-TMP (Poland) type scanning electron microscope (SEM) coupled with energy-dispersive X-ray spectrometer (EDS) and EDAX (PHOENIX) Genesis 2000 with a Be detector were used for microstructure observation of the interface and element distribution of samples, respectively.
2.2.2 X-ray diffractometry (XRD)
X-ray diffraction patterns were obtained with a Rigaku diffractiometer (D/MAX-2200) operated at 36 kV and 30 mA by using Ni-filtered Cu kα radiation (λ=0.154 06 nm) at a rate of 5 (?)/min from 2θ=20? to 80?. The samples were analyzed without previous treatment after deposition on an agate mortar. The identification of crystalline phases was made by matching the JCPDS files.
2.2.3 X-ray photoelectron spectroscopy (XPS)
In XPS (PHI 5500) analysis Al Kα radiation with energy of Al rake and power of 200 W was used. The analyzer resolution was 1 eV. An Ar+ ion gun was used to sputter clean specimen surfaces. The photoelectron spectra were calibrated using C 1s signal detected at a binding energy of 284.8 eV from adventitious carbon. The continuum spectrum was fitted according to the Gaussian-Lorentzian files.
2.2.4 pH of carbon surface
0.4 g of dry carbon powder was added to 20 mL of water and the suspension was stirred overnight to reach equilibrium. Then, the pH of the suspension was measured [7-9].
2.2.5 Boehm titration
The contents of acidic and basic surface groups were determined according to Boehm method [7-9, 18]. 1 g of carbon sample was placed in 25 mL of each of the following 0.1 mol/L solution, respectively: NaOH, Na2CO3, NaHCO3 and HCl. The vials were sealed and shaken for 24 h. Each of titrate was pipetted and the excess of base or acid was titrated with HCl or NaOH, as required. The contents of acidic sites of various types were calculated under the assumption that NaOH neutralizes all acidic groups (carboxylic, phenolic and lactonic groups): Na2CO3 neutralizes carboxylic and lactonic; and NaHCO3 only neutralizes carboxylic groups. The number of surface basic sites was calculated from the amount of HCl that reacted with carbon.
2.3 Desulphurization test
Catalytic activity tests were performed in a fixed- bed quartz reactor (4 mm×100 mm, diameter×length) under atmospheric pressure. COS from gas cylinder (1% COS in N2) was diluted with N2 (99.99%) and 0.5% O2 to the concentration of 1 200-1 500 mg/m3. The delivery of feed gases to the reactor was controlled by means of mass flow controllers except H2O, which was introduced through a saturator. The mass of catalysts used in the measurement was 0.4 g and the gas hourly space velocity (GHSV) was set to 1 000 h-1 and controlled by mass flow controllers. The temperature of this reactor was controlled to (50±1) ℃ over its entire length by water-bath with circulating pump. The conversion rate of COS was determined by analyzing the inlet and outlet concentration of COS using an online GC-508 gas chromatography with a flame photometric detector (FPD). The detectability of H2S and COS with GC-508 used in this work was 0.01 mg/m3. When the COS concentration of outlet gases reached 5% inlet gases, inlet stream of the outlet gases stopped. A blank measurement was carried out in order to examine the reactor adsorption of COS. No conversion rate was detected at temperatures below 50 ℃.
3 Results and discussion
3.1 Effect of different metal oxides on catalytic hydrolysis of COS
The effects of three metal precursors, Fe(NO3)3·6H2O, Al(NO3)3·6H2O and Zn(NO3)2·6H2O, with the content of 5.0% (mass fraction) for COS conversion rate were evaluated. The results are shown in Fig.1.
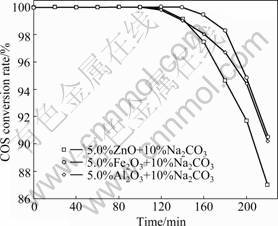
Fig.1 Effect of different metal oxides on catalytic hydrolysis of COS
For sample Fe-SC/AC, a 100% conversion rate is observed for about 140 min, while no H2S is detected during 220 min. For sample Al-SC/AC, a 100% conversion rate is observed for about 120 min, but the emission of H2S is detected almost at that time. When sample Zn-SC/AC is used,the same time of 100% conversion rate as that of sample Al-SC/AC is observed, however, H2S is not detected in 220 min. The above results suggest that the COS removal efficiency for Fe-loaded AC catalysts is higher than that of Zn- and Al-loaded AC. The activity follows the order Fe-SC/AC>Al-SC/AC>Zn-SC/AC. The high removal efficiency of COS can last for much longer period for Fe-SC/AC catalysts. Therefore, Fe is chosen as the metal species for later study.
