J. Cent. South Univ. Technol. (2007)06-0788-05
DOI: 10.1007/s11771-007-0150-9
Preparation, characterization and photocatalytic behavior of WO3-TiO2/Nb2O5 catalysts
TONG Hai-xia(童海霞), CHEN Qi-yuan(陈启元), HU Hui-ping(胡慧萍),
YIN Zhou-lan(尹周澜), LI Jie(李 洁), ZHOU Jian-liang(周建良)
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China,)
Abstract: TiO2/Nb2O5 photocatalyst loaded with WO3 (WO3-TiO2/Nb2O5) was prepared by a modified hydrolysis process, and characterized by X-ray diffractometry, transmission electron microscopy, Raman spectra and UV-Vis diffuse refraction spectroscopy. The photocatalytic activity of WO3-TiO2/Nb2O5 was investigated by employing splitting of water for O2 evolution. The results indicate that WO3 loading can pronouncedly improve the photocatalytic activity of TiO2/Nb2O5 by using Fe3+ as an electron acceptor under UV irradiation. The optimum molar fraction of the loaded WO3 is 2%, and the largest speed of O2 evolution for 2% WO3-TiO2/Nb2O5 catalyst is 151.8 ?mol/(L?h).
Key words: photocatalysis; load; oxygen evolution; rutile TiO2; Nb2O5; WO3
1 Introduction
Nowadays, because of the high efficiency, cleanness and other advantages of hydrogen energy, more and more attention is paid to studying photocatalytic decomposition of water. FUJISHIMA and HONDA[1] used N-type semiconductor TiO2 to split water and produce H2 in the last century. As titania with large band gap is cheap, nontoxic, stable and reclaimable[2], it is a very promising kind of semiconductor material that can be photo-excited to generate electron-hole pairs on its surface and create a strong oxidation ability when it absorbs light with energy higher than its band gap and the electrons in the valence band are excited to the conduction band. However, pure titania is of low photoactivity and slow speed of photocatalytic reaction of water, leading to be difficult to be practically used. So it is necessary to improve its photoactivity and enhance the speed of photocatalytic reaction of water. Generally, the photocatalytic activity of semiconductor can be improved through doping or loading semiconductor, such as Ru3+/TiO2[3], TiO2-Al2O3, V2O5/TiO2-Al2O3[4], TiO2- La2O3[5], TiO2-WO3[6], Pt-TiO2[7], NiO and WO3- TiO2[8-15]. And most of these doped semiconductors are used to photo-decompose organic pollutants, and some of them are used to split water with low photocatalytic efficiency[3]. ZHANG et al[9] reported that WO3 thin films sputtered on TiO2 can improve the speed of the photocatalytic degradation of methylene blue, but there is no further report about its application in water splitting. The reaction of photocatalytic splitting water is carried out via two half reactions: one is the photo-reduction with H2 evolution, the other is the photo-oxidation with O2 evolution, and the later is more difficult to achieve. In the present study, by using Fe3+ as electron acceptors, the catalyst WO3-TiO2/Nb2O5 prepared by a modified hydrolysis process and used for the photocatalytic oxidation of water with O2 evolution was investigated.
2 Experimental
2.1 Preparation of photocatalyst
TiO2 colloid solution was prepared by the hydrolysis of tetrabutyl titanate (Ti(OC4H9)4). Ti(OC4H9)4 (A.R. grade) was dissolved in ethanol (A.R. grade), and the mixture was added to distilled water drop by drop under the stirring of a magnetic stirrer. Then niobium oxide (Nb2O5) was added into the solution (molar ratio of Nb2O5 to Ti was 1:100). The solution was stirred for 2 h and then deposited for 6-8 h before being dried at 373 K to obtain a white precursor. The white precursor was ground in a carnelian mortar and loaded in a navicular quartz vessel to be calcined at 1 223 K in air for 5 h. The white precursor was ground again to obtain rutile TiO2 doped with 1%Nb2O5 (molar fraction), i.e. TiO2/Nb2O5.
TiO2/Nb2O5 was dipped in the ammonium tungstate solution, then dried and calcined at 973 K in air for 5 h. A series of catalysts with molar fraction of the loaded WO3 of 0, 1%, 2%, 5%, 10% and 15% were obtained respectively.
2.2 Characterization of catalysts
X-ray diffractometry (XRD) was used to check the coexistence of different crystal phases of the catalysts by a HATCHI D/max 2 250 powder X-ray diffractometer. The diffractograms were recorded with Cu Ka radiation (0.154 056 nm) over a 2θ range from 10° to 90°. A plumbaginous counter with monochromator was used. The X-ray tube was operated at 40 kV and 300 mA.