3.2 Effect of calcination temperature on catalytic hydrolysis of COS
The calcination temperature has a great effect on the characteristics of the catalysts. It also affects the crystallinity and oxidation states. Fig.2 shows the effect of the calcination temperature on the hydrolysis of COS. The temperatures for calcination were studied in order to enhance the catalyst degree of scatter. It is clear from the data shown in Fig.2 that the activity of the catalysts is found to increase with increasing calcination temperature from 400 to 500 ℃ and then decrease at 600 ℃. The results indicate that the calcination temperature is an important factor to for hydrolysis of COS.
The phase and crystalline orientation of Fe-SC/AC were determined by XRD and presented in Fig.3. XRD patterns specify the calcination temperature. The peak intensity of the three samples is changed by increasing the temperature, but these changes are comparatively weak. This is due to the poor crystallinity and the amount
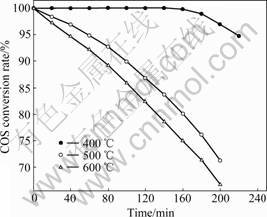
Fig.2 Effect of calcination temperatures on catalytic hydrolysis of COS
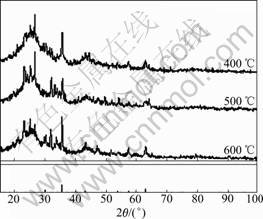
Fig.3 XRD patterns of Fe-SC/AC at different temperatures
of metal oxide is less than that of AC. In the XRD peak patterns, peaks with strong intensity appear at 2θ=30.20?, 35.56?, 51.20? and 62.92?. These diffraction peaks match to the contents of Fe2O3, which exists in the AC used as active component. The amount of Fe2O3 increases with increasing calcination temperature. After calcining at 400 ℃, the metal oxides can be considered as mixed oxides of Fe2O3 and Fe3O4, most of which exist in the form of Fe2O3. When the calcination temperature rises up to 500 ℃, the oxidation state changes. Most diffraction peaks belong to Fe2O3 except some small weak peaks that are attributed to Fe3O4. However, when the metal oxide is calcined at 600 ℃, the oxidation state is Fe2O3 without Fe3O4.
In Ref.[19] it was proposed that the oxidized decomposition temperature of the AC was higher than 450 ℃ and decomposition rates increased with increasing temperature. It also suggested that metal nitrate precursors were decomposed at 250-450 ℃ based their experiments. According to the XRD in this work and Ref.[19], there may be certain relationship between the activity and the crystallinity of the oxidation state. A plausible explanation can be found in the mechanism of crystal growth of spinel phase during calcination which is affected by the presence of Fe2O3. At the same time, the loss of AC due to calcination at high temperature (600 ℃) can cause the decrease of activity. It can be inferred that the major metal oxide is Fe2O3 after calcining at 500 ℃, which is the optimum calcination temperature for Fe-SC/AC catalysts.
3.3 Effect of Fe2O3 content on catalytic hydrolysis of COS
In order to clarify the importance of metal oxides in hydrolysis of COS, the relationship between the activity of catalytic hydrolysis of COS and the content of metal oxides in catalyst was investigated. Fe(NO3)3 solution of various concentrations were used to obtain Fe-SC/AC catalysts with various amounts of metal loading. The relationship between Fe2O3 content and the activity of catalytic hydrolysis of COS is shown in Fig.4. There is a significant difference of activity among the catalysts. The activity of catalytic hydrolysis of COS decreases with the increase of Fe2O3 content. Although Fe2O3 loading is enhanced from 2.5%-10.0% after loading four times, the activity of Fe-SC/AC is even worse. Very similar trend in the relationship between the conversion rate and Fe2O3 loading reveals that there is an optimal value of Fe2O3 loading of around 2.5%-5.0%. In addition, H2S is not detected in the tail gas at 220 min. However, as Fe loading continuously increases to 5.0%, the catalytic hydrolysis efficiency decreases significantly.
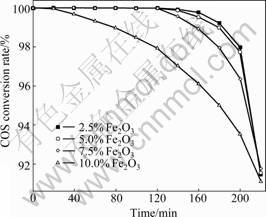
Fig.4 Effect of Fe2O3 content (mass fraction) on catalytic hydrolysis of COS
The results show that surface loading will occupy adsorption sites provided by the interface of AC, the adsorption quantity of Fe will be maintained at a constant value, and the catalytic efficiency cannot be enhanced. Therefore, the surface adsorption sites of AC will be excessively overlapped when Fe2O3 content of exceeds 7.5%, that is, the pore surface of AC can be blocked by the excessive metal oxide. So the conversion rate of COS decreases obviously. The results indicate that COS may be more effectively removed by AC and H2S can also be removed simultaneously.
SEM images of fresh and exhausted catalysts are presented in Fig.5. Significant changes are observed after reacting at 50 ℃. From Fig.5(a), the particle size distribution is uneven and the presence of a granular structure is fresh Fe-SC/AC. Simultaneously, it is observed that the exhausted AC are of the platy structures and the micropores are blocked obviously. By comparison, the catalysts tarnish after reaction.
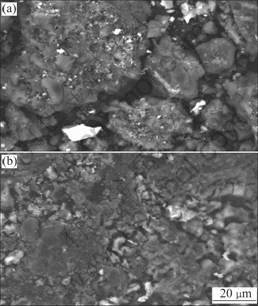
Fig.5 SEM images of modified carbon catalysts: (a) Fresh; (b) Exhausted
Fig.6 shows the results of the samples analyzed by using XPS. The S 2p photoelectron lines were studied and fitted, corresponding to binding energy of three main compounds at 164.44, 169.78 and 171.28 eV. The binding energy of 164.44 eV is attributed to element sulfur. Furthermore, there are two kinds of SO42- species on the activated carbon surface at 169.78 and 171.28 eV respectively and the SO42- species at 171.28 eV is predominant. It can be inferred that the two kinds of sulfates are Na2SO4 and Fe2(SO4)3.
LIU et al [1] investigated oxygen poisoning mechanism of catalytic hydrolysis of COS over Al2O3 at room temperature. The results showed that surface hydrogen thiocarbonate (HSCO2-) species as a key intermediate and the accumulation of sulfate on catalysts led to the poisoning of Al2O3 in the presence of oxygen. In Ref.[20] the evidence was also obtained, using IR spectroscopy, that HSCO2- formed during the reaction process, and this intermediate could readily decompose to form H2S and CO2. Based on these studies and the SEM and XPS results, it is indicated that the product of H2S can be converted to surface SO42- species and element sulfur by the interaction with metal compounds in the presence of oxygen, and the activities of catalysts decrease with the increase of SO42- species and element sulfur accumulated on the AC surface. Therefore, the deactivation of Fe-SC/AC as a function of the capacity is mainly attributed to the accumulation of deposited solid sulfur inside the pores of the catalyst.
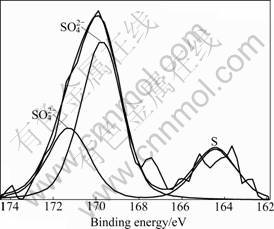
Fig.6 XPS 2p photoelectron peaks on Fe-SC/AC
3.4 Effect of basic density on catalytic hydrolysis of COS
Many investigations showed that the basic sites were catalytically important [1-3]. In order to determine whether basic sites make any contribution to the conversion rate observed in the field, a series of experiments were conducted to ascertain the reactivity of COS. The effect of basic density on catalytic hydrolysis of COS was investigated by comparing with various catalysts, which were 8% Na2CO3 and 8% NaHCO3, 8% KOH loaded on activated carbons and were referred to as Fe-SC/AC, Fe-SB/AC and Fe-CP/AC, respectively. All the samples were calcined at 500 ℃.
As shown in Fig.7, the conversion rate of COS increases with increasing relative density of basic capacity loaded onto AC. In the case of Fe-SB/AC sample, a 100% conversion rate is observed for about 140 min, followed by a decrease of COS conversion rate to about 91% at 220 min. Excellent activity is observed for sample Fe-CP/AC since a 100% conversion rate is detected for more than 220 min. Unlike samples Fe-SC/AC and Fe-SB/AC, less than 95% conversion rate COS of Fe-CP/AC is measured during 220 min test. The activity follows the order: KOH>Na2CO3>NaHCO3. On the other hand, H2S is not detected during 220 min test for these samples, indicating 100% adsorption for H2S. The fact that H2S is an acidic gas also suggests that the presence of basic environment plays a significant role in its removal. The above experimental results indicate that —OH species on the surface is the key species for
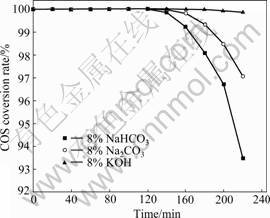
Fig.7 Effect of basic density on catalytic hydrolysis of COS
the catalytic hydrolysis of COS over AC at low temperatures.