The morphology and grain size of the catalysts were examined by transmission electron microscopy(TEM) using a JEM-1230 instrument made by JEOL Company in Japan.
UV-Vis diffuse refraction spectroscope measure- ments were carried out on a Beijing Purkinje TU-1901 UV/Vis spectrophotometer equipped with a diffuse reflectance accessory and an IS19-1 integrating sphere, and BaSO4 powder was used as reference.
Raman spectra were recorded on a RT1-30 spectrophotometer. The line with wavelength of 4 880 nm from an argon ion laser was used as the excitation source. The laser power applied was 326 mW, and the raster width was 201 ?m. The scan range was from 100 to 1 100 cm-1.
The results were examined by HATCHI SP-2305 gas chromatography which was equipped with a thermal conductivity detector. The argon was used as the carrier gas and the fixed phase was molecule sieve with size of 0.5 nm.
2.3 Test of photocatalytic activity
The photocatalytic reaction was performed through using a closed gas-circulating system with an inner irradiation reactor. A light source (250 W high-pressure Hg lamp) was covered with a glass jacket made up of quartz. The reactor temperature was kept constant at 293 K by using cooling water. A mixture of catalyst (2 g), distilled water (640 mL) and a required amount of Fe2(SO4)3 in the reactor was completely degassed and then argon gas was introduced into the system. The catalyst powder was suspended by using a magnetic stirrer. The product gas oppressed the liquid in the reactor into the graduated cylinder through the catheter 8 in Fig.1. So the volume of product gas can be obtained through the graduated cylinder indirectly. The evolution of O2 was detected by gas chromatography. The sketch of photocatalytic reactor is shown in Fig.1.
3 Results and discussion
3.1 Characterization of catalyst
Fig.2 shows the XRD patterns for the catalysts TiO2/Nb2O5 calcined at 1 223 K for 5 h and then at 973 K for 5 h, TiO2/Nb2O5 calcined at 1 223 K for 5 h
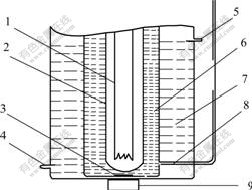
Fig.1 Sketch of photocatalytic reactor
1-Hg lamp; 2-Glass jacket; 3-Magnetic bar; 4-Cooling water inlet; 5-Cooling water outlet; 6-Mixture of reactor; 7-Cooling water; 8-Catheter(Outlet); 9-Magnetic stirrer
and 15%WO3-TiO2/Nb2O5 calcined at 1 223 K for 5 h and then at 973 K for 5 h. Some distinct peaks for Nb2O5 can be observed in the second pattern. This indicates that when the precursor is calcined at 1 223 k for 5 h Nb2O5 is still not doped into the lattice of TiO2 entirely. However, after calcined at 1 223 K for 5 h and then at 973 K for 5 h, no distinct peaks for Nb2O5 are observed in the first and the third patterns, which indicates that Nb2O5 is doped into the lattice of TiO2 wholly. Therefore, the catalyst of TiO2/Nb2O5 is also calcined at 1 223 K for 5 h and then at 973 K for 5 h before the photocatalytic experiments. According to Scherrer equation D = kλ/(Δbcosθ), the average size of TiO2/Nb2O5 (calcined at 1 223 K for 5 h and then at 973 K for 5 h) was calculated to be about 55.3 nm.
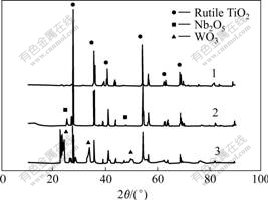
Fig.2 X-ray diffraction patterns of calcined TiO2/Nb2O5 and 15%WO3-TiO2/Nb2O5
1-TiO2/Nb2O5 calcined at 1 223 K for 5 h and then at 973 K for 5 h;2-TiO2/Nb2O5 calcined at 1 223 K for 5 h; 3-15% WO3-TiO2/Nb2O5 calcined at 1 223 K for 5 h and then at 973 K for 5 h
Fig.3 shows TEM image of 2%WO3-TiO2/Nb2O5 nanoparticles, indicating that TiO2 particles modified by WO3 and Nb2O5 are almost with regular geometric shape, and the particles size distribution is narrow. The particles disperse well. The even size of the particles is about 55 nm from the TEM image using statistic method. This is in a good agreement with the result from Scherrer equation.