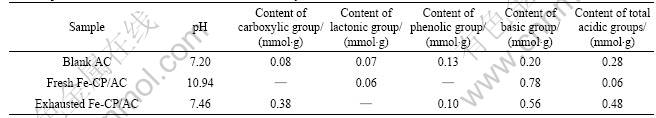
To understand what governs the process of catalytic hydrolysis of COS on carbon surface, a deeper insight on samples under investigation and the more detailed changes in surface chemistry are seen from Boehm titration results. There are significant changes in their chemistry, reported in Table 1 as pH and the amount of carboxylic, lactonic, phenolic and total acidic groups present on each carbon surface. The average effect of the amount and density of basic groups reflects pH on the surface. As expected, the larger the amount of basic groups, the higher the pH of the carbon surface after impregnation. Contrary to the before and exhausted samples, the amount of all acids increase dramatically. The fresh Fe-CP/AC sample has the highest basic 0.78 mmol/g among the three samples studied, corresponding to its pH of 10.94. For the total amount of acidic and basic groups, the amount of acidic groups of exhausted sample is the highest and that of the basic groups decreases (from 0.78 to 0.56 mmol/g); at the same time, this is reflected by a reduction in pH (from 10.94 to 7.46). The data clearly show that the total acidity increases and the basic groups decrease after COS hydrolysis and H2S adsorption. The apparent discrepancy between an increase in the number of groups and a decrease in the concentration of OH ions can be explained by the fact that the pH of the surface is a result of dissociation of both acidic and basic groups present on the surface. The low average pH of carbon surface suppresses the dissociation of H2S and creation of HS-. Those ions, when present in low concentration in small pores, are oxidized to sulfur oxides from which sulfuric acid forms. The results indicate that the effect of strong acids that are contributed to the concentration of H+ is more significant than that of weak acids [6, 8]. Generally speaking, the basic sites of carbon surfaces are related to the hydrolysis of COS.
Table 1 pH and Boehm titration results of surface chemistry
4 Conclusions
(1) Three metal precursors (Fe, Zn, Al) loaded on AC are prepared for the hydrolysis of COS. The activity follows the order: Fe-SC/AC>Al-SC/AC>Zn-SC/AC. The high removal efficiency of COS can last for much longer period for Fe-SC/AC catalysts at 50 ℃.
(2) The phase and crystalline orientation of Fe-SC/AC are determined by XRD. The results show that the optimum calcination temperature is 500 ℃ for Fe-SC/AC catalysts.
(3) The conversion rate of COS decreases with the increase of Fe2O3 content. The optimum Fe2O3 content is 2.5%-5.0%.
(4) The conversion rate of COS increases with increasing relative density of basic capacity loaded onto AC. The activity follows the order: KOH>Na2CO3>NaHCO3. Boehm titration results show that the amount of basic groups decreases from 0.78 to 0.56 mmol/g and that of acidic groups increases from 0.06 to 0.48 mmol/g after reaction. The basic sites of carbon surfaces are beneficial to the hydrolysis of COS.
(5) The COS and product H2S can be removed simultaneously by Fe-CP/AC catalysts. XPS results show that element sulfur and SO42- species are accumulated on the surface, which may contribute to catalysts poisoning.
References
[1] LIU Jun-feng, LIU Yong-chun, XUE Li, YU Yun-Bo, HE Hong. Oxygen poisoning mechanism of catalytic hydrolysis of COS over Al2O3 at room temperature [J]. Acta Physico-Chimica Sinica, 2007, 23: 997-1002.
[2] DANIELACHE S O, JOHNSON M S, NANBU S, GRAGE M M L, MCLINDEN C, YOSHIDA N. Ab initio study of sulfur isotope fractionation in the reaction of COS with OH [J]. Chemical Physics Letters, 2008, 450: 214-220.
[3] WANG Li, WANG Shu-dong, YUAN Quan, LU Guan-zhong. COS hydrolysis in the presence of oxygen: Experiment and modeling [J]. Journal of Natural Gas Chemistry, 2008, 17: 93-97. (in Chinese)
[4] ZHANG Yi-qun, XIAO Zhong-bin, MA Jian-xin. Hydrolysis of carbonyl sulfide over rare earth oxysulfides [J]. Applied Catalysis B: Environmental, 2004, 48: 57-63.
[5] SAKANISHI K, WU Z H, MATSUMURA A, SAITO I, HANAOK T, MINOWA T, TADA M, IWASAKI T. Simultaneous removal of H2S and COS using activated carbons and their supported catalysts [J]. Catalysis Today, 2005, 104: 94-100.