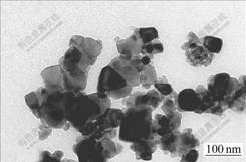
Fig.3 TEM image of 2%WO3-TiO2/Nb2O5 nanoparticles
The Raman spectra of WO3-TiO2/Nb2O5 samples are shown in Fig.4. It is known that rutile TiO2 has two characteristic peaks at around 440 and 612 cm-1[16]. WO3 has four characteristic peaks at around 807, 715, 324 and 270 cm-1[17] and its major peak is at 807 cm-1[16]. Fig.4 shows that two characteristic peaks of rutile TiO2 at around 440 and 612 cm-1 are in agreement with literature report, but characteristic peaks of WO3 at around 715 and 324 cm-1 are overlapped by the characteristic peaks of rutile TiO2 at around 440 and 612 cm-1, respectively[18]. A new peak at 807 cm-1 appears when the molar fraction of WO3 is more than 2%, which indicates the existence of crystalline WO3[13], because a peak of WO3 at 807 cm-1 is assigned to W-O stretching mode[19]. It also indicates that WO3 loaded on the surface of TiO2 is more than one layer when the molar fraction of WO3 is more than 2%. By using the XPS measurements for WO3-TiO2, SCHOLZ et al[20] reported that when WO3 disperses on the surface of TiO2, and the mass fraction of W reaches about 3.8%, the monolayer coverage is 1.11, and its molar fraction of WO3 loaded on the surface of TiO2 at that time is about 1.74%. With the increase of the concentration of the loaded WO3, its characteristic peaks become more and more obvious.
Fig.5 shows the UV-Vis diffuse reflectance spectra of the catalysts TiO2/Nb2O5, 2%WO3-TiO2/Nb2O5, 5%WO3-TiO2/Nb2O5 and 10%WO3-TiO2/Nb2O5. It shows that after being loaded the catalyst of TiO2/Nb2O5 exhibits a lower reflection percent for visible light. But their reflection percentages to UV-light are the same. The result indicates that TiO2/Nb2O5 loaded with WO3 absorbs visible light more conveniently than that of TiO2/Nb2O5. The band gap of WO3 is about 2.7 eV, which is narrower than that of rutile TiO2 (3.0 eV) [21]. Therefore, it can easily absorb light with lower energy. However, the transition of electrons from the WO3 valance band to its conduction band is not observed in Fig.5.
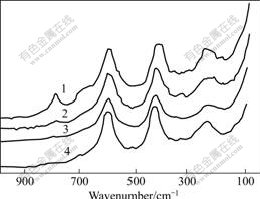
Fig.4 Raman spectra of WO3-TiO2/Nb2O5 catalyst samples
1-10% WO3-TiO2/Nb2O5; 2-2% WO3-TiO2/Nb2O5;
3-1% WO3-TiO2/Nb2O5; 4-TiO2/Nb2O5
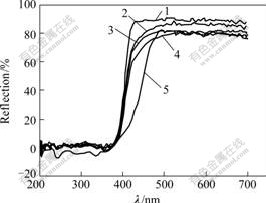
Fig.5 Diffusion reflectance UV-Vis spectra of catalysts
1-TiO2/Nb2O5; 2-2% WO3-TiO2/Nb2O5; 3-10%WO3-TiO2/Nb2O5; 4-5%WO3-TiO2/Nb2O5; 5-WO3
3.2 Photocatalytic activity of catalysts
The photocatalytic activity of WO3-TiO2/Nb2O5 was studied through the splitting of water for O2 evolution. The results are shown in Fig.6. It shows that the rate of O2 evolution using WO3-TiO2/Nb2O5 as catalyst is faster than that of TiO2/Nb2O5 within 12 h. With the increase of the quantity of loaded WO3, the rate of O2 evolution also increases. It reaches the maximum rate of 151.8 ?mol/(L?h) when the molar fraction of the loaded WO3 is 2%. The rate of O2 evolution declines when the molar fraction of the loaded WO3 is more than 5%. Therefore, TiO2/Nb2O5 loaded with WO3 in a suitable amount can improve the photocatalytic activity of TiO2/Nb2O5.
Generally, the photocatalytic activity of photo- catalyst is determined by its ability of light absorbing, the efficiency of separation between photoelectrons and holes, the transfer rate of charge carriers[22]. HUANG et al[23] have found that metallic ions doping can introduce defects into the crystal lattices or change the crystallinity and improve the photocatalytic efficiency. The rutile TiO2 doped with Nb2O5 for photo-catalytic oxidation of water with O2 evolution has been researched. The result indicates that the rutile TiO2 doped with 1%Nb2O5 shows the highest photocatalytic efficiency. So in this paper WO3 was loaded on rutile TiO2 doped with 1% Nb2O5.
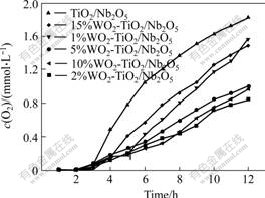
Fig.6 Dependence of photocatalytic O2 evolution on molar fraction of loaded WO3
When the surface of the semiconductor is irradiated by light whose energy is higher than the band gap of this semiconductor, electrons will obtain much energy to jump onto the conduction band and become free electrons named photoelectrons. Thus holes are left in the valence band.
Because the band gap of WO3 is lower than that of rutile TiO2, electrons can be gathered in the conduction band of WO3 and holes are left in the valence band of TiO2 after the photo-irradiation of the catalyst. The produced photoelectrons and holes can be separated efficiently. During the process of photocatalytic reactions, photo-electrons in the conduction band of WO3 can be captured by electron acceptor Fe3+, and anions OH– in water can release their electrons in the valence band of TiO2 with O2 evolution. Fig.7 shows the sketch of the electron transfer in the photocatalytic reaction.
The maximum rate for O2 evolution via water splitting for photocatalyst WO3-TiO2/Nb2O5 is 151.8 ?mol/(L·h) when the molar fraction of the loaded WO3 is 2% using Fe3+ as an electron acceptor under UV irradiation, which can be explained as follows: when the UV-light irradiates on the surface of photocatalyst WO3- TiO2/Nb2O5, the photoelectrons yield and then jump from the valance band of TiO2 to its conduction band. The stored photoelectrons in the atomic nucleus have three paths to go. The first is to luminescence and then vanish; the second is to go back to the valance band of TiO2 and compound with holes; the third is to jump to the conduction band of WO3 and compound with Fe3+ (in Fig.7). Loading WO3 can make less photoelectrons choose the second path and improve the separation for photoelectrons-holes and the transfer of charge carriers. So the photocatalytic activity of TiO2 is increased. This conclusion is in a good agreement with the result in Refs.[24-25]. ZHANG et al[24] and LI et al[25] reported that because the energy level of Ti3d electrons is close to that of W5d electrons and therefore Ti4+ and W6+ can interact, some quantity of WO3 that is doped into TiO2 can make the absorbance spectrum of TiO2 red-shifted and the light absorbing strengthened. However, the transfer center of electrons becomes the recombination center of them with over-loaded WO3, and the efficiency of separation for photoelectrons and holes can be reduced, which leads to the decrease of the photocatalytic activity of TiO2.
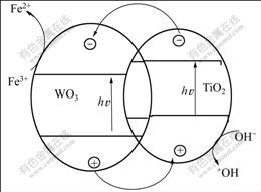
Fig.7 Sketch of electrons transfer in photocatalytic reaction
4 Conclusions
1) TiO2/Nb2O5 photocatalysts is synthesized using hydrolysis process. Nb2O5 is doped into the crystal lattice of TiO2 after calcined twice. And then TiO2/Nb2O5 is dipped in the ammonium tungstate solution, dried, and calcined. At last WO3-TiO2/Nb2O5 powders were obtained.
2) Loading WO3 can improve the photocatalytic activity of TiO2/Nb2O5. A maximum rate of water splitting with WO3 loaded TiO2/Nb2O5 as photocatalyst is 151.8 ?mol/(L·h) when the molar fraction of the loaded WO3 is 2% using Fe3+ as an electron acceptor under UV irradiation for 12 h.
References
[1] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38.
[2] HOFFMAN M R, MARTIN S T, CHOI W. Environmental applications of semiconductor photocatalysis[J].Chem Rev, 1995, 95(1): 69-96.
[3] KAMAT P V. Photochemistry on nonactive and reactive (semiconductor) surfaces[J]. Chem Rev, 1993, 93(1): 267-271.
[4] JU Jian-feng, SHI Lei, LI Cheng-jun, et al, Preparation of TiO2-WO3 nanopowder and its photocatalysis for formaldehyde degradation[J]. Fine Chemicals, 2004, 21(3): 181-184. (in Chinese)
[5] OHNO T, TANIGAWA F, FUJIHARA K, et al. Photocatalytic oxidation of water by visible light using ruthenium-doped titanium dioxide powder[J]. Photochemistry and Photobiology A: Chem, 1999, 127: 107-110.
[6] SANG C M, HIROAKI M. Photocatalytic production of hydrogen from water using TiO2 and B/TiO2[J]. Catalysis Today, 2000, 58: 125-132.
[7] CHOI W Y, TERMIN A, HOFFMANN M R. The role of metal-ion dopants in quantum-size TiO2-correlation between photoreactivity and charge-carrier recombination dynamics [J]. Phys Chem, 1994, 98(51): 13669-13679.
[8] CHENG P, ZHENG M P, JIN Y P. Preparation and characterization of silica-doped titania photocatalyst through sol-gel method[J]. Mater Letters, 2003, 57: 2989-2994.
[9] ZHANG Qi, LI Xin-jun, LI Fang-bai, Effect of preparation process of WO3/TiO2 films on photo-catalytic activity under visible light[J]. The Chinese Journal of Nonferrous Metals, 2002, 16(12): 1299-1303. (in Chinese)
[10] BENJARAM M R, PAVANI M S, ETTIREDDY P R. Surface characterization of La2O3-TiO2 and V2O5/La2O3-TiO2 catalysts[J]. J Phys Chem, 2002, 106: 5695-5700.
[11] LI Fang-bai, GU Guo-bang, LI Xin-jun, et al. Preparation of WO3/TiO2 nanopowder and its photocatalytic behavior[J]. Phys Chem Trans, 2000, 11(16): 997-1002.
[12] CAI Nai-cai, WANG Ya-ping, CAO Yin-liang. A study of supported Pt-TiO2 photocatalyst[J]. Chinese Journal of Catalysis, 1999, 20(2): 7-18.
[13] BIN X, LIN D, Yin F, YI C. A study on the dispersion of NiO and/or WO3 on anatase of catal[J]. Journal of Catalysis, 2000, 193: 88-95.
[14] KAZUHIRO S, RINTARO Y, HITOSHI K, et al. Photocatalytic decomposition of water into H2 and O2 by a two-step photoexcitation reaction using a WO3 suspension catalyst and an Fe3+/Fe2+ redox system[J]. Chem Phys Lett, 1997, 277: 387-391.
[15] BENJARAM M R, BISWAJIT C. X-ray photoelectron spectroscopy study of V2O5 dispersion on a nanosized Al2O3-TiO2 mixed oxide[J]. Langmuir, 2001, 17: 1132-1137.
[16] ENGWEILER J, HARF J, BAIKER1 A. WOx/TiO2 catalysts prepared by grafting of tungsten alkoxides: Morphological properties and catalytic behavior in the selective reduction of NO by NH3[J]. Journal of Catalysis, 1996,159: 259-269.
[17] DO Y R, LEE K, DWIGHT K. The effect of WO3 on the photocatalytic activity of TiO2[J]. Journal of Solid State Chemistry, 1994, 108(1): 198–204.
[18] CHAN S S, WACHS I E, MURREL L L. Insitu laser Raman-spectroscopy of supported metal-oxides[J]. Phys Chem, 1984, 88(24): 5831-5835.
[19] RAMANL N C, SULLIVAN D L, EKERDT J G. Selective oxidation of 1-butene over silica-supported Cr(VI), Mo(VI), and W(VI) oxides[J]. Catal, 1998, 176: 143-154.
[20] SCHOLZ A, SCHNYDER B, WOKAUN A. Influence of calcinations treatment on the structure of grafted WOx species on titania[J]. Journal of Molecular Catalysis A: Chemical, 1999, 138: 249.
[21] HAN Wei-ping. Catalytic Chemistry Introduction[M]. Beijing: Science Press, 2003: 272-310. (in Chinese)
[22] ZHANG Qi, LI Xin-jun, LI Fang-bo, et al. Investigation on visible-light activity of WOx/TiO2 photocatalyst[J]. Phys Chem Trans, 2004, 20(5): 507-511. (in Chinese)
[23] HUANG Cui-ying, YOU Wan-sheng, DANG Li-qin, et al. Effect of Nd3+ doping on photocatalytic activity of TiO2 nanoparticles for water decomposition to hydrogen[J]. Chinese Journal of Catalysis, 2006, 3(27): 203-209. (in Chinese)
[24] ZHANG Peng-yi, YU Gang, JIANG Zhan-peng. Review of semiconductor photocatalyst and its modification[J]. Advance in Environment Science, 1997, 5(3): 1-10. (in Chinese)
[25] LI Fang-bai, GU Guo-bang, LI Yong-jin. Enhanced rates of photocatalytic behavior using WO3/TiO2 coupled semiconductor nanopowder[J]. Environment Science, 1999, 20(4): 75-78. (in Chinese)
(Edited by CHEN Wei-ping)
Foundation item: Project(2002AA327140) supported by National High-Tech Research and Development Program of China
Received date: 2007-03-29; Accepted date: 2007-05-18
Corresponding author: CHEN Qi-yuan, PhD, Professor; Tel:+86-731-8877364; E-mail: cqy@mail.csu.edu.cn