[6] XIAO Yong-hou, WANG Shu-dong, WU Di-yong, YUAN Quan. Catalytic oxidation of hydrogen sulfide over unmodified and impregnated activated carbon [J]. Separation and Purification Technology, 2008, 59: 326-332.
[7] BAGREEV A, BANDOSZ T J. H2S adsorption/oxidation on unmodified activated carbons: Importance of prehumidification [J]. Carbon, 2001, 39: 2303-2311.
[8] BAGREEV A, ADIB F, BANDOSZ T J. pH of activated carbon surface as an indication of its suitability for H2S removal from moist air streams [J]. Carbon, 2001, 39: 1897-1905.
[9] BAGREEV A, MENENDEZ J A, DUKHNO I, TARASENKO Y, BANDOSZ T J. Bituminous coal-based activated carbons modified with nitrogen as adsorbents of hydrogen sulfide [J]. Carbon, 2004, 42: 469-476.
[10] WEST J, WILLIAMS B P, YOUNG N C, RHODES C, HUTCHINGS G J. Ni- and Zn-promotion of γ-Al2O3 for the hydrolysis of COS under mild conditions [J]. Catalysis Communications, 2001, 2(3/4): 135-138.
[11] WEST J, WILLIAMS B P, YOUNG N C, RHODES C, HUTCHINGS G J. New directions for COS hydrolysis: Low temperature alumina catalysts [J]. Studies in Surface Science and Catalysis, 1998, 119: 373-378.
[12] WILLIAMS B P, YOUNG N C, WEST J, RHODES C, HUTCHINGS G J. Carbonyl sulphide hydrolysis using alumina catalysts [J]. Catalysis Today, 1999, 49: 99-104.
[13] CLARK P D, DOWING N I, HUANG M. Conversion of CS2 and COS over alumina and titania under Claus process condition: Reaction with H2O and SO2 [J]. Applied Catalysis B: Environmental, 2001, 31: 107-112.
[14] LAPERDRIX E, JUSTIN I, COSTENTIN G, SAUR O, LAVALLEY J C, ABOULAYT A, RAY J L, NEDEZ C. Comparative study of CS2 hydrolysis catalyzed by alumina and titania [J]. Applied Catalysis B: Environmental, 1998, 17: 167-173.
[15] SPARKS D E, MORGAN T, PATTERSON P M, TACKETT S A, MORRIS E, CROCKER M. New sulfur adsorbents derived from layered double hydroxides (Ⅰ): Synthesis and COS adsorption [J]. Applied Catalysis B: Environmental, 2008, 82: 190-198.
[16] TOOPS T J, CROCKER M. New sulfur adsorbents derived from layered double hydroxides (Ⅱ): DRIFTS study of COS and H2S adsorption [J]. Applied Catalysis B: Environmental, 2008, 82: 199-207.
[17] LIANG Mei-sheng, LI Chun-hu, GUO Han-xian, XIE Ke-chang. Study on reaction kinetics of COS hydrolysis at lower temperature [J]. Chinese Journal of Catalysis, 2002, 23(4): 357-362. (inChinese)
[18] QIU Jie-shan, WANG Yan-bin, DENG Yi-zhao. Measurement of the acidic groups in activated carbon surface and their effect on adsorption behaviors [J]. Carbon Techniques, 1996, 4: 11-17. (inChinese)
[19] SCHWICKARDI M, JOHANN T, SCHMIDT W, BUSCH O, SCHUTH F. High surface area metal oxides from matrix assisted preparation in activated carbons [J]. Scientific Bases for the Preparation of Heterogeneous Catalysts, 2002, 143: 93-100.
[20] FIEDOROW R, LEAUTE R, DALLA-LANA I G. A study of the kinetics and mechanism of COS hydrolysis over alumina [J]. Journal of Catalysis, 1984, 85(2): 339-348.
(Edited by CHEN Wei-ping)
Foundation item: Project(50908110) supported by the National Natural Science Foundation of China; Project(2008AA062602) supported by the National High-Tech Research and Development Program of China; Project(20090451431) supported by China Postdoctoral Science Foundation; Project(2007PY01-10) supported by Young and Middle-aged Academic and Technical Back-up Personnel Program of Yunnan Province, China
Received date: 2009-11-02; Accepted date: 2010-04-28
Corresponding author: TANG Xiao-long, Professor; Tel: +86-871-5170905; E-mail: txl-km@163.